Introduction
Engineered nanomaterials (ENMs) provide novel solutions to address various limitations in pharmaceuticals, agriculture, engineering, electronics, etc. At present the agriculture sector is under tremendous pressure for enhancing the crop-yield to meet the food demands of increasing human population across the world. According to several international surveys and reports [1-4], various challenges come in the way for enhancing crop production in a sustainable manner. Besides the natural climatic causes, knowledge gap and poor decision making to use modern technology dominate the reasons for low agricultural productivity. As a consequence, across the globe, many agricultural sectors are still facing issues like dilatory uptake of fertilizers and pesticides [5], persistence of unwanted and harmful chemicals in crops [6], retention of agro-chemicals in fertilizers/pesticides in the soil resulting in deterioration of soil quality [7]; and subsequent run off to further environmental components like water bodies [8]. The research reports from the field of agriculture-nano-biotechnology suggest that nano-science can address several of the contemporary issues and help achieve improved yield from the limited resources in a sustainable manner [9-11]. Agricultural nanotechnology can provide tailor-made solutions for particular agro-climatic zones or soil types through development of nano-nutrients, nanocomposites [11], nano-fertilizers, and nano-pesticides [12]. In addition, the use of novel bio-sensors along with modern information technology helps in site specific crop management [9]. This enables the farmers to determine the accurate amounts of resources such as water, fertilizers, insecticides and pesticides. In practicing so, eventually there is optimal utilization of these resources, reduction in cost, balance in soil health, prevention in additional run –off and most importantly controlling the adverse impact on the environment [13]. The on-field applications have also been extended to food packaging and storage, where nanotechnology reportedly aid in anti-microbial activities [14]. The recent research activities for addressing the pressing demands in agriculture by using a sustainable approach have yielded a variety of engineered nano materials (ENMs), produced via different synthesis routes (physical, chemical or biological [15]). However, these developments have also sparked debates regarding the probable health and environmental impacts associated with the use of nanomaterials for agricultural improvement [16]. This is largely due to several inconsistent research reports that suggest beneficial or harmful nature of certain nano-materials [17]. The inconsistency between the reports has largely arisen due to absence of complete data regarding physicochemical characteristics of ENMs and associated interactions with the biotic and abiotic components of environment [18,19].
A rapidly developing approach aiming at minimizing ENM associated health and environment risk is via the use of biological entities (as such or their extracts) to design desired nanomaterials. The biologically synthesized nanomaterials are hypothesized to be more benign towards human health and environment as compared to chemically synthesized nanomaterials [20], but a detailed safety analysis and risk assessment is a big research lacuna at present. ENMs synthesized through biological routes (biogenic ENMs) have an additional matrix of biomolecules on the surface by the virtue of the synthesis method (Figure 1). The biomolecular components such as proteins, carbohydrates, phytochemicals, etc. from the synthesizing extracts tend to cap the naïve particles [21,22]. The physicochemical properties and behavior of the biogenic ENMs can be characterized accurately under idealized conditions, however, when exposed to the environment, these tend to acquire different properties due to interaction with various biotic and abiotic factors [23]. Herein, proteins and other biomolecules interact with surface of ENMs, forming a biomolecular corona that critically affects their biological and technical identities [24].The bimolecular corona may impact the in vitro and in vivo applications of ENMs, and therefore the mechanistic understanding of the biophysical forces regulating their interactions with various biological and environmental factors is required to estimate/predict influence of the biogenic ENMs on humans and environment.
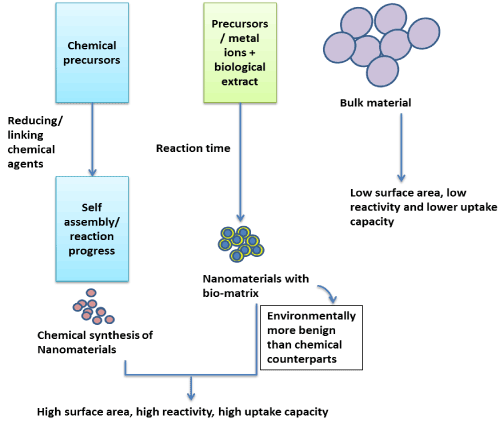
Figure 1. Schematic showing properties of chemically synthesized and biogenic ENMs as compared to bulk form for agricultural applications.
ENMs dispersal in abiotic and biotic environment
The ENMs, when applied in agricultural fields, can find their way to the environmental components: soil, water and air. Interactions of bio-corona with different factors such as different electrolytes with monovalent and divalent ions, natural organic matter (NOM) [25], terrestrial and aquatic plants and animal species; present in the natural environment determine the fate of the ENMs transformations such as dissolution, agglomeration, sedimentation, interaction and release of surface moieties within the immediate surrounding [26,27]. This could greatly affect the pathway and extent of emission and consequently the impact on the human and environment. Yet, it is still an unsolved question to accurately determine the relevant concentrations of applied biogenic ENMs that will be released from one stratum to another at any given time [28]. It becomes difficult to predict the relevant concentration of biogenic ENMs once released. Such a challenge is due to very little data on prevalence of biogenic ENM as a prospective, commercially available agricultural product [29,30]. Several risk assessment approaches have been used to simulate and calculate predicted environmental concentrations (PECs) of chemically synthesized ENMs but there is an unfilled gap about the biogenic ENMs [31-33].
Most of the inferences on transformation and emission of nanomaterials have been drawn by using chemically synthesized ENMs [34-36]. However, only a few experiments have been carried out under natural conditions to analyze the fate of the biogenic ENMs [37]. Research needs to be expedited to identify environmental transportation pathways of biogenic ENMs, along with subsequent evaluation of their behaviour, fate, dissolution, residence time and concentrations within different media – soil, water and air. For this different mass flow models can be developed to predict the concentration of nanomaterials from manufacturing and after application to various compartments of the environment. Simulations may serve as an important area of focus to assess the physical and chemical transformations of ENMs once adapted in agricultural practices.
ENM Characterization and Transformation
Risk assessment and life cycle analysis of the ENMs, especially biogenic ENMs, is a growing field and demands a comprehensive understanding of ENM characterization and transformation. A significant contribution on transformation and fate of environmentally released ENMs is made by studying complex interaction of ENMs with different model systems and mesocosm studies, but several challenges harbor the strategies to study ENM safety [38,39]. Many of these challenges center on the tension between understanding the mechanism of interaction or the ENM transformation as it relates to more complex whole organism or ecosystem models [40,41]. The complexity enhances when there is an intervention of biogenic ENM having an additional bio-corona [42] (Figure 2).
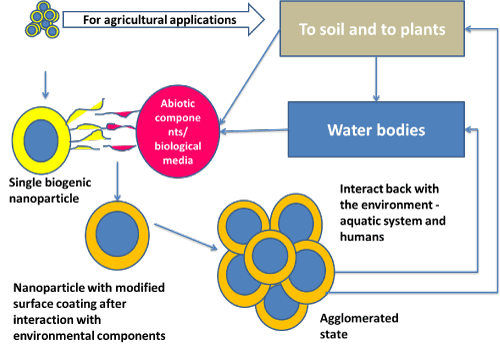
Figure 2. Interaction of agriculturally relevant biogenic ENM within the environment – a schematic.
The most common techniques used to characterize ENMs are X-ray based (diffraction, absorption spectroscopy and photoelectron spectroscopy, energy-dispersive X-ray spectroscopy), electron microscopy (transmission and scanning), atomic force microscopy, spectroscopy (UV-vis, infrared, atomic absorption and nuclear magnetic), zeta potential and dynamic light scattering measurements, etc [43]. However, these provide only a static picture of the ENM without consideration of the relevant biological environment [44] and interaction of different biological macromolecules present in bio-media and bio-corona. Such characterization is either performed where the biological environment is ignored, in water or organic solvent alone or under vacuum, or in oversimplified models systems, such as adding BSA to model protein coverage [44]. As, the available techniques are insufficient to characterize the ENMs all before, during and after the applications in agricultural fields [45,46], new characterization methods are needed to be studied. These novel techniques can then may be exploited to understand translocation and transfer of the biogenic ENMs protein adsorption (i.e. opsonisation) on the surface of the particle or particle aggregation/agglomeration that may impact the uptake/clearance mechanisms [28,44].
Molecular toxicology provides a basis for much of the nano-safety assessments for all sorts of nanomaterials and their bulk counterparts. However, in relation to risk of cytotoxicity posed by a biogenic ENM as compared to its bulk or chemically synthesized ENM counterparts, one distinguishing feature of biogenic ENM is the difference in their physical characteristics, including size, crystallinity and importance of surface charge and surface chemistry. When brought in contact with the environment, the ENMs irrespective of their inherent physical nature undergo a transformation. This is attributed to high surface area and thus high reactivity of ENMs towards various molecules resulting in heterogeneous surface composition over them [47,48]. ENM-transformations are the result of a myriad of reactions or processes, including aggregation/agglomeration, redox reactions, dissolution, and reactions with naturally occurring macromolecules and biomolecules. These dynamic transformations in turn affect the transport, fate, and toxicity of ENMs in the body or environment, and therefore, it is critical to understand and characterize these transformations. Typically, physiological or environmental conditions are simulated in the laboratory by modeling ionic strength and protein or (NOM) content, which are also the key players in ENM transformation in natural environment, particularly soil and water bodies [44].
In these cases, the size of ENMs is an important determinant to reactivity, transport, and toxicity, and while the primary particle size is always estimated (with electron microscopy); ENMs tend to agglomerate in different solutions and biological media which leads to an interaction between a biological system and the aggregate sized material instead of the nano-material. Dynamic light scattering techniques are most commonly employed to study stability of ENMs in solution [49] or as an aerosol [50]. With respect to aggregation, the presence of bio-macromolecules present on the surface of ENM and also in media may have varied effects [51]. In the presence of physiological factors like varying pH and salinity, the biomolecules behave in a dissimilar manner and undergo several biophysical alterations which perturbs their native assembly and may result is agglomerative behavior [25]. Therefore, the capped/biogenic ENMs respond differently to the exposed environment as compared to uncapped ENMs [51].
Understanding aggregation is critical for characterizing transport of biogenic ENMs through the body of human and other model organisms and environmental compartments. In the body, greater aggregation yields larger particles that are cleared from the body by the mononuclear phagocyte system [52], and in the environment, less aggregation yields lower rates of sedimentation and greater mobility [44]. Therefore, understanding the interaction of ENMs under natural conditions (e.g. salinities, pH, and molecular species) may enable a better assessment of exposure and transport. These aggregation studies bring to light the importance of the ENM surface and localized environment around that surface in the transformation of the material [36,53,54]. Proteins and other biomolecules like fatty acids can act upon the biogenic ENM leading to free energy change of the surface and/or can influence other ENM transformations [55].
Other kind of ENMs (e.g. chemically synthesized metallic ENMs, such as, formulations of Au, Ag, Ti, Fe, Zn and Cu [56]) experience a similar speciation either in the dissolution to ions or chemical reactions that, in turn, could affect other physicochemical changes associated with them and ultimately their translocation and toxicity. The interplay between the different, dynamic forms of ENMs that may be acquired after interaction with the environmental factors discussed above and the importance of their state on subsequent transport and toxicity necessitates careful time-dependent characterization of biogenic ENMs in environmentally relevant conditions [28].
Considerations of Model Systems
For understanding a complete functional impact of biogenic ENMs on humans and environment, choice of relevant model system and the investigation with them is important. Increasing the complexity of a model system makes the risk assessment difficult and demands the need for the development of advanced methods to address this challenge. In particular, it becomes the necessity of the hour to develop a toxicological methodology with specific in vitro techniques and models’ systems that are high-throughput, reproducible (both intra and inter lab), close to mimic human system, as well as predictive of toxic response in vivo. In the realm of biological model systems, complex culture systems allow for more reliable results and are worthy of further exploration. Common biological and ecological model systems are discussed below:
Biological Model’s
Biological studies often utilize two categories of model systems: in vivo wherein a whole organism is used, and in vitro, with cells derived from live tissues/ organs or immortal cell lines.
For in vitro studies, there is a wide range in model systems. Choices must be made when selecting a model cell - between use of primary culture or immortal cell lines (i.e. cells isolated directly from an animal or a self-propagating cell line) and use of cells of human origin or animal origin. Also an assessment needs to be made to see if the physiological function of the model cell is relevant, and whether the model cell is found in the tissue likely exposed to the biogenic ENM [43]. Ideally to study human toxicity, primary culture of human cells are the best suitable, but these models are generally restricted to commercially available cells capable of continuous propagation or cell types isolated from small samples of donor blood such as mononuclear cells [57], B lymphocytes, and T lymphocytes [58]. An alternative is to use primary culture of cells from animal models that require less regulation [59]. The major advantages of using the primary cells are their functional and genetic fidelity corresponding to systemic research. Such organ specific primary cells can be derived from the relevant animal models to understand the relevance of systemic exposure to ENMs. Culturing of cells from live model organisms yields a heterogeneous mixture of cells that then typically require density gradient or flow cytometry sorting to isolate the model cell of interest. These separation techniques often damage cells in the process of isolation. Additionally, primary culture cells are often obtained in limited numbers and have a limited lifetime in culture [60]. Due to these key disadvantages, cell lines are commonly used for in vitro testing. A plethora of immortal cell lines is commercially available for use as both cancer cell models and immortalized representations of normal cells. The major drawback of using immortal cell lines is that the mutations required for the immortalization of the lines may affect the way the cells respond to ENMs [61,62]. To improve the reliability of interpretations from immortal models, studies can be compared using different cell lines that have the same physiological function. An alternative method can be the use of co-culture models to yield a better representation of in vivo conditions [63,64], although the presence of two cells in culture may lead to cross contamination and thus, can complicate interpretation.
In vivo studies provide vital information to assess the health and safety aspects of biogenic ENMs as they are capable to demonstrate a systemic response once the model organism is exposed to biogenic ENMs [65]. Depending on the potential application of the ENM, various animal models or physiological mechanisms can be studied, such as zebrafish embryo development [66], rabbit ocular toxicity [67], rat pulmonary toxicity [68] or immune cell distributions in mice [43]. These types of studies have several limitations including the long experimental time required, the high cost, and the ethical concerns regarding the treatment of laboratory animals [43]. Yet, in vivo studies are necessary to explore ENM bio-distribution and determine appropriate cell types to be used in in vitro studies.
In vitro methods are relatively faster, inexpensive and minimize ethical concerns as compared to in vivo studies; however, many of these methods require more extensive validation with in vivo studies to evaluate their toxicological predictive capability and reproducibility.
Ecological Models
Ecological model organisms range from single celled microorganisms to plants and higher order animals. The nano-eco-toxicity generally follows a hierarchical study and thus, choses typical model system based on the strata of food web. Microorganisms form the lower most strata of a typical food chain and are omnipresent in the different ecosystems. Besides their ubiquitous presence, it is evident since long that they play important roles in nutrient cycling. Nano-safety studies have included commonly used research species such as Escherichia coli [69,70], Bacillus subtilis [71] and Pseudomonas aeruginosa, [72] Nitrosomonas europaea [73]. The breadth of choices in these monoculture systems has led to some challenges within the field in generalizing experimental results. That is, the deepest studies utilize the common microbial species that may not be environmentally relevant, while more environmentally relevant species have been considered less thoroughly. To overcome this issue, some research groups have pursued toxicity studies on naturally- sampled bacteria [74-76].
Although the research involving micro-organisms give an insight of impact of ENM on biotic environment, a detailed inference can be made only when plant and higher order organism are studied for the effects of ENMs on them.
For understanding the implication of agriculturally useful biogenic ENMs and to define their eco-nano-toxicity plants play a relevant candidate role. Plants have a ubiquitous interaction with ENM contained abiotic components viz. air, water and soil. Additionally, they have a critical role to play in inter-species transfer and bio-distribution of ENMs attributed to their consumption by organisms at all the hierarchical levels of food chain. Notably, most of the nano-safety work related to ENM for agriculture has focused on edible plants (pumpkin, radish and cucumber) and crops (maize, wheat, soybean, tobacco and rice) [77,78].
The ecosystems functions in a regulated manner with a number of aquatic and terrestrial animals. These make to the major components in the food web as food sources. An in-depth comprehension of the impact of biogenic ENMs can be directly related to human health. The Organization for Economic Co-operation and Development (OECD) defines guidelines that consider Japanese medaka [79] and zebrafish as standard organisms for aquatic toxicity testing [80,81]. The ability to quickly reproduce and having a completely sequenced genome, makes zebrafish (a freshwater fish), [82] and medaka (saline habitats) important for understanding genetic impacts of biogenic ENMs in the environment [83].
Conclusion
Globally, an exponential release in nano-enabled product development has been observed since 2000s [84], with a projected growth to over half a million tons by 2020 [85,86]. Agriculture being the prime area to fulfill the global food requirement will entail the applications of next generation nanotechnology [16]. This makes it certain that there has been and will be a continuous release of ENMs in the environment, which will lead to significant human exposure. As an inference, it can be deduced that a thorough understanding on impact of ENM exposure is needed to assess the biological and ecological implications – both beneficial and harmful. It becomes critical within the field of nano-safety to develop specific technology that can enable the understanding of impact of biogenic ENM on a mechanistic level. Development of methods for clear elucidation of the kind of bio-interaction and the effect thereof in various biotic and abiotic components of the environment related to biogenic ENMs is needed. While it can be assessed that the biological and ecological toxicology studies have different aspects, the challenges that are faced by the nano-safety community demands interdisciplinary efforts to overcome them. However, the dose dependent response should be the preliminary factor to be considered while commenting on the toxic effects of biogenic ENMs, as required dose of such ENMs in agriculture fields could be much lesser as compared to the bulk materials. Also due to virtue of transfer and transformation across the different trophic levels in the environment, there are chances that the biogenic ENM concentration may get reduced to an inconsequential amount, thereby decreasing the probability of an ill-effect to infinitesimal.
References
- Aiken GR, Hsu-Kim H, Ryan, JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45: 3196-3201.
- Amde M, Liu JF, Tan ZQ, Bekana D (2017) Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments: a review. Environ Pollut 230: 250-267.
- Arias-Estévez M, López-Periago E, Martínez-Carballo E, Simal-Gándara J, Mejuto JC, et al. (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agriculture, ecosystems & environment 123: 247-260.
- Arnaout CL, Gunsch CK (2012) Impacts of silver nanoparticle coating on the nitrification potential of Nitrosomonas europaea. Environ Sci Technol 46: 5387-5395.
- Barbero CA, Yslas EI (2017) Ecotoxicity effects of nanomaterials on aquatic organisms: nanotoxicology of materials on aquatic organisms. Applying nanotechnology for environmental sustainability 330-351.
- Batley GE, Kirby JK, McLaughlin MJ (2012) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46: 854-862.
- Beloin-Saint-Pierre D, Turner DA, Salieri B, Haarman A, Hischier R (2018) How suitable is LCA for nanotechnology assessment? Overview of current methodological pitfalls and potential solutions: 65th LCA Discussion Forum, Swiss Federal Institute of Technology, Zürich, May 24, 2017. Int J life cycle Assesst 23: 191-196.
- Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, et al. (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano letts 6: 866-870.
- Bruckner S, Rhamouni S, Tautz L, Denault JB, Alonso A, et al. (2005). Yersinia phosphatase induces mitochondrially dependent apoptosis of T cells. J biological Chem 280: 10388-10394.
- Chaudhry N, Dwivedi S, Chaudhry V, Singh A, Saquib Q, et al. (2018) Bio-inspired nanomaterials in agriculture and food: Current status, foreseen applications and challenges. Microbial pathogen 123: 196-200.
- Christian P, Von der Kammer F, Baalousha M, Hofmann T (2008) Nanoparticles: structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17: 326-343.
- Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, et al. (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323: 1014-1015.
- Das P, Williams CJ, Fulthorpe RR, Hoque ME, Metcalfe CD, et al. (2012) Changes in bacterial community structure after exposure to silver nanoparticles in natural waters. Environ Sci Technol 46: 9120-9128.
- Douglas Gollin DL, Waugh M, Saeed S (2012) The Agricultural Productivity Gap in Developing Countries. International Growth Center.
- Dransfield I, Buckle A, Savill JS, McDowall A, Haslett C, et al. (1994) Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. the journal of immunology 153(3), 1254-1263.
- Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, et al. (2006) Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano lett 6: 1522-1528.
- Fischer HC, Chan WC (2007) Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol18: 565-571.
- Fresta M, Fontana G, Bucolo C, Cavallaro G, Giammona G, et al. (2001) Ocular tolerability and in vivo bioavailability of poly (ethylene glycol)(PEG)‐coated polyethyl‐2‐cyanoacrylate nanosphere‐encapsulated acyclovir. J Pharm Sci 90: 288-297.
- Furutani-Seiki M, Wittbrodt J (2004) Medaka and zebrafish, an evolutionary twin study. Mechanisms of Development 121: 629-637.
- Gavankar S, Suh S, Keller AF (2012) Life cycle assessment at nanoscale: review and recommendations. International journal of life cycle assessment 17: 295-303.
- Ge Y, Schimel JP, Holden PA (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45: 1659-1664.
- Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, et al. (2010) Food security: the challenge of feeding 9 billion people. Science 1185383.
- Gollin D, Lagakos D, Waugh M. (2011) The agricultural productivity gap in developing countries. Unpublished manuscript.
- Gordon S (2016) Phagocytosis: an immunobiologic process. Immunity 44: 463-475.
- Gottschalk F, Scholz RW, Nowack B (2010) Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ modelling & software 25: 320-332.
- Guo Z, Cui K, Zeng G, Wang J, Guo X (2018) Silver nanomaterials in the natural environment: An overview of their biosynthesis and kinetic behavior. Sci total Environ 643: 1325-1336.
- Gupta H (2018) Role of nanocomposites in agriculture. Nano hybrids and composites 81-89.
- Haase A, Lynch I (2018) Quality in Nanosafety-Towards a reliable nanomaterial safety assessment. Nanoimpact 11: 67-68.
- Hayami Y (1969) Sources of agricultural productivity gap among selected countries. Am J Agricultural Econs 51: 564-575.
- He X, Aker WG, Leszczynski J, Hwang HM (2014) Using a holistic approach to assess the impact of engineered nanomaterials inducing toxicity in aquatic systems. J Food Drug Anal 22: 128-146.
- Hischier R, Walser T (2012) Life cycle assessment of engineered nanomaterials: state of the art and strategies to overcome existing gaps. Sci Total Environ 425: 271-282.
- Hu X, Sun A, Kang W, Zhou Q (2017) Strategies and knowledge gaps for improving nanomaterial biocompatibility. Environ Int 102: 177-189.
- Huang F, Gilbert B, Zhang H, Banfield JF (2004) Reversible, surface-controlled structure transformation in nanoparticles induced by an aggregation state. Phys Rev lett 92: 155501.
- Ingham RE, Trofymow J, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecological monographs 55: 119-140.
- Justino CI, Rocha-Santos TA, Duarte AC (2011) Sampling and characterization of nanoaerosols in different environments. Trends in Anal Chem 30: 554-567.
- Kah M, Kookana RS, Gogos A, Bucheli TD (2018) A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat Nanotechnol 13: 677-684.
- Kasahara M, Naruse K, Sasaki S, Nakatani Y, Qu W, et al. (2007). The medaka draft genome and insights into vertebrate genome evolution. Nature 447: 714.
- Kaur G, Dufour JM (2012) Cell lines: Valuable tools or useless artifacts. 2: 1-5.
- Khan R, Inam MA, Zam SZ, Park DR, Yeom IT (2018) Assessment of key environmental factors influencing the sedimentation and aggregation behavior of zinc oxide nanoparticles in aquatic environment. Water 10: 660.
- Khati P, Gangola S, Bhatt P, Kumar R, Sharma A (2018) Application of nanocompounds for sustainable agriculture system. Microbial biotechnol Environ monitoring and cleanup 194-211.
- Klaine S, Alvarez P, Batley G, Fernandes T, Handy R, et al. (2008) Critical review of nanomaterials in the environment. Environ Toxicol Chem 27: 1825-1851.
- Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XHN (2007) In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 1: 133-143.
- Lei C, Sun Y, Tsang DCW, Lin D (2018) Environmental transformations and ecological effects of iron-based nanoparticles. Environ pollut 232: 10-30.
- Levard C, Hotze EM, Lowry GV, Brown GE (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46: 6900-6914.
- Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J nanomater 2011.
- Lorsch JR, Collins FS, Lippincott-Schwartz J (2014) Fixing problems with cell lines. Science 346: 1452-1453.
- Love SA, Maurer-Jones MA, Thompson JW, Lin YS, Haynes CL (2012) Assessing nanoparticle toxicity. Ann Rev Anal Chem 5: 181-205.
- MacCuspie RI (2011) Colloidal stability of silver nanoparticles in biologically relevant conditions. J Nanoparticle Res 13: 2893-2908.
- Makarov V, Love A, Sinitsyna O, Makarova S, Yaminsky I, et al. (2014) Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta naturae 6: 20.
- Marquis BJ, Haynes CL (2008) The effects of co-culture of fibroblasts on mast cell exocytotic release characteristics as evaluated by carbon-fiber microelectrode amperometry. Biophys Chem 137: 63-69.
- Maurer-Jones MA (2012) Immunogenicity and ecotoxicity of engineered nanoparticles.
- Maurer-Jones MA, Bantz KC, Love SA, Marquis BJ, Haynes CL (2009) Toxicity of therapeutic nanoparticles. Nanomedicine 4: 219-241.
- Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of Engineered Nanoparticles in the Environment. Anal Chem 85: 3036-3049.
- Monopoli MP, Åberg C, Salvati A, Dawson KA (2012) Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7: 779.
- Mudunkotuwa IA, Grassian VH (2011) The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit 13: 1135-1144.
- Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42: 4447-4453.
- Neagu M, Piperigkou Z, Karamanou K, Engin AB, Docea AO, et al. (2017) Protein bio-corona: critical issue in immune nanotoxicology. Arch Toxicol 91: 1031-1048.
- OCDE (2013). Test No. 236: Fish Embryo Acute Toxicity (FET) Test.
- Padilla S, Cowden J, Hinton DE, Johnson R, Flynn K, et al. (2009) Use of medaka in toxicity testing. Curr Protoc Toxicol 39: 1-10.
- Pan C, Kumar C, Bohl S, Klingmueller U, Mann M (2009) Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics 8: 443-450.
- Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, et al. (2010) Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol 76: 7981-7989.
- Pereda M, Marcovich N, Ansorena M (2018) Nanotechnology in food packaging applications: barrier materials, antimicrobial agents, sensors, and safety assessment. Handbook of ecomaterials 1-22.
- Piacenza E, Presentato A, Turner RJ (2018) Stability of biogenic metal (loid) nanomaterials related to the colloidal stabilization theory of chemical nanostructures. Crit Rev Biotechnol 1-20.
- Postlethwait JH, Johnson SL, Midson CN, Talbot WS, Gates M, et al. (1994) A genetic linkage map for the zebrafish. Science 264: 699-703.
- Pradhan A, Seena S, Pascoal C, Cássio F (2011) Can metal nanoparticles be a threat to microbial decomposers of plant litter in streams? Microbial ecology 62: 58-68.
- Praetorius A, Scheringer M, Hungerbühler K (2012) Development of environmental fate models for engineered nanoparticles: a case study of TiO2 nanoparticles in the Rhine river. Environ Sci Technol 46: 6705-6713.
- Raliya R, Saharan V, Dimkpa C, Biswas P (2017) Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J Agric Food Chem 66: 6487-6503.
- Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR (2009) Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol 43: 4227–4233.
- Rudolph M, Erler J, Peuker UA (2012) A TGA–FTIR perspective of fatty acid adsorbed on magnetite nanoparticles–decomposition steps and magnetite reduction. Colloids and surfaces a: physicochemical and engineering aspects 397, 16-23.
- Saptarshi SR, Duschl A, Lopata AL (2015) Biological reactivity of zinc oxide nanoparticles with mammalian test systems: an overview. Nanomedicine 10: 2075-2092.
- Saratale RG, Saratale GD, Shin HS, Jacob JM, Pugazhendhi A, et al. (2018) New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollu Res25: 10164-10183.
- Savci S (2012) An agricultural pollutant: chemical fertilizer. Int J Environ Sci development 3: 73.
- Sayes CM, Marchione AA, Reed KL, Warheit DB (2007) Comparative pulmonary toxicity assessments of C60 water suspensions in rats: few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano lett 7: 2399-2406.
- Sayes CM, Reed KL, Warheit DB (2007) Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci 97: 163-180.
- Schultz AG, Boyle D, Chamot D, Ong KJ, Wilkinson KJ, et al. (2014) Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing. Environ Chemi 11: 207-226.
- Scott NR, Chen H, Cui H (2018) Nanotechnology applications and implications of agrochemicals toward sustainable agriculture and food systems. J Agric Food Chem 66: 6451-6456.
- Sharma VK, Siskova KM, Zboril R, Gardea-Torresdey JL (2014) Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv Colloid Interface Sci 204: 15-34.
- Shin HK, Seo M, Shin SE, Kim KY, Park JW, et al. (2018) Meta-analysis of Daphnia magna nanotoxicity experiments in accordance with test guidelines. Environ Sci: nano 5: 765-775.
- Singh A, Singh N, Afzal S, Singh T, Hussain I (2018) Zinc oxide nanoparticles: a review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J Mater Sci 53(1), 185-201.
- Society R (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. Royal Society.
- Stensberg MC, Wei Q, McLamore ES, Porterfield DM, Wei A, et al. (2011) Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 6: 879-898.
- Unrine JM, Colman BP, Bone AJ, Gondikas AP, Matson CW (2012) Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles. Part 1. Aggregation and dissolution. Environ Sci Technol 46: 6915-6924.
- White JC, Gardea-Torresdey J (2018) Achieving food security through the very small. Nature nanotechnol 13: 627.
- Yang Y, Mathieu JM, Chattopadhyay S, Miller JT, Wu T, et al. (2012) Defense mechanisms of Pseudomonas aeruginosa PAO1 against quantum dots and their released heavy metals. ACS nano 6: 6091-6098.
- Yin J, Wang Y, Gilbertson LM (2018) Opportunities to advance sustainable design of nano-enabled agriculture identified through a literature review. Environ Sci: nano 5: 11-26.
- Zhang W, Xiao B, Fang T (2018) Chemical transformation of silver nanoparticles in aquatic environments: mechanism, morphology and toxicity. chemosphere 191: 324-334.


