Abstract
In recent decades, the miniaturization of processes towards micro/ nanoscale has emerged as a new and general approach for all engineering disciplines, and for various reasons, chemical engineering has been a good ground for the growth of this technology. The miniaturization of tools and technologies at the micro and nano scales has led to a revolution in chemical engineering, as exemplified by the advent of microchips in the computing and computer industries. These activities, which took place mainly during the past two decades, owe much to human achievements in the ability to fabricate microscale equipment, as well as the invention of laboratory devices to identify and monitor microstructures at the nanometer scale. To meet the diverse and emerging needs of the chemical industry, modern chemical engineering is essential to meet the needs of the market in terms of producing products with micro-scale characteristics, as well as overcoming the operational and environmental constraints of traditional macro-scale industrial processes. In this review article, many recent developments in the profession of chemical engineering are discussed from the perspective of downsizing equipment and their applications, and the need for a thorough revision in the educational contents of this engineering discipline in line with the growth of micro/nanotechnology has been pointed out.
Keywords
Chemical Engineering Curriculum, Micro/Nano Scale, NanoTechnology, Microfluidics, Process Intensification, Electrokinetic Phenomena
Introduction
Chemical engineering is the technique of applying basic sciences (mathematics, physics, chemistry and biology) to implement physical and chemical processes in industrial plants. This discipline is a branch of engineering sciences that deals with the design, manufacture, and operation of processes in the chemical industries [1]. Chemical industries are those industries in which chemical, physical, or biological reactions convert raw materials into industrially valuable products. The main area of activity of a chemical engineer is supervising the three sections of mixing, reaction engineering, and separation. Accordingly, many industries such as refineries, petrochemicals, wood and paper, food, pharmaceuticals and medical equipment, cellulose, polymer, inorganic chemical, and many other industries directly benefit from the applications of chemical engineering. This part of chemical engineering, which is related to large-scale chemical industrial processes, is called process engineering. The separation processes used by a chemical engineer (such as distillation, extraction, etc.) are called unit operations and include mass, heat, and momentum transfer. These processes usually combine to complete the chain of chemical synthesis or separation of materials. The three basic physical laws in chemical engineering are the principles of mass, energy, and momentum conservations. Material and energy transfer in a process is evaluated using mass and energy balance for the whole unit, unit operation or part of it. Chemical engineers apply the principles of thermodynamics, reaction kinetics, and transfer phenomena to perform an industrial process [2-4].
In recent years, as a result of the increasing synergistic advancement of science and technology, new aspects of science and technology have emerged every day that has not been discussed until recent decades. Recent advances in nanoscience and nanotechnology have made it possible to produce very tiny tools and equipment that enable humans to control the movement of very small volumes of fluids or particles suspended in them. With this equipment, many difficult, complex, and parallel processes can be performed in a short time, at low cost, and more easily. With this equipment, many difficult, complex, and parallel processes can be performed more easily in a short time, and at a low cost. By manipulating materials and equipment at the micro and nano scales, researchers have been able to produce new materials with advanced and intensive properties. The miniaturization of tools and technologies at the micro and nano scales has led to a revolution in chemical engineering, as exemplified by the advent of microchips in the computing and computer industries [5]. Chemical microsystems have special potential. To meet the diverse and emerging needs of the chemical industry, modern chemical engineering is necessary to meet the needs of the market as well as the production of products with micro-scale characteristics in order to overcome the process and environmental constraints of traditional large-scale processes. To this end, it is important to understand the relations and differences among small-scale phenomena to the characteristics and behavior of large-scale processes [6].
Traditional areas of expertise in chemical engineering, including transfer phenomena, process design and analysis, and commercialization skills, have evolved, and new areas of expertise in this engineering major at molecular and micro/nanometer scales, especially in biological systems, in It is increasing [7]. In this regard, many industrial process equipment related to the chemical industry, called unit operation equipment, previously used in traditional chemical engineering on macro and meso scales, are downsizing, an activity that began decades ago by pioneers miniaturizing the equipment [8]. These include mixers, reactors, pumps, tubes, and valves, now referred to as micromixers, micro-reactors, micropumps, micro/nanotubes, micro-valves, and more [6]. Table 1 shows a number of unit operation equipment in both macro and micro/nano scales.
Equipment |
Macro scale |
Micro scale |
Turbine
(a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work) |

|

|
Reactor
(a device for containing and controlling a chemical reaction) |

|
 
A Leaf-(Inspired Chemical MicroReactor)
|
Mixer
(a device for mixing the materials) |

|

|
Pipe
(a tubular section or hollow cylinder, usually but not necessarily of circular cross-section, used mainly to convey substances which can flow) |

|

|
Pump
(a device that moves fluids (liquids or gases), or sometimes slurries, by mechanical action) |

|

|
Valve
(a device or natural object that regulates, directs or controls the flow of a fluid (gases, liquids, fluidized solids, or slurries) by opening, closing, or partially obstructing various passageways) |

|

|
Table 1.A number of equipment and devices for unit operation in macro and micro scales
Micro/nanoscale systems were initially thought to be merely scaled-down examples of large-scale systems. Advances in micro-and nanotechnology have proven that the problem is far more complex than downsizing the geometry of the device, and a better understanding of the properties is needed. For example, in micro-and nanoscale systems, where the dimensions of the system are very tiny, the surface-to-volume ratio is very high, and therefore capillary and electrokinetic effects, which may be negligible in large-scale processes, become very important. In fact, effective forces and mechanisms change with scale, and this unique feature has led scientists to use such systems to control fluid flow. For example, for a cube, if one dimension is reduced 10 times, its volume is reduced 1000 times. Therefore, the force of gravity, which is proportional to mass, is also reduced 1000 times. At the same time, the frictional force caused by contact with the surrounding objects is reduced by only a factor of 100 because this force depends on the surface. As objects get smaller, surface forces such as friction become much more important than gravity. For further explanation, scaling analysis for fluid forces, electromagnetic forces, electrostatic forces, and surface tension is shown in Figure 1 [9].
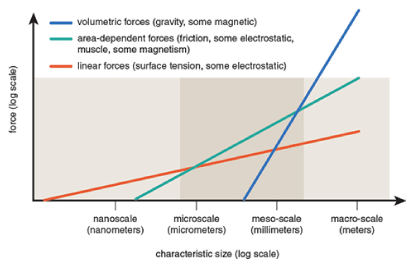
Figure 1. Scaling of fluid forces, electromagnetic forces, electrostatic forces and surface tension
This review article examines the relationship and differences between phenomena at the macro and micro/nanoscales and discusses many recent developments in chemical engineering regarding equipment downsizing and their applications. Other cases mentioned in this article are the shortcomings of traditional chemical engineering textbooks that have been compared with the new texts required by this engineering discipline.
Motivations towards equipment downsizing
There are many specific motivations that justify human determination to use micro/ nanosystems. One of the major advantages of micro-systems is that they have a high surface-to-volume ratio. However, in addition to the high surface-to-volume ratio, the high rate of heat and mass transfer in these systems has led scientists to use micro and nanoscale equipment to perform separation, reaction, and tracking processes. Other incentives for equipment downsizing include process intensification; Optimization and integrated control of strategies; Ability to integrate multiple features into one device; Leading processes towards cleaner and cheaper technologies (reducing the cost of purchasing materials for smaller equipment), lower energy consumption, higher safety and less waste of raw materials.
For example, downsizing chemical reactors has a positive effect on chemical processes. In fact, as the surface-to-volume ratio increases, so does the rates of mass and heat transfer, which prevents hot spots from forming in micro/nano-reactors, where intense exothermic reactions occur. Also in such systems, the possibility of creating dead or blind spaces is very weak. These reactors are used in situations where there is a need to produce small quantities of specific products. Therefore, in situations where it is necessary to react at very high temperatures or pressures, or in situations where the reaction environment is toxic and hazardous, the use of micro-reactors is much safer than conventional reactors [10]. It is very easy to control the flow and concentration of fluids in micro-reactors; therefore, using these types of equipment, it is possible to produce products with predetermined characteristics with high efficiency. For this reason, the use of micro-reactors in the synthesis of nanostructured materials, whose size, size distribution, geometry, and structure are very important, has been widely welcomed [11].
Factors facilitating the entry of engineering disciplines into the field of micro and nano
Recent advances in micro/ nanotechnology owe much to new human capabilities in measuring and controlling individual structures at the micro/nanoscale. The increasing development of new tools for the characterization of materials and their manufacturing technology has played a very important and fundamental role in human progress towards micro/ nanotechnology. These tools enable the eyes to see and the fingers to control the nanostructures. In fact, micro/nanoscale process management owes much to technological advances in the following two areas:
Manufacture of laboratory instruments with the ability to measure, analyze, and observe at the micro/ nanoscale
In various engineering and medical sciences, the subject of measurement and characterization is of key importance. So that the physical and chemical properties of materials depend on the raw materials used as well as the microstructure obtained from the manufacturing process. For example, to identify raw materials, it is obvious that the type and amount of impurities, geometry and particle size distribution, crystal structure, and the like affect the nature and quality of the product. Therefore, the more powerful measuring and characterizing devices are available, the more accurate control of raw materials would take place. In addition, to study microstructures, microscale identification and analysis tools are needed. In microstructure technology, the microscopic structure of materials, the type of phases, their geometry, size, quantity, and distribution are studied in detail. Until the beginning of the new century, devices could not analyze particles on a scale smaller than micro, but now with the advancement of science, devices with the ability to measure particle size with high accuracy at the nanometer scale and even smaller have been made [12]. Table 2 provides a number of different techniques used to characterize micro/nanoparticles.
Measurement technique |
Characterization |
Sensitivity |
Reference |
Atomic Force Microscopy (AFM) |
Measurable properties: Geometric morphology, adhesion distribution, friction, surface impurities, material of different surface points, elasticity, magnetism, chemical bonds, surface electric charge distribution, and electrical polarization |
8 μm to 1 nm |
[13] |
Nuclear Magnetic Resonance (NMR) |
Identification of chemical molecules, atomic composition, Effect of ligands on nanoparticle shape, nanoparticle size |
Up to 1 nm |
[14] |
Transmission Electron Microscopy TEM) |
Determining the size and shape of particles |
Up to 1 nm |
[15] |
X-ray Photoelectron Spectroscopy (XPS) |
Identify elements on the surface, identify the experimental formula for analyzing the composition of samples |
≥ 100 nm |
[16] |
Fourier Transformed Infrared Spectroscopy (FTIR) |
Determining the structure and measuring chemical species |
-- |
[17] |
Dynamic Light Scattering (DLS) |
Determining the distribution of particles in solutions and suspensions |
-- |
[18] |
Brunauer-Emmett-Teller (BET) |
Surface area, and characterization of nanopores |
1 to 300 nm |
[19] |
Table 2. Some of the micro/nanoparticle characterization techniques
Manufacture of micro-scale process equipment (such as microfluidic devices)
The second factor that led to the entry of engineers into the world of nanotechnology was the acquisition of tools for the production of small-scale parts and machines. Today, micro/ nanoscale channels and equipment can be fabricated using a variety of methods, and various techniques for making microfluidic devices are available to engineers. For example, photolithography, soft lithography, thermoforming techniques, etching, engraving, and laser photoablation may be used [13-20]. Qamar and Shamsi recently conducted a review of microfluidic equipment fabrication on flexible substrates such as paper and plastic [21].
Photolithography is the process of applying light to transfer geometric patterns, usually from a transparent mask, to a substrate through a layer of light-sensitive emulsion called a protective coating. Photolithography is essentially the generalized form of photography technology. First, something like a negative photographic film is made of the overall layout of the design. This negative, called a mask here, is used to replicate the design on a substrate. In photolithography, after placing a layer of light-sensitive polymer on the substrate surface, a homogeneous light beam passes through a mask and creates a pattern on the polymer. In the photolithographic process, after creating the design on the interface polymer, the lighted areas, with their resistance to corrosion, mediate the transfer of the design to the substrate; This process is very similar to the UV sealing technique [22].
Soft lithography is a set of techniques that uses a soft elastic material, usually polydimethylsiloxane (PDMS), to transfer designs to the substrate material. The main process involves the construction of elastic microchannels. These microchannels are designed in a special program and then printed on a transparent mask with high resolution or molded on a conventional chrome mask to be used as a mold or seal for the soft material. Polymer seals can also be used to make nanostructures [23].
Laser cutting is another technology that uses laser beams to cut objects. Laser cutting is done by using a laser beam from a strong laser output on objects that want to be cut. A small part of the object to be cut is melted, burned, or sublimated and removed from the body by the pressure of the gas, and finally, the cut surface is created with excellent quality [23,24]. Figure 2 shows an example of a microfluidic device made by laser engraving [25].
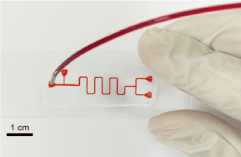
Figure 2. View of a microfluidic device with a T-shaped channel made by laser cutting
Chemical engineering activities and services in the field of micro/nanotechnology
Miniaturized devices are used in many fields such as chemical processes, propulsion and power generation, electronics cooling, the aerospace industry, inkjet printers, and biomedical industries and chemical engineering plays a key role in many of these fields [26-30]. In the following, some applications of chemical engineering in the field of process miniaturization have been studied.
Microextraction
Liquid-liquid extraction or solvent extraction is a separation method in a two-phase system in which the extraction of the substance is based on its inclination to one of these two phases. The system usually consists of an aqueous phase and an organic phase. Due to the flexibility and scalability of the solvent extraction process, this process is being used in various applications in industry such as the extraction of metal ions and organic materials [31]. Conventional liquid-liquid extraction processes are usually performed in large columns or tanks with a capacity of hundreds of liters, and the phase contact and mass transfer rate are controlled using packings, flow distributors, or special agitators. Following the contact of the phases, the phase separation takes place by gravity and the phase free of the extracted substance is discarded. In these systems, intense mixing is required to shorten the time period that chemicals must travel to reach the aqueous-organic interface, and maximize the contact surface [32,33]. The need to mix phases for effective extraction requires the use of large volumes of aqueous and organic phases that prevent the use of solvent extraction devices for low volume materials, such as expensive chemicals or biochemicals, trace chemicals, and extraction of molecules at low concentrations.
To avoid these limitations and to use solvent extraction on a much smaller scale, various substrates have been developed, such as microchannels that use very small volumes of fluid. In fact, micro-and nano-scale extraction systems can be used in such situations. The tiny size of these systems has minimized the diffusion path, so that two-phase mixing is no longer necessary. Also, the ratio of the contact surface of fluids to their volume is very large; Therefore, the mass transfer takes place at a high rate. It should be noted that using such systems, the extraction process can be performed continuously [10, 34-36]. Figure 3 shows a micro-extraction system in which the interface between two parallel aqueous-organic streams forms the mass transfer surface [37,38].
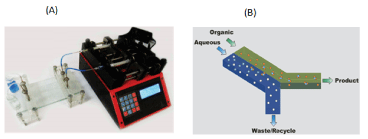
Figure 3. (A) Microfluidic system for microextraction, (B) Schematic of solute extraction mechanism
Solvent extraction microfluidic equipment has been developed for a wide range of applications, including metal ion separation and DNA purification [39-47]. Sarkar, et al. investigated liquid-liquid biphasic flow patterns in microchannels [48]. Dai, et al. performed micro-solvent extraction of copper ions from water using a microchannel device. They used kerosene as a solvent [45]. Jahromi, et al. performed micro-extraction of calcium ion using a type of crown ether as a solvent in a Y-shaped microchannel and investigated the hydrodynamics of two-phase flow in the microchannel. Micro-extraction technology showed significant potential in reducing the reaction time and increasing the efficiency of calcium ion extraction compared to traditional extraction devices. So that in 9.5 seconds the extraction reached an efficiency of 18.5% [49] Priest et al. performed parallel flow micro-solvent extraction using a type II amine as the solvent, as well as the excretion of the solution obtained from the refined feed containing high amounts of Pt chloride in a Y-shaped microchannel. Also, the simultaneous effect of scale-up was investigated by increasing the number of microfluidic devices and various operational parameters on the rate of platinum extraction and recovery [50]. Abbasi, et al. in a study extracted and removed color pollutants from industrial effluents [37]. In another study, they studied the removal of drug contaminants from effluents [51]. Recently, research has been conducted on the extraction of chromium from the aqueous phase by rotating microchannels. As mentioned, these systems can also be used to separate DNA. Samla, et al. studied DNA extraction from a biological sample (blood) in a microfluidic medium. The latter activity can be very useful because of its vital role in clinical diagnosis and genetic analysis [52].
Micromixing
The mixing process is used to mix a volume of fluid in a chemical reaction, heat transfer, mass transfer, or multi-phase combination in the industry. The mixing mechanism is done in two ways, micro and macro. In micro mechanism, molecular scale is involved [53,54]. Laboratory micromixers and shakers are tools used to homogenize mixtures. These devices are an important part of laboratory equipment in the food, beverage, cosmetics, electronics, as well as laboratories related to life sciences, water and wastewater, and biotechnology [55]. Shakers mix materials by shaking. Shaker plate moves back and forth or rotates. The micromixer performs the operation of mixing fluid masses in two scales. There are several things that distinguish between shaker and micromixer. Micromixers are one of the most important components of a microfluidic device and are classified according to laboratory applications. On a large scale, fluids are mixed by convection, like mixing milk and coffee by stirring. In microsystems, fluids do not mix by convection, but when two fluid streams meet in a microchannel, they flow in parallel without creating a turbulent flow, and mixing is done by the penetration of molecules at the contact surface between the two fluids [56,57]. Figure 4 shows a microfluidic mixer [58].
Micromixers are divided into active and inactive categories. Passive type, without an external driving force and work only through channel geometry (like T and Y micromixers). On the other hand, in active micro-mixers, mixing is provided by an external driving force (such as mixing caused by micro blades). Figure 5 shows the classification of micromixers [59].
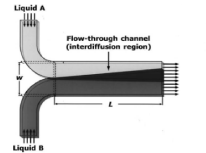
Figure 4. Schematic of the T-shaped microfluidic mixer
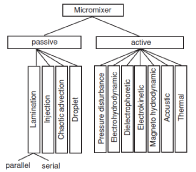
Figure 5. Schematic of the classification of micromixers
Nahr, et al. investigated the effect of the geometry of a microchannel on fluid flow and mixing. In this study, flow characteristics and mixing efficiency in three different microchannel geometries were investigated. The results showed that the mixing behavior is strongly dependent on the channel geometry [60]. In a study using flow imaging and ethanol-soluble red and green inks, Koch, et al. tested horizontal micromixers [61]. In a theoretical study, Liu et al. numerically investigated the mixing of two fluids (water and glycerin water solution) in three-dimensional spiral and checkered micromixers at Reynolds numbers 1 and 10 and showed that the mixing performance was inversely related to glycerol content [62]. In another study, using phenolphthalein and sodium hydroxide solutions, Liu et al. experimentally tested the mixing performance of three-dimensional microchannels in two Reynolds numbers 6 and 70 and showed that increasing the Reynolds number increases the mixability in the microchannel [63]. Shamsoddini, et al. simulated a micromixer equipped with a blade using the hydrodynamic method of incompressible particles and evaluated the performance of the micromixer in terms of mixing efficiency and type of blade state. They showed that the performance of the cross blade was better than that of the straight blade [64]. Nguyen and Wu conducted a review study on micromixers, their types, and applications [59]. Another study by Cai, et al. Examines recent advances in passive and active micromixers for the production of various microfluidic chips [65].
Synthesis of micro and nanoparticles
Due to the wide applications of nanoparticles in various fields, the synthesis of these materials is very important. Synthesis of micro and nano particles is done using micro and nano reactor equipment. At the macroscopic scale, the chemical reactor is a chamber that makes it possible to perform a reaction in a certain volume [66]. One of the advantages of using a reactor is the possibility of precise control of reaction conditions such as solvent, temperature, and stirring speed. At the micro and nano scales, chambers can also be created that separate a certain volume of the reaction mixture from the bulk medium. If a chemical reaction is trapped inside such a chamber, then the chamber is considered a micro or nano-reactor. The advantages of using these reactors include more control over the reaction, selectivity, separation of toxic and unstable substances from the mass environment, and consequently reduction of system toxicity [67]. Microreactors generally consist of a network of micro-sized channels deposited on a solid substrate. Microreactors are made of materials such as ceramics, glass, polymeric materials, stainless steel, silicon, etc [68-70]. In a general classification, nanoreactors are classified into two groups: natural nanoreactors and synthetic nanoreactors. Natural nanoreactors include bacterial protein microparticles, protein cages, and viruses. Synthetic nanoreactors are more diverse and include molecules, macromolecules, nanostructures, and porous solids. The application of micro / nano-reactors has made significant progress in the last two decades [71].
The high surface-to-volume ratio of micro / nano-reactors results in more efficient chemical reactions (higher mass transfer and higher heat transfer). Also, this high surface-to-volume ratio causes the reactants to communicate more, and as a result, the chemical reaction takes place much faster. Therefore, the residence time in these reactors is less than conventional systems [72]. Researchers believe that by using micro-reactors, about 30% of chemical and pharmaceutical products can be produced more efficiently. Due to the small size of the micro-reactors, the reaction is faster, and the reaction is easier to control. Therefore, the use of micro-reactors greatly reduces production costs. Reactions in these small spaces can be very carefully controlled, a feature of micro-reactors that allows reactions to be performed in a safe, clean, and, of course, highly efficient environment [73]. The technologies of micro-reactor and micro-process are mainly used in laboratories to synthesize organic matter, and small-scale research has been conducted, both in academia and in industry.
Wagner and colleagues were the first to synthesize gold nanoparticles using a micro-reactor. In this study, ascorbic acid was used as a reducing agent and polyvinylpyrrolidone as a stabilizer (to prevent the accumulation of nanoparticles). The size of nanoparticles produced in this study was reported in the range of 15-24 nm [74]. Singh, et al. succeeded in chemically synthesized gold and silver nanoparticles in a micro-reactor. The advantages of this micro-reactor were continuous flow, low solvent content, faster reaction, less loss, and better reaction control [75]. Lin and colleagues were able to synthesize silver nanoparticles using a continuous flow tubular micro-reactor [76]. Krishnadasan, et al. synthesized cadmium selenide nanoparticles using 170 μm wide, 80 m deep, and 40 cm long a glass micro-reactor [77]. A typical glass micro-reactor is shown in Figure 6 [69]. Song et al synthesized cobalt nanoparticles using a polymeric micro-reactor [78]. Wang, et al. synthesized 10 nm titania nanoparticles using ceramic / glass microreactors [79]. Appalakutti, et al. synthesized copper chromite nanoparticles with a size in the range of 192–300 nm in a continuous flow micro-reactor. In this study, copper nitrate and chromium nitrate were used as precursors [80]. In a recent study, the extensive synthesis of inorganic nanoparticles (Au, Ag, Se, and mixed oxides of Cu, Co, Ni, Ge, and Ta) in parallel nanoreactors was investigated by Jibril, et al. [81].

Figure 6. A typical glass micro-reactor
Energy production
One of the methods of energy production on the micro and nanoscale is the use of micro-fuel cells. Micro-fuel cells are devices that use electrochemical reactions to generate electrical energy [82,83]. Using a silicon chip, Pattekar et al designed a micro-reactor for the methanol regeneration reaction to produce hydrogen. This hydrogen is used in micro-fuel cells. The efficiency of this cell was 85-90% and its power generation was 8-10 watts. They mounted equipment such as a temperature and pressure sensor, and a filter catalyst on a microreactor chip to try to apply conventional equipment in non-microreactors to the microreactor. The result was proper control and monitoring of the reaction. Figure 7 is a picture of a microreactor made by this group [84]. A recent review study of microfluidic fuel cells with different types of fuel was conducted by Wang, et al. In this study, paper-based, non-pumped microfluidic cells are introduced, as well as the prospects for the development and future applications of micro-fuel cells [85].

Figure 7. Micro-reactor image made by Pattekar et al.
Another method of energy production is the use of electrostatic properties in micro-and nanochannels. When the inner walls of the nano-channel have an electric charge, they exhibit ion-selective behavior. In other words, due to the electric charge of the wall, ions in the electrolyte possessing an opposite charge to the wall are absorbed by the wall, and ions of similar charge are expelled from it. This phenomenon indicates the presence of an electrostatic effect in ionic channels. Similarly, an electrical double layer is formed near the surface of the channel. In addition, while the electrolyte solution is flowing from the high-concentration electrolyte tank to the low-concentration tank in the nanochannel, a diffusive flow occurs in the flow direction. This phenomenon is reverse electrodialysis [86].
Kang et al claimed that by using alumina nanopores in the potassium chloride concentration gradient, a significant power density (the amount of power per unit volume) could be obtained [87]. In addition to theoretical studies, the application of reverse electrodialysis was also experimentally investigated. Kim, et al. Showed that power density can be obtained from a silica nanopore [88]. Guo, et al. performed reverse electrodialysis experimentally on single-ion-selective nanopores and obtained maximum power output. One of the remarkable mechanisms of electrodialysis is the overlap of electrical double layers [89]. Hsu, et al. studied the rectification of ionic current in a conical nanopore. They noticed the effect of electroosmotic flow on current rectification [90]. In another study, they looked at the production of electricity by reverse electrodialysis through a conical nanopore [91]. Yeh et al. Investigated the power generated by the cone geometry of a negatively charged channel for different types of electrolytes [92]. In a study by Khatibi, et al. they modeled the electrokinetic process and the generation of electricity from a cone-shaped nanochannel coated with a polyelectrolyte layer using the Poisson-Nernst-Planck equation for ion transport and electrical potential, as well as the Navier-Stokes equation for electroosmotic flow. Figure 8 shows a schematic of the energy generation process by conical nanochannels. As can be seen, ionic species are transferred from the high concentration tank across the channel to the low concentration tank [86].
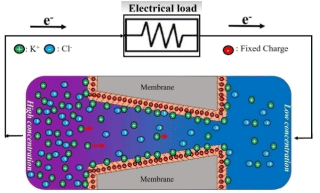
Figure 8. Schematic of the energy generation process in soft nanopores
Pumping and creation of fluid flow
Micropumps are used to move fluids on a micro and nanoscale. Micropumps have been developed for many different applications, including fluid flow, drug delivery and biomedical experiments, cell culture, and more [93]. Figure 9 shows an example of the application of fluid flow in a microfluidic system for drug delivery. In this device, the drug is embedded in tanks with micro dimensions [94].
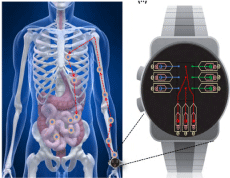
Figure 9. Wrist microfluidic system for drug delivery
The first generation of micropumps was first reported in 1975 and was soon developed by Smith and Van Lintel in the early 1980s, making them the first real MEMS micropumps. This sparked interest in reducing the size of the pump to new operational dimensions [95-97]. Micropumps can be divided into mechanical and non-mechanical categories. Mechanical systems include moving parts [98]. Recently, attempts have been made to design non-mechanical micropumps that, due to their independence from an external driving force, can be used in areas where external driving force cannot be used. One of these types of pumps is capillary pumps that play an important role in microfluidics because they do not require external driving force. Glass capillaries and porous media, including nitrocellulose paper, can be integrated into microfluidic chips. Capillary pumping is widely used in lateral flow testing [99-101]. Recently, new capillary pumps have been developed with a constant pumping flow rate independent of liquid viscosity and surface energy [102]. Another non-mechanical micropump is the electroosmotic micropump. An electroosmotic pump (EOP) is used to generate fluid flow or pressure using an electric field [103]. Figure 10 shows the differences in velocity profiles generated by electroosmotic and pressure-driven flows [104].
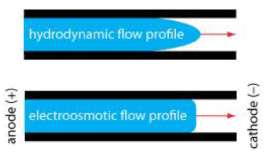
Figure 10. Velocity profiles generated by pressure-driven flow (parabolic) and electroosmotic flow (almost plug)
Electroosmosis has been used in various fields such as high-performance liquid chromatography (HPLC) [105,106], cooling of microelectronic equipment [107], drug delivery [108-110], etc. In a review study, Wang, et al. studied electroosmotic pumps and their applications in microfluidic systems [111]. In another study, Chakraborty and Ray investigated the flow rate control by applying pulsed electric fields to circular microchannels using some of the intrinsic properties of the electroosmosis phenomenon [112]. Sadeghi, et al. studied the effect of combining both electroosmotic and pressure-driven flows in a flat microchannel parametrically [113]. Another method of creating fluid flow is to use the diffusioosmotic phenomenon in micro and nanochannels. Diffusioosmotic flow is an electrokinetic phenomenon that uses a concentration gradient to stimulate flow instead of conventional driving forces. Hoshyargar, et al. studied the diffusioosmotic current in the microchannel. Their results showed that in certain conditions, the velocity profile in this type of flow can be flatter than that of the electroosmotic stream [114,115].
Separation and detection of particles and molecules
One of the best and most feasible methods for separating molecules and analyzing a wide range of ionized analytes is the use of electrophoresis [116]. Electrophoresis is one of the major electrokinetic phenomena discovered by Reuss in 1807 [117,118]. He observed that clay particles were dispersed in water by an electric field [118]. The term electrophoresis refers to the movement of charged compounds or particles in a solution (liquid medium) under the influence of an electric current. Molecules move at different speeds depending on the type of molecule, the size, as well as the different electrical charges. Electrically charged chemical compounds depending on their type of electric charge move towards the pole of the anode or cathode. The electrophoresis separation technique is much more convenient and less expensive than other methods and has undeniable advantages. Analytes that are of particular interest are peptides, amino acids, nucleic acids and oligonucleotides, DNA, nucleotides, organic acids, and small anions and cations in body fluids and tissues [119-124].
One type of electrophoresis that is the oldest type of this technique is called surface electrophoresis. In this method, a porous thermoplastic paper layer consisting of cellulose, acetic acid, and polymer gel is used [125,126].
Another type of electrophoresis is capillary electrophoresis (CE). In 1960, Hjerten described the first capillary electrophoresis device. He used this device to prove various theoretical concepts in capillary electrophoresis and was able to separate mineral ions, proteins, nucleic acids, and microscopic organisms using this technique [127]. This technique, which is mainly used in pharmaceutical and therapeutic chemistry, is used to separate large and small molecules in very thin tubes. In this method, separation becomes possible by high voltages [128,129].
Numerous theoretical and experimental studies in the field of separation have been performed using electrophoresis, especially capillary electrophoresis. Kohl, et al. studied the techniques and applications of two-dimensional separation systems based on capillary electrophoresis [130]. In a review study, Lu, et al. investigated the application of capillary electrophoresis in the separation of glycans [131]. In a review study, Heller, et al. Examined the principles and mechanisms of DNA separation by capillary electrophoresis [132]. Ganjizade, et al. Modeled the DNA sequencing in polyelectrolyte-coated nanopores [133]. Figure 11 shows a schematic of the capillary electrophoresis process for the separation and arrangement of molecules [134].
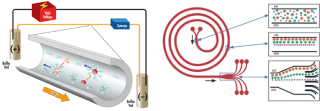
Figure 11. Schematic of capillary electrophoresis process for separation and sequencing of molecules
Numerous other studies have been performed on particle analysis using capillary electrophoresis. In a review study, Ramos-Payan et al examined the application of capillary electrophoresis in the identification and analysis of high complexity samples such as biological fluids, single cells, etc. [135]. Paul, et al. Reviewed recent advances in the analysis of antibiotics by capillary electrophoresis. This study briefly describes the high degree of counterfeiting of antimicrobial drugs with a focus on its immediate health consequences [136].
Coating
One of the most important limiting factors for human beings from time immemorial to achieve their dreams has been the limitation in finding the right materials with the desired properties. Man has learned over time to meet his needs by improving the properties of materials. By manipulating and improving the properties of various available materials, new capabilities can be added to the products. Today, with the use of advanced technologies, new materials have been produced or the properties of existing materials have been improved. In this regard, coating technology is sometimes used to improve physical, chemical, and mechanical properties. One of the cases in which nanotechnology is currently widely and effectively used is coating processes as well as the production of nanostructured materials. Studies on nanocoatings show that their properties are significantly improved in many cases compared to conventional coatings. Nano-coatings have a better thermal expansion coefficient, higher hardness and toughness, and more resistance to corrosion, abrasion, and erosion compared to micro-coatings. If the microstructure of the coating reaches the dimensions of nanometers (up to 10 nanometers), maximum hardness is obtained. Nano-coatings can have a hydrophobic or hydrophilic surface layer. Velayi, et al. used super-hydrophobic nano-zinc oxide to coat the stainless steel membrane and showed that the super-hydrophobic capability could be used to separate water and oil [137]. In another study, they synthesized super-hydrophobic Co3O4 surfaces with micro/nano hierarchical structures on a stainless-steel grid. They investigated the chemical stability and self-cleaning properties of the obtained super-hydrophobic surface. The results of their evaluations showed that the super-hydrophobic surface of Co3O4 exhibited excellent self-cleaning performance [138].
Application of micro / nanostructured coatings is possible with different methods such as laser, thermal spraying, chemical, and electrochemical deposition, etc. [139-141]. One of these methods is electrophoretic deposition, which is a two-step process in which charged particles suspended in a suspension under the influence of an electric field move towards the electrode with the opposite charge and then become deposited as a dense film on the electrode. This method has the ability to create a coating with very good uniformity and control the thickness of the coating [142-145]. In one study, Abdollahi, et al. Used electrophoretic deposition to form ZSM-5 zeolite layers about a few microns thick on porous alumina. They succeeded in hydrothermally modifying the dense uniform zeolite membrane and showed that the thickness and density of the membrane can be modified by changing the Si / Al ratio of ZSM-5 zeolite particles [146]. In another study, Saberi, et al. studied the electrophoretic deposition of zirconia nanoparticles suspended in a mixture of different solvents (ethanol, butanol, and isopropanol) and triethanolamine as a dispersant. Their results showed that the corrosion resistance increases with decreasing voltage applied during the electrophoretic deposition process [147].
Another application of the coating is to create a thin layer on the surface of the micro/nanochannel walls in microfluidic equipment. In a theoretical and experimental study, Monteferrante, et al. investigated the effect of a polymer coating on the surface of a capillary tube on electroosmotic current. The density of the polymer, the thickness, and the charge of the capillary tube change as a function of pH and it is possible to reverse the flow under acid pH conditions. Therefore, by covering the surface of the capillary tube with a polymer layer, the electroosmotic current can be controlled [148]. In another study, Khatibi et al. Showed that coating conical nanostructures with dense polyelectrolyte layers significantly improved ionic current rectification [149]. Another benefit of coating is the superhydrophobicity of the inner surfaces of micro/nanochannels and ducts, which has always been of interest to researchers. Superhydrophobic surfaces reduce the fluid flow pressure gradient and can significantly reduce fluid transfer costs [150,151]. Speyer and Pastorino studied droplet transfer in a nanochannel coated with hydrophobic semi-flexible polymer brushes. They found that as the stiffness of the polymer chains increased, the droplet transfer rate decreased [152]. Figure 12 shows a schematic of a microfluidic device with channels covered by a polymer layer [153].
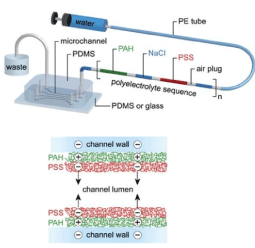
Figure 12. Schematic of a microfluidic device with microchannels covered by a polymer layer
Micro/nano scale transport phenomena
In general, process design and control in micro/nano scale systems are influenced from channel geometry in regulating temperature, pressure, and fluid velocity distribution. The classification of microchannels is presented in Table 3 [154].
The range of channel dimention |
Definition |
3mm < Dc |
Conventional channels |
200 μm < Dc ≤ 3 mm |
Minichannels |
10 μm < Dc ≤ 200 μm |
Microchannels |
1 μm < Dc ≤ 10 μm |
Transitional Microchannels |
0.1 μm < Dc ≤ 1 μm |
Transitional Nanochannels |
Dc ≤ 0.1 μm |
Nanochannels |
Table 3.General schemes for channel classification
Therefore, to build such micro devices, it is important to understand the basic mechanisms involved in fluid flow and heat transfer properties in microchannels, because their behavior affects the transport phenomena for many microelectromechanical systems (MEMS) and microfluidic applications. Working on a micro or nano scale involves considering issues, features, and phenomena that may not matter at all on a macro scale. These characteristics are completely different for gas and liquid flows. In gas microflows, we encounter four important effects of compressibility, viscous heating, thermal creep and rarefaction. Liquid flows, on the other hand, are exposed to other micro-scale attributes such as surface tension and electrokinetic phenomena [155].
As size decreases, some common theories about the transport of fluid, energy, and mass need to be revised for validation [156]. As scale decreases, buoyancy, gravity, and inertia forces become less important, and viscous and surface forces become more and more prevalent. At the micro scale, the relative importance of forces in a two-phase water/oxygen flow stream is reported as follows [157].
Surface forces> Viscose forces > Gravity forces > Inertial forces > Floating forces
In solving transport phenomena issues, most phenomena depend on many variables, the analysis of which using the original sample, and this number of variables, is costly and time consuming. This problem is solved using dimensional analysis. Thus, instead of using individual variables, we obtain the relevant dimensionless numbers and use them, resulting in a reduction in the number of variables. On the other hand, using the law of similarity resulting from dimensional analysis, the data of a small sample can be converted into design data of a real sample. The law of similarity can also be used to make connections between three transport phenomena. In calculations related to macro-scale transfer phenomena, dimensionless numbers such as Nusselt, Schmidt, Reynolds, Prandtl, Sherwood, etc. are used. Table 4 lists some common dimensionless numbers used in chemical engineering.
Equation |
Definition |
Dimensionless |
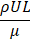
|
Inertia/Viscous |
Reynolds |
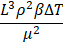
|
Buoyancy/Viscous |
Grashoff |
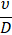
|
kinematic viscosity/Mass diffusivity |
Schmidt |

|
kinematic viscosity/Thermal diffusivity |
Prandtl |
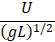
|
Inertia/ Gravitational |
Froude |
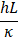
|
Convective/Conductive heat transfer |
Nusselt |
Table 4. Some common dimensionless and widely used numbers in chemical engineering
It is also common on the micro and nano scales to use dimensionless numbers for easier classification and understanding of flow behavior [158]. In these scales, several dimensionless numbers are more important, some of which are listed in Table 5. These dimensionless numbers are defined according to the structural features of the microsystems.
Equation |
Definition |
Dimensionless |
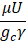
|
Viscous/Interfacial |
Capillary |
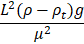
|
Gravitational/Interfacial |
Bond |
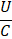
|
Flow velocity/the local speed of sound |
Mach |
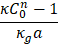
|
Mean free path/ Representative physical length scale |
Knudsen |

|
Reaction rate/Diffusive mass transfer rate |
Damköhler |
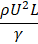
|
Inertia/Interfacial |
Weber |
Table 5. Some new and important dimensionless numbers in microscale systems
The Reynolds number (Re) in micro dimensions is usually much smaller than 1, which indicates the importance of viscous forces in these dimensions. This indicates a significant pressure drop in the flow and eliminates the possibility of instability to mix in the system. In addition to the Reynolds number, the capillary number (Ca) (ratio of viscosity to surface force) is also very useful in describing and characterizing the behavior of multiphase flows in micro and nano-channels. Flow patterns in multiphase systems are classified as segmented flow (drop-based) and parallel flow [159]. When the viscosity of the flow is constant, Weber number (We), which is a combination of Reynolds and Capillary numbers, can be used. Another important dimensionless number is the Mach number (Ma). In micro-and nanoscale systems, the flow of a compressible fluid such as air can also become incompressible due to the low flow velocity, if the local Mach number is less than 0.3. A scale of Ma <0.3 is required to eliminate compressibility but is not sufficient and conditions such as sharp density changes must be avoided so that the fluid flow is almost incompressible. Then incompressible equations can be used in both gas and liquid [157,158].
When a dilute gas is used as a fluid in microchannels, the velocity on the wall is not necessarily zero; In other words, the condition of sliding velocity and temperature jump for the fluid on the solid boundary may be established. The criterion for detecting and classifying gas flow is the Knudson dimensionless number (Kn) [160] . Due to the fact that gas flow within the slip regime within microchannels is one of the important topics in fluid mechanics and applied in industry, so the study of Knudson number in these systems is also important. In a numerical study, Hettiarachchi et al. Investigated the gas flow heat transfer in a rectangular microchannel. Considering the sliding conditions and temperature jump for the wall, they showed that sliding speed increases the Nusselt number (Nu) and temperature jump decreases this number, so the effects of both can reduce or increase the Nusselt number [161] . Another dimensionless number that can be important in micro and nano scales is the Damcohler number (Da). This number indicates the relationship and ratio of the duration of a chemical reaction (chemical reaction rate) to the rate of transport phenomena in a chemical system. In micro-reactors, this number, which is defined as the ratio of the reaction rate to the diffusion rate, acts as an indicator to check the performance of the reactor [162] .
Fluid mechanics at the micro/nanoscale
There are differences in small-scale fluid flow modeling that result from 1) Deviation from the assumption of continuity for gas flow 2) Increase the effect of some additional forces such as electrokinetic forces 3) Uncertainty about the use of empirical factors from larger scale experiments and 4) Uncertainty in micro dimensional measurements including geometric dimensions and operational parameters [154] .
Channels and microchannels are used in various types of devices that deal with single-phase fluid flow. Early applications included micromachines such as micropumps, microvalves, and microsensors. This has been addressed with advances in biology and life sciences due to the need for biological materials such as proteins, DNA, cells, and chemicals. Later, the field of micromixers attracted a lot of attention with the development of micro-reactors; Where two chemicals are mixed before entering the reaction chamber. The main component of micro/ nano-devices are micro-channels in which the establishment of flow requires the application of high-pressure gradients. In fact, among micromachine and microfluidic systems, microchannels are recognized as one of the basic elements for fluid transport. In addition to connecting various process chambers, microchannels are used to transport reactants, separate physical particles, control liquids, mix chemicals, and cool computer chips. The fluid transfer is done in several ways in the microchannels used in these devices. Two important methods of transporting liquids on a small scale are pressure-driven flows and electroosmotic flows.
Fluid flow within microchannels is present in most natural systems (such as the brain, lungs, kidneys, blood vessels) as well as man-made systems (such as turbines, heat exchanger cooling systems, and nuclear reactors for distillation units). The following four factors are important for fluid mechanics on a small scale:
- Micro/nanofluid flows are usually slow due to small length scales.
- Boundary conditions are more important in micro/nanosystems due to the large surface-to-volume ratio.
- Slip conditions may not always apply.
- Chemical composition at surfaces can affect the fluid mechanics of micro/nanoscale.
As mentioned earlier, electrokinetic phenomena become important on a small scale. Electrokinetics affects the transport phenomena and their governing equations. Electrokinetic transport phenomena can be used for flow control in microfluidic systems containing species and particles. The Navier-Stokes equation becomes in the form of Eq. 1 considering the electrokinetic effects:
-∇p+η∇^2 u-ρ∇ψ=ρ Du/Dt |
(Eq.1) |
where , , , and are the pressure, viscosity, velocity, and density of the fluid, respectively. The parameter represents the time and of the electrostatic potential. The third term from the left (bolded) exerts the electrokinetic effect of microstructural considerations.
In nanofluid systems, similar to micro systems, in addition to volumetric forces losing their importance, intermolecular forces also join the group of forces influencing fluid behavior. A fundamental difference in the study of nano-dimensional fluid transfer with macro and even micro dimensions is that the assumption of fluid continuity is challenged. Therefore, in its study, it is no longer possible to use the relevant equations governing the fluid with the assumption of continuity, such as Navier-Stokes. Instead, appropriate simulation methods such as the Boltzmann lattice method, molecular dynamics, and kinetic Monte Carlo method should be used to understand fluid transfer. All of these methods require appropriate computational capabilities [163,164] .
Heat transfer at the micro/nanoscale
Heat transfer at the nanoscale is different from macro and micro scales. In structures with characteristic lengths comparable to the mean free path and wavelengths of heat carriers (electrons, photons, and molecules), classical laws are no longer valid, and new methods are used to predict heat transfer at the nanoscale. Although much work has been done recently in this area, there is still a need to better understand the thermal phenomena in nanostructures. In addition, the knowledge of better control and operation of heat carriers in small structures can open up new avenues for discovering creative applications.
Increasing the surface-to-volume ratio, which is a general feature of micro/ nanosystems, leads to the increase of convective and radiative heat transfer rates. For example, the flow inside microchannels has high heat transfer coefficients [155,165,166] . This is explained by the fact that in the region where the flow is fully expanded, the Nusselt number is constant and is defined as Eq. 2.
Where , and are the convective heat transfer coefficient, hydraulic diameter, and thermal conductivity of the fluid, respectively. The heat transfer coefficient is inversely proportional to the hydraulic diameter and increases with decreasing hydraulic diameter.
Tuckerman and Pease were the first to propose the use of microchannels to cool electronic components [167] . Qu and Mudawar numerically studied the flow and heat transfer inside rectangular microchannels and gave a detailed description of the characteristics of mean and local heat transfer, temperature, Nusselt number and heat flux [168] . Li, et al. performed accurate simulations of heat transfer in silicon microchannels and investigated the effect of microchannel geometric parameters and physical properties of the fluid on the flow and heat transfer by simplifying the 3D heat transfer model [169] . Peterson and Liu developed a three-dimensional model of flow and heat transfer within parallel microchannels [170] . Liu, et al. Also investigated the effect of viscosity on heat transfer within microchannels. They investigated the effect of viscosity and thermal conductivity on flow characteristics and heat transfer [171] .
There are issues in the transfer of heat by fluids on a small scale. For example, if the hydrodynamic diameter of the system is less than 10 micrometers, the macro-scale results should be used with caution. Also, when the hydrodynamic diameter reaches 100 nm, the interactions and molecular interactions between the fluid and the solid wall must be considered [160] .
Considering the electrokinetic effects, the energy equation also changes (the bolded term is added to the equation). The energy equation can be expressed for the flow of isotropic, Newtonian, and incompressible fluid in the presence of an external electric field as follows [172] :
k∇^2 T+ρ_E Eu=ρC_v DT/Dt
|
(Eq.3) |
where , , and are temperature, electric field, heat capacity and time, respectively.
Mass transfer at the micro/nanoscale
The mass transfer equation considering the electrokinetics effect for an electrolyte solution is expressed as the Nernst Planck equation (Eq. 4).
∇.(-D_i ∇n_i-z_i D_i/(K_B T) n_i e∇ψ+un_i )=J_i |
(Eq.4) |
Where , , , , and are the diffusion coefficient, number density, electric charge, mass flux of ion i, Boltzmann constant and electron charge, respectively.
In a study by Fadaei et al., they studied the mass transfer of ionic species from nanopores [173] . Zhao, et al. experimentally investigated the effect of different parameters on the mass transfer rate in a T-shaped microchannel. Mass transfer coefficients were calculated and the effect of different parameters was studied. Studies have shown that a decrease in channel height or a decrease in channel length at a constant Reynolds number, or a decrease in volumetric flux will lead to an increase in the overall average mass transfer coefficient [174].
The necessity for change in the chemical engineering curriculum
Due to the growing need of industry and society for systems with micro/nano dimensions and their various applications in everyday life, the need for training of experienced professionals to design and develop these systems is more felt. In the last few decades, due to rapid developments in microelectronics and biotechnology, applied research in the field of micro-coolers, micro-biochips, micro-reactors, and micro-fuel cells, all of which are microfluidic systems, is expanding at an extraordinary rate. On the other hand, chemical engineering textbooks taught in universities are only able to meet the needs of large-scale processes, and the knowledge gained from them does not help much to know the mechanisms, behavior, and performance of small-scale processes. It should be noted that the basic equations of traditional physics and chemistry do not simulate the evolution of small scales (especially nanoscale) well.
The main sources for chemical engineering courses in many countries are still the heat transfer textbooks by Holman [175] and by Incropera, et al. [176], thermodynamics by Van Wylen [177] and by Smith, et al. [178], fluid mechanics by Streeter, et al. [179] and by Munson, et al. [180], mass transfer by Treybal [181], unit operations by McCabe, et al. [182], reactor design by Levenspiel [183] and Fogler, et al. [184]. Most of these books were written in the relatively distant past and can only meet the needs of large-scale processes and large-scale equipment design (Table 6). In the past two decades, due to significant advances in micro/ nanotechnology, the need to develop courses focusing on micro/ nanoscale transport phenomena is felt more. In fact, the use of design principles is essential for the development of micro-scale (micro/nano) systems and methods for the production and commercialization of nanotechnology-based products.
Ref |
|
Author, Year |
Book |
[176] |

|
Frank P. Incropera, David P. DeWitt, Theodore L. Bergman, Adrienne S. Lavine; 1985 |
Introduction to Heat Transfer |
[181] |

|
RE Treybal; 1955 |
Mass Transfer Operations |
[179] |

|
Victor L. Streeter, K.W. Bedford, E. Benjamin Wylie; 1983 |
Fluid Mechanics |
[185] |

|
R. B. Bird, W. E. Stewart, E. N. Lightfoot, Robert E. Meredith; 1961 |
Transport Phenomena |
[182] |

|
WL McCabe, JC Smith, P Harriot; 1986 |
Unit operation of chemical engineering |
[178] |

|
JM. Smith, HC. Van Ness, MM Abbott; 1950 |
Introduction to chemical engineering thermodynamics |
[183] |

|
O. Levenspiel; 1962 |
|
Table 6. Some common books used in the field of chemical engineering
Transport phenomena, i.e., fluid mechanics, heat transfer, and mass transfer are fundamental topics in disciplines such as chemical engineering. The existing textbooks on these subjects, which are often taught at the undergraduate or graduate level, focus more on the behavior of large-scale systems, and so far limited textbooks for studying the micro and nano fields required to design and build microelectronic, microfluidic, and micro-reactors have been compiled [7].
Among the important topics on the microscale are topics related to electrostatic principles (origin of electrostatic forces, surface charge and repulsive forces, density of opposing ions at the surface, electrostatic forces in the presence of electrolytes, the concept of electric double layer, and Debye length), surface and electrokinetic phenomena, etc. must be included in the chemical engineering curriculum. For instance, the book Electrokinetics and colloid transport phenomena, written by Masliyah, as one of the pioneers of microtechnology in chemical engineering, can be a good reference for this purpose [185,186]. Therefore, it is suggested that courses in micro/nanofluid flow as well as in micro-scale heat transfer be added to the undergraduate courses in chemical engineering. Table 7 presents some useful books that fit the needs of the day, just for example, for use in chemical engineering.
|
Author, Year |
Book |

|
V Hessel, H Löwe, S Hardt; 2004 |
Chemical micro process engineering: fundamentals, modelling and reactions |

|
X Jiang, C Bai, M Liu; 2019 |
Nanotechnology and Microfluidics |

|
YM Joshi, S Khandekar; 2015 |
Nanoscale and microscale phenomena: Fundamentals and applications |

|
DY Tzou; 2014 |
Macro-to microscale heat transfer: the lagging behavior |

|
SS Elnashaie, F Danafar, HH Rafsanjani; 2015 |
Nanotechnology for Chemical Engineers |

|
SK Mitra, S Chakraborty; 2016 |
Microfluidics and nanofluidics handbook: fabrication, implementation, and applications |

|
BV Dzyubenko, YA Kuzma-Kichta, AI Leontiev, II Fedik, LP Kholpanov; 2008 |
Intensification of Heat and Mass Transfer on Macro-, Micro-, and Nanoscales |
Table 7. Some useful books for micro and nano-scale chemical engineering
On the other hand, interdisciplinary courses and the integration of interdisciplinary knowledge required to design and build micro/nanodevices can also help educate people in the basic areas of micro/nanoscience. For example, an interdisciplinary course from three engineering groups (chemical, mechanical, and plastics engineering) can cover the principles of micro / nano-scale transport phenomena required for the production of nano-devices (Figure 13) [7].
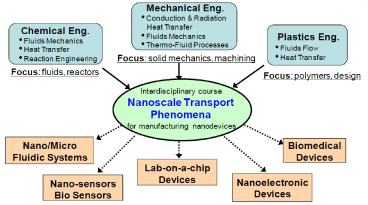
Figure 13. Interdisciplinary approach to creating a new transport phenomena course in the manufacture of micro/ nanoscale equipment
Conclusion and outlook
Modern chemical engineering encompasses a scope beyond conventional process engineering. In recent decades, the miniaturization of processes into micro/ nano-scale has emerged as a new approach for all engineering disciplines, and chemical engineering has been a good ground for the growth of this technology. Systems miniaturization is growing rapidly, and new ideas have emerged in recent decades. Today, in addition to dealing with the world of micro-scale, chemical engineering has moved beyond its traditional lacquer and found close connections with biology, medical engineering, and most engineering disciplines. To meet new and emerging needs in chemical engineering, the curriculum in this engineering major need to be quickly revised to include phenomena related to micro-and nano-scales. One of the most important parts of the curriculum that needs to be fundamentally revised is the courses on transfer phenomena and in particular the topic of heat transfer, which may require fundamental changes in theory and governing equations to predict system behavior. Research topics in the field of chemical engineering will inevitably focus on nanomaterial synthesis, molecular engineering, downsizing, intensification, and process integration over the coming decades.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Research Council of Iran University of Science and Technology is highly appreciated for supporting this research.
References
- Solen KA, Harb JN (2011) Introduction to Chemical Engineering: Tools for Today and Tomorrow, 5th ed, Wiley.
- Nnaji UP (2019) Introduction to Chemical Engineering: For Chemical Engineers and Students, Wiley.
- Bakshi BR (2019) Toward sustainable chemical engineering: The role of process systems engineering. Annu Rev Chem Biomol Eng 10: 265-288. [Crossref]
- Rangaiah GP, Feng Z, Hoadley AF (2020) Multi-objective optimization applications in chemical process engineering: Tutorial and review. Processes 8: 508.
- Duan C, Wang W, Xie Q (2013) Review article: Fabrication of nanofluidic devices. Biomicrofluidics 7: 026501. [Crossref]
- Charpentier JC (2017) Modern chemical engineering in the framework of globalization, sustainability, and technical innovation. Ind Eng Chem Res 46: 3465-3485.
- Gu Z, Budhlall BM (2013) A New Interdisciplinary Engineering Course on Nanoscale Transport Phenomena, Proc, 120th ASEE Ann. Conf., Atlanta, GA.
- Cross WT, Ramshaw C (1986) Process intensification: laminar flow heat transfer. Chem Eng Res Des 64: 293-301.
- Wood RJ (2014) The challenge of manufacturing between Macro and Micro: classic ways of folding paper into dynamic shapes--origami, pop-up books--inspire methods to engineer millimeter-scale machines. Am Sci 102: 124-132.
- Assmann N, Ładosz A, Rudolf von Rohr P (2013) Continuous micro liquid‐liquid extraction. Chem Eng Technol 36: 921-936.
- Marre S, Jensen KF (2010) Synthesis of micro and nanostructures in microfluidic systems. Chem Soc Rev 39: 1183-1202.
- Mourdikoudis S, Pallares RM, Thanh NTK (2018) Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 10: 12871-12934.
- Pletikapić G, Žutić V, Vinković Vrček I, Svetličić V (2012) Atomic force microscopy characterization of silver nanoparticles interactions with marine diatom cells and extracellular polymeric substance. J Mol Recognit 25: 309-317.
- Marbella LE, Millstone JE (2015) NMR techniques for noble metal nanoparticles. Chem Mater 27: 2721-2739.
- Hu C, Zhang Z, Liu H, Gao P, Wang ZL (2006) Direct synthesis and structure characterization of ultrafine CeO2 nanoparticles. Nanotechnology 17: 5983.
- Baer DR (2020) Guide to making XPS measurements on nanoparticles. J Vac Sci Technol 38: 31201.
- Kontopoulos I, Presslee S, Penkman K, Collins MJ (2018) Preparation of bone powder for FTIR-ATR analysis: the particle size effect. Vib Spectrosc 99:167-177.
- Milani S, Baldelli Bombelli F, Pitek AS, Dawson KA, Radler J (2012) Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard corona. ACS Nano 6: 2532-2541.
- Hwang N, Barron AR (2011) BET surface area analysis of nanoparticles. Connexions Proj 1-11.
- Limongi T, Tirinato L, Pagliari F, Giugni A, Allione M, et al. (2017) Fabrication and applications of micro/nanostructured devices for tissue engineering. Nano-Micro Lett 9: 1-13.
- Qamar AZ, Shamsi MH (2020) Desktop fabrication of lab-on-chip devices on flexible substrates: A brief review. Micromachines 11: 126.
- Tabeling P (2005) Introduction to microfluidics, OUP Oxford.
- Maurya DK, Ng WY, Mahabadi KA, Liang YN, Rodríguez I (2007) Fabrication of lab‐on chip platforms by hot embossing and photo patterning. Biotechnol J Healthc Nutr Technol 2: 1381-1388.
- Prakash S, Kumar S (2015) Fabrication of microchannels: a review. Proc Inst Mech Eng Part B J Eng Manuf 229: 1273-1288.
- Isiksacan Z, Guler MT, Aydogdu B, Bilican I, Elbuken C (2016) Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J Micromechanics Microengineering 26: 35008.
- https://www.intechopen.com/books/advanced-biomedical-engineering/recent-developments-in-cell-based-microscale-technologies-and-their-potential-application-in-persona
- Darabi J, Ekula K (2003) Development of a chip-integrated micro cooling device. Microelectronics J 34: 1067-1074.
- Ahammed N, Asirvatham LG, Wongwises S (2016) Thermoelectric cooling of electronic devices with nanofluid in a multiport minichannel heat exchanger. Exp Therm Fluid Sci 74:81-90.
- Osiander R, Darrin MAG, Champion JL (2018) MEMS and microstructures in aerospace applications, CRC press.
- Stone HA, Kim S (2001) Microfluidics: basic issues, applications, and challenges. AIChE J 47: 1250-1254.
- Ohno K, Tachikawa K, Manz A (2008) Microfluidics: applications for analytical purposes in chemistry and biochemistry. Electrophoresis 29: 4443-4453.
- Chen H, Fang Q, Yin XF, Fang ZL (2005) Microfluidic chip-based liquid-liquid extraction and preconcentration using a subnanoliter-droplet trapping technique. Lab Chip 5: 719-725.
- Cai ZX, Fang Q, Chen HW, Fang ZL (2006) A microfluidic chip based liquid-liquid extraction system with microporous membrane. Anal Chim Acta 556: 151-156.
- Maurice A, Theisen J, Gabriel JCP (2020) Microfluidic lab-on-chip advances for liquid-liquid extraction process studies. Curr Opin Colloid Interface Sci 46: 20-35.
- Ciceri D, Perera JM, Stevens GW (2014) The use of microfluidic devices in solvent extraction. J Chem Technol Biotechnol 89: 771-786.
- Xu C, Xie T (2017) Review of microfluidic liquid-liquid extractors. Ind Eng Chem Res 56: 7593-7622.
- Abbasi A, Seifollahi Z, Rahbar-Kelishami A (2021) Experimental work on decontamination of wastewaters containing organic dye by liquid phase micro extraction method. Sep Sci Technol 56.
- Davidson N (2012) International Solvent Extraction Conference-ISEC 2011. Platin Met Rev 56: 177-180.
- Abdel-Rehim M, Pedersen-Bjergaard S, Abdel-Rehim A, Lucena R, Moein MM, et al. (2020) Microextraction approaches for bioanalytical applications: An overview. J Chromatogr A 1616: 460790. [Crossref]
- Zhang Y, Park S, Yang S, Wang TH (2010) An all-in-one microfluidic device for parallel DNA extraction and gene analysis. Biomed Microdevices 12: 1043-1049. [Crossref]
- Wu J, Kodzius R, Cao W, Wen W (2014) Extraction, amplification and detection of DNA in microfluidic chip-based assays. Microchim Acta 181: 1611-1631.
- Shaw KJ, Thain L, Docker PT, Dyer CE, Greenman J, et al. (2009) The use of carrier RNA to enhance DNA extraction from microfluidic-based silica monoliths. Anal Chim Acta 652: 231-233. [Crossref]
- Muhammed A, Hussen A, Kaneta T (2021) Speciation of chromium in water samples using microfluidic paper-based analytical devices with online oxidation of trivalent chromium. Anal Bioanal Chem 1-9. [Crossref]
- Alahmad W, Varanusupakul P, Kaneta T, Varanusupakul P (2019) Chromium speciation using paper-based analytical devices by direct determination and with electromembrane microextraction. Anal Chim Acta 1085: 98-106. [Crossref]
- Dai S, Luo J, Li J, Zhu X, Cao Y, et al. (2017) Liquid-liquid microextraction of Cu2+ from water using a new circle microchannel device. Ind Eng Chem Res 56: 12717-12725.
- Abbasi A, Rahbar-Kelishami A, Ghasemi MJ (2018) Development of a microfluidic-chip system based on parallel flow for intensified Gd (III) extraction from nitrate media using cationic extractant. J Rare Earths 36: 1198-1204.
- Farahani A, Rahbar-Kelishami A, Shayesteh H (2021) Microfluidic solvent extraction of Cd (II) in parallel flow pattern: Optimization, ion exchange, and mass transfer study. Sep Purif Technol 258: 118031.
- Sarkar PS, Singh KK, Shenoy KT, Sinha A, Rao H, et al. (2012) Liquid-liquid two-phase flow patterns in a serpentine microchannel. Ind Eng Chem Res 51: 5056-5066.
- Jahromi PF, Karimi-Sabet J, Amini Y (2018) Ion-pair extraction-reaction of calcium using Y-shaped microfluidic junctions: An optimized separation approach. Chem Eng J 334: 2603-2615.
- FH Kriel, Holzner G, Grant RA, Woollam S, Ralston J, et al. (2015) Microfluidic solvent extraction, stripping, and phase disengagement for high-value platinum chloride solutions. Chem Eng Sci 138: 827-833.
- Abbasi A, Rahbar-Kelishami A, Seifollahi Z, Ghasemi MJ (2020) Intensified decontamination of amoxicillin drug wastewater assisted by liquid-phase micro extraction method. Environ Technol 1-10. [Crossref]
- Samla G, Gan KB, Then SM (2017) Solid phase microextraction based micro-device for extraction of PCR amplifiable DNA. Int J Nanoelectronics Mater 10: 75-92.
- Mao Z, Yang C (2017) Micro-mixing in chemical reactors: a perspective. Chinese J Chem Eng 25: 381-390.
- Nienow AW, Edwards MF, Harnby N (1997) Mixing in the process industries, Butterworth-Heinemann.
- Lee CY, Fu LM (2018) Recent advances and applications of micromixers. Sens Actuators B Chem 259: 677-702.
- Capretto L, Cheng W, Hill M, Zhang X (2011) Micromixing within microfluidic devices. Microfluidics 27-68. [Crossref]
- Lee CY, Chang CL, Wang YN, Fu LM (2011) Microfluidic mixing: a review. Int J Mol Sci 12: 3263-3287. [Crossref]
- Gambhire S, Patel N, Gambhire G, Kale S (2016) A review on different micromixers and its micromixing within microchannel. Int J Curr Eng Technol 4: 409-413.
- Nguyen NT, Wu Z (2005) Micromixers-a review. J Micromech Microeng 15: R1. [Crossref]
- Naher S, Orpen D, Brabazon D, Poulsen C, Morshed M (2011) Effect of micro-channel geometry on fluid flow and mixing. Simul Model Pract Theory 19: 1088-1095.
- Koch M, Witt H, Evans AGR, Brunnschweiler A (1999) Improved characterization technique for micromixers. J Micromech Microeng 9: 156.
- Liu ZY, Kim BJ, Sung HJ (2004) Two-fluid mixing in a microchannel. Int J Heat Fluid Flow 25: 986-995.
- Liu RH, Stremler MA, Sharp KV, Olsen MG, Santiago JG, et al. (2000) Passive mixing in a three-dimensional serpentine microchannel. J Microelectromech Syst 9:190-197.
- Shamsoddini R, Sefid M, Fatehi R (2014) ISPH modelling and analysis of fluid mixing in a microchannel with an oscillating or a rotating stirrer. Eng Appl Comput Fluid Mech 8: 289-298.
- Cai G, Xue L, Zhang H, Lin J (2017) A review on micromixers. Micromachine 8: 274. [Crossref]
- Khodashenas B, Zadghaffari R, Jafari SD (2015) Process intensification approach for the synthesis of metal nanoparticles: a mini review. Orient J Chem 31: 249-257.
- Renggli K, Baumann P, Langowska K, Onaca O, Bruns N, et al. (2011) Selective and responsive nanoreactors. Adv Funct Mater 21: 1241-1259.
- Dietrich TR, Freitag A, Scholz R (2005) Production and characteristics of microreactors made from glass. Chem Eng Technol Ind Chem Equipment‐Process Eng 28: 477-483.
- Watts P, Haswell SJ (2005) The application of microreactors for small scale organic synthesis. Chem Eng Technol Ind Chem Equipment‐Process Eng 28: 290-301.
- Singh A, Malek SK, Kulkarni SK (2010) Development in microreactor technology for nanoparticle synthesis. Int J Nanosci 9: 93-112.
- Kamali R (2017) Synthesis of Organic Compounds and biological activity in the presence of Nano-reactors. Int J Adv Biotechnol Res 8:1766-1774.
- Salomon P (2005) Product-Technology Roadmap for Microsystems A Nexus Task Force Report, Berlin, WTC Wicht Technologie Consulting.
- Li Y, Yan L, Liu Y, Qian K, Liu B, et al. (2015) High-efficiency nano/micro-reactors for protein analysis. Rsc Adv 5:1331-1342.
- Wagner J, Kirner T, Mayer G, Albert J, Köhler JM (2004) Generation of metal nanoparticles in a microchannel reactor. Chem Eng J 101: 251-260.
- Singh A, Shirolkar M, Lalla NP, Malek CK, Kulkarni SK (2009) Room temperature, water-based, microreactor synthesis of gold and silver nanoparticles. Int J Nanotechnol 6: 541-551.
- Lin XZ, Terepka AD, Yang H (2004) Synthesis of silver nanoparticles in a continuous flow tubular microreactor. Nano Lett 4: 2227-2232.
- Krishnadasan S, Tovilla J, Vilar R, DeMello AJ, DeMello JC (2004) On-line analysis of CdSe nanoparticle formation in a continuous flow chip-based microreactor. J Mater Chem 14: 2655-2660.
- Song Y, Modrow H, Henry LL, Saw CK, Doomes EE, et al. (2006) Microfluidic synthesis of cobalt nanoparticles. Chem Mater 18: 2817-2827.
- Wang H, Nakamura H, Uehara M, Miyazaki M, Maeda H (2002) Preparation of titania particles utilizing the insoluble phase interface in a microchannel reactor. Chem Commun 1462-1463.
- Appalakutti S, Sonawane S, Bhanvase BA, Mittal V, Ashokkumar M (2015) Process intensification of copper chromite (CuCr2O4) nanoparticle production using continuous flow microreactor. Chem Eng Process Intensif 89: 28-34.
- Jibril L, Chen PC, Hu J, Odom TW, Mirkin CA (2019) Massively Parallel Nanoparticle Synthesis in Anisotropic Nanoreactors. ACS Nano 13: 12408-12414.
- Morse JD (2007) Micro‐fuel cell power sources. Int J Energy Res 31: 576-602.
- Zhang Y, Wilkinson DP, Taghipour F (2020) Development and Characterization of a Micro Redox Fuel Cell. J Electrochem Soc 167: 114514.
- Pattekar AV, Kothare MV(2004) A microreactor for hydrogen production in micro fuel cell applications. J Microelectromech Syst 13: 7-18.
- Wang Y, Luo S, Kwok HYH, Pan W, Zhang Y, Zhao X, Leung DYC (2021) Microfluidic fuel cells with different types of fuels: A prospective review. Renew Sustain Energy Rev 141: 110806.
- Khatibi M, Sadeghi A, Ashrafizadeh SN (2021) Tripling the reverse electrodialysis power generation in conical nanochannels utilizing soft surfaces. Phys Chem Chem Phys 23: 2211-2221.
- Kang BD, Kim HJ, Lee MG, Kim DK (20150 Numerical study on energy harvesting from concentration gradient by reverse electrodialysis in anodic alumina nanopores. Energy 86: 525-538.
- Kim SJ, Ko SH, Kang KH, Han J (2010) Direct seawater desalination by ion concentration polarization. Nat Nanotechnol 5: 297-301.
- Guo W, Cao L, Xia J, Nie F, Ma W, et al. (2010) Energy harvesting with single‐ion‐selective nanopores: a concentration‐gradient‐driven nanofluidic power source. Adv Funct Mater 20: 1339-1344.
- Hsu JP, Yang ST, Lin CY, Tseng S (2017) Ionic current rectification in a conical nanopore: influences of electroosmotic flow and type of salt. J Phys Chem C 121: 4576-4582.
- Hsu JP, Lin SC, Lin CY, Tseng S (2017) Power generation by a pH-regulated conical nanopore through reverse electrodialysis. J Power Sources 366: 169-177.
- Yeh HC, Chang CC, Yang RJ (2014) Reverse electrodialysis in conical-shaped nanopores: salinity gradient-driven power generation. RSC Adv 4: 2705-2714.
- Iverson BD, Garimella SV (2008) Recent advances in microscale pumping technologies: a review and evaluation. Microfluid Nanofluidics 5: 145-174.
- https://www.intechopen.com/chapters/29689
- Nguyen NT, Huang X, Chuan TK (2002) MEMS-micropumps: a review. J Fluids Eng 124: 384-392.
- Laser DJ, Santiago JG (2004) A review of micropumps. J Micromech Microeng 14: R35.
- Woias P (2005) Micropumps—past, progress and future prospects. Sens Actuators B Chem 105: 28-38.
- Abhari F, Jaafar H, Yunus NAM (2012) A comprehensive study of micropumps technologies. Int J Electrochem Sci 7: 9765-9780.
- Liu H, Zhang X, Hong Z, Pu Z, Yao Q, et al. (2017) A bioinspired capillary-driven pump for solar vapor generation. Nano Energy 42: 115-121.
- Guan YX, Xu ZR, Dai J, Fang ZL (2006) The use of a micropump based on capillary and evaporation effects in a microfluidic flow injection chemiluminescence system. Talanta 68: 1384-1389.
- Fathi S, Mohseni SS, Mehrizi AA (2020) Flow rate controlling by capillary micropumps in open biomicrofluidic devices, in: 27th Natl. 5th Int. Iran. Conf. Biomed. Eng., IEEE: pp. 187-191.
- GuoW, Hansson J, Van Der Wijngaart W (2018) Capillary pumping independent of the liquid surface energy and viscosity. Microsyst Nanoeng 4: 1-7.
- Dehghan Manshadi MK Khojasteh D, Mohammadi M, Kamali R (2016) Electroosmotic micropump for lab‐on‐a‐chip biomedical applications. Int J Numer Model Electron Networks, Devices Fields 29: 845-858.
- Flores JV (2017) Methods used to determine the zeta potential of colloids in wastewater. J Sci Eng 1: 19-32.
- Chen L, Ma J, Guan Y (2003) An electroosmotic pump for packed capillary liquid chromatography, Microchem J 75:15-21.
- Chen L, Ma J, Guan Y (20040 Study of an electroosmotic pump for liquid delivery and its application in capillary column liquid chromatography. J Chromatogr A 1028: 219-226.
- Jiang L, Mikkelsen J, Koo JM, Huber D, Yao S, et al. (2002), Closed-loop electroosmotic microchannel cooling system for VLSI circuits, IEEE Trans. Components Packag Technol 25: 347-355.
- Olenev E, Al-Haidri W, Lebedinskaya O (2020) A Method of Deep Delivery of Drugs into The Biological Tissues, 2020 Ural Symp Biomed Eng Radioelectron Inf Technol, pp. 89-92.
- Rajabi F, Bakhshi A, Kazemi G (2021) Drug delivery applications of mechanical micropumps, International Conference on Applied Researches in Science & Engineering, Amsterdam, Netherlands.
- Chen L, Choo J, Yan B (2007) The microfabricated electrokinetic pump: a potential promising drug delivery technique. Expert Opin Drug Deliv 4: 119-129.
- Wang X, Cheng C, Wang S, Liu S (2009) Electroosmotic pumps and their applications in microfluidic systems. Microfluid Nanofluidics 6: 145-162.
- Chakraborty S, Ray S (2008) Mass flow-rate control through time periodic electro-osmotic flows in circular microchannels. Phys Fluids 20: 83602.
- Sadeghi A, Yavari H, Saidi MH, Chakraborty S (2011) Mixed electroosmotically and pressure-driven flow with temperature-dependent properties. J Thermophys Heat Transf 25: 432-442.
- Hoshyargar V, Ashrafizadeh SN, Sadeghi A (2017) Mass transport characteristics of diffusioosmosis: potential applications for liquid phase transportation and separation. Phys Fluids 29: 12001.
- Hoshyargar V, Ashrafizadeh SN, Sadeghi A (2016) Diffusioosmotic flow in rectangular microchannels. Electrophoresis 37: 809-817.
- Marina ML, Ríos A, Valcárcel M (205) Analysis and detection by capillary electrophoresis, Elsevier.
- Felix C, Yaroshchuk A, Pasupathi S, Pollet BG, Bondarenko MP, et al. (2014) Electrophoresis and stability of nano-colloids: History, theory and experimental examples. Adv Colloid Interface Sci 211: 77-92.
- Ashrafizadeh SN, Seifollahi Z, Ganjizade A, Sadeghi A (2020) Electrophoresis of spherical soft particles in electrolyte solutions: A review. Electrophoresis 41: 81-103. [Crossref]
- Huang Y, Huang C, Hu C, Chang H (2006) Capillary electrophoresis‐based separation techniques for the analysis of proteins. Electrophoresis 27: 3503-3522. [Crossref]
- Zhang L, Dang F, Baba Y (2003) Microchip electrophoresis-based separation of DNA. J Pharm Biomed Anal 30: 1645-1654. [Crossref]
- Lo CK, Paau MC, Xiao D, Choi MMF (2008) Application of capillary zone electrophoresis for separation of water‐soluble gold monolayer‐protected clusters. Electrophoresis 29: 2330-2339. [Crossref]
- Zhai SL, Luo GS, LiU JG (2001) Aqueous two-phase electrophoresis for separation of amino acids. Sep Purif Technol 21: 197-203.
- Lee PY, Costumbrado J, Hsu CY, Kim YH (2012) Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp 62: 9400-9405.
- Swinney K, Bornhop DJ (2000) Detection in capillary electrophoresis. Electrophor An Int J 21: 1239-1250.
- Kim JE, Kim BR, Woo KS, Kim JM, Park JI, et al. (2011) Comparison of capillary electrophoresis with cellulose acetate electrophoresis for the screening of hemoglobinopathies. Korean J Lab Med 31: 238.
- Hanauer M, Pierrat S, Zins I, Lotz A, Sönnichsen C (2007) Separation of nanoparticles by gel electrophoresis according to size and shape. Nano Lett 7: 2881-2885. [Crossref]
- Hjerten S (1990) Zone broadening in electrophoresis with special reference to high‐performance electrophoresis in capillaries: an interplay between theory and practice. Electrophoresis 11: 665-690.
- Grossman PD, Colburn JC (2012) Capillary electrophoresis: Theory and practice, Academic Press.
- Voeten RLC, Ventouri IK, Haselberg R, Somsen GW (2018) Capillary electrophoresis: trends and recent advances. Anal Chem 90: 1464-1481.
- Kohl FJ, Sánchez‐Hernández L, Neusüß C (2015) Capillary electrophoresis in two‐dimensional separation systems: Techniques and applications. Electrophoresis 36:144-158.
- Lu G, Crihfield CL, Gattu S, Veltri LM, Holland LA (2018) Capillary electrophoresis separations of glycans. Chem Rev 118: 7867-7885.
- Heller C (2001) Principles of DNA separation with capillary electrophoresis. Electrophoresis 22: 629-643.
- Ganjizade A, Ashrafizadeh SN, Sadeghi A (2019) Significant alteration in DNA electrophoretic translocation velocity through soft nanopores by ion partitioning. Anal Chim Acta 1080: 66-74.
- Shields CW, Reyes CD, López GP (2015) Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 15: 1230-1249.
- Ramos‐Payán M, Ocaña‐Gonzalez JA, Fernández‐Torres RM, Llobera A, Bello‐López MA (2018) Recent trends in capillary electrophoresis for complex samples analysis: A review. Electrophoresis 39: 111-125.
- Paul P, Sänger-van de Griend C, Adams E, Van Schepdael A (2018) Recent advances in the capillary electrophoresis analysis of antibiotics with capacitively coupled contactless conductivity detection. J Pharm Biomed Anal 158: 405-415.
- Velayi E, Norouzbeigi R (2020) A mesh membrane coated with dual-scale superhydrophobic nano zinc oxide: Efficient oil-water separation. Surf Coatings Technol 385: 125394.
- Nikosokhan R, Norouzbeigi R, Velayi E (2021) Preparation of Co3O4 self-cleaning nanocoatings: Investigation of ZnO seeded steel meshes. Surf ad Interfaces 23: 100912.
- Fekry AM, Azab SM (2020) The development of an innovative nano-coating on the surgical 316 L SS implant and studying the enhancement of corrosion resistance by electrochemical methods using Ibandronate drug. Nano-Struct Nano-Obj 21: 100411.
- Abdeen DH, El Hachach M, Koc M, Atieh MA (2019) A review on the corrosion behaviour of nanocoatings on metallic substrates. Mater 12: 210.
- Keeney M, Jiang XY, Yamane M, Lee M, Goodman S, et al. (2015) Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J Mater Chem B 3: 8757-8770.
- Corni I, Neumann N, Novak S, König K, Veronesi P, et al. (2009) Electrophoretic deposition of PEEK-nano alumina composite coatings on stainless steel. Surf Coatings Technol 203: 1349-1359.
- Maleki-Ghaleh H, Rekabeslami M, Shakeri MS, Siadati MH, Javidi M, Talebian SH, Aghajani H (2013) Nano-structured yttria-stabilized zirconia coating by electrophoretic deposition. Appl Surf Sci 280: 666-672.
- Barzegar B, Ashrafizadeh SN, Ashrafizadeh F (2004) Preparation of catalytic coating of titanium oxide by means of electrolytic deposition. Iran J Mater Sci Eng 1: 9-15.
- Ashrafizadeh SN, Barzegar B, Ashrafizadeh F (2003) Characterization of catalytic ceramic coatings produced by electrolytic deposition., in: Ecomater Ecoprocesses Int Symp Light Met as Held 42 Nd Annu.Conf. Metall CIM, pp. 123-130.
- M. Abdollahi M, Ashrafizadeh SN, Malekpour A (2007) Preparation of zeolite ZSM-5 membrane by electrophoretic deposition method. Microporous Mesoporous Mater 106: 192-200.
- Saberi F, Boroujeny BS, Doostmohamdi A, Baboukani AR, Asadikiya M (2018) Electrophoretic deposition kinetics and properties of ZrO2 nano coatings. Mater Chem Phys 213: 444-454.
- Monteferrante M, Sola L, Cretich M, Chiari M, Marini Bettolo Marconi U, Melchionna S (2015) Controlling electroosmotic flows by polymer coatings: A joint experimental-theoretical investigation. J Chem Phys 143: 184907.
- Khatibi M, Ashrafizadeh SN, Sadeghi A (2020) Covering the conical nanochannels with dense polyelectrolyte layers significantly improves the ionic current rectification. Anal Chim Acta 1122: 48-60.
- Stevens KA, Crockett J, Maynes DR, Iverson BD (2017) Two-phase flow pressure drop in superhydrophobic channels. Int J Heat Mass Transf 110: 515-522.
- Choi C, Yu DI, Kim M (2011) Surface wettability effect on flow pattern and pressure drop in adiabatic two-phase flows in rectangular microchannels with T-junction mixer. Exp Therm Fluid Sci 35: 1086-1096.
- Speyer K, Pastorino C (2017) Droplet transport in a nanochannel coated by hydrophobic semiflexible polymer brushes: the effect of chain stiffness. Langmuir 33:10753-10763.
- Bauer WAC, Fischlechner M, Abell C, Huck WTS (2010) Hydrophilic PDMS microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions. Lab Chip 10: 1814-1819.
- https://www.intechopen.com/books/1989
- https://www.intechopen.com/chapters/13428
- Chen C, Ma M, Jin K, Liu JZ, Shen L, et al. (2011) Nanoscale fluid-structure interaction: Flow resistance and energy transfer between water and carbon nanotubes. Phys Rev E 84: 46314. [Crossref]
- Shui L (2007) Two-Phase Flow in Micro and Nanofluidic Devices, Zutphen, Netherlands, Wohrmann Print Service.
- Ogden S, Bodén R, Do-Quang M, Wu ZG, Amberg G, Hjort K (2014) Fluid behavior of supercritical carbon dioxide with water in a double-Y-channel microfluidic chip. Microfluid Nanofluidics 17: 1105-1112.
- Tang J, Smit M, Vincent‐Bonnieu S, Rossen WR (2019) New capillary number definition for micromodels: The impact of pore microstructure. Water Resour Res 55: 1167-1178.
- Kandlikar S, Garimella S, Li D, Colin S, King MR (2005) Heat transfer and fluid flow in minichannels and microchannels, Elsevier.
- Hettiarachchi HDM, Golubovic M, Worek WM, Minkowycz WJ (2008) Three-dimensional laminar slip-flow and heat transfer in a rectangular microchannel with constant wall temperature. Int J Heat Mass Transf 51: 5088-5096.
- Aoki N, Hasebe S, Mae K (2006) Geometric design of fluid segments in microreactors using dimensionless numbers. AIChE J 52:1502-1515.
- Karimipour A, Nezhad AH, D’Orazio A, Shirani E (2012) Investigation of the gravity effects on the mixed convection heat transfer in a microchannel using lattice Boltzmann method. Int J Therm Sci 54: 142-152.
- Karimipour A, Afrand M, Akbari M, Safaei MR (2012) Simulation of fluid flow and heat transfer in the inclined enclosure. Int J Mech Aerosp Eng 6: 86-91.
- Tzou DY (2004) Macro-to microscale heat transfer: the lagging behavior, Wiley.
- Zhang ZM, Zhang ZM (2007) Nano/microscale heat transfer, Switzerland AG, Springer.
- Tuckerman DB, Pease RFW (1982) Ultrahigh thermal conductance microstructures for cooling integrated circuits, In: IEEE-Electronics Lab Symp, pp. 145-149.
- Qu W, Mudawar I (2002) Analysis of three-dimensional heat transfer in micro-channel heat sinks. Int J Heat Mass Transf 45: 3973-3985.
- Li, J, Peterson GP, Cheng P (2004) Three-dimensional analysis of heat transfer in a micro-heat sink with single phase flow. Int J Heat Mass Transf 47: 4215-4231.
- Li J, Peterson GP (2007) 3-Dimensional numerical optimization of silicon-based high performance parallel microchannel heat sink with liquid flow. Int J Heat Mass Transf 50: 2895-2904.
- Liu JT, Peng XF, Yan WM (2007) Numerical study of fluid flow and heat transfer in microchannel cooling passages. Int J Heat Mass Transf 50: 1855-1864.
- Mour M, Das D, Mullick AN (2010) Characteristics of Fluid Flow through Microchannels, In: AIP Conf Proc, American Institute of Physics: pp. 71-79.
- Fadaei F, Shirazian S, Ashrafizadeh SN (2011) Mass transfer modeling of ion transport through nanoporous media. Desalination 281: 325-333.
- Zhao Y, Chen G, Yuan Q (2007) Liquid-liquid two‐phase mass transfer in the T‐junction microchannels, AIChE J 53: 3042-3053. doi:10.1002/aic.11333.
- Holman JP (2008) Heat Transfer, Tata McGraw-Hill Education.
- Incropera FP, DeWitt DP (1990) Introduction to heat transfer, Wiley.
- Van Wylen GJ, Sonntag RE (1986) Fundamentals of classical thermodynamics, Wiley.
- Smith JM (1950) Introduction to chemical engineering thermodynamics, ACS Publications.
- Streeter VL, Wylie EB, Bedford KW (2002) Fluid Mechanics, New York, McGraw-Hill.
- Munson BR, Young DF, Okiishi TH (1996) Fundamentals of fluid mechanics, Wiley.
- Treybal RE (1980) Mass transfer operations, New York , McGraw-Hill.
- McCabe WL, Smith JC, Harriot P (1986) Unit operation of chemical engineering, New York, McGraw Hil.
- Levenspiel O (1996) Chemical reaction engineering, Wiley.
- Fogler S (1998) Chemical reactions engineering, Albright’s Chem. Eng. Handb.
- Bird RB, Stewart WE, Lightfoot EN, Meredith RE (1961) Transport phenomena, Wiley.
- Masliyah JH, Bhattacharjee S (2006) Electrokinetic and colloid transport phenomena, Wiley.
- Hessel V, Löwe H, Hardt S (2004) Chemical micro process engineering: fundamentals, modelling and reactions, Wiley.
- Jiang X, Bai C, Liu M (2020) Nanotechnology and microfluidics, Wiley Online Library.
- Joshi YM, Khandekar S (2015) Nanoscale and microscale phenomena: Fundamentals and applications, Springer.
- Elnashaie SS, Danafar F, Rafsanjani HH (2015) Nanotechnology for chemical engineers, Springer.
- Mitra SK, Chakraborty S (2016) Microfluidics and nanofluidics handbook: fabrication, implementation, and applications, CRC press.
- Dzyubenko BV, Kuzma-Kichta YA, Leontiev AI, Fedik II, Kholpanov LP (2008) Intensification of heat and mass transfer on macro-, micro-, and nanoscales, Moscow TSNIIatominform.








































