The aim of this study was to investigate the interface between zirconia and zinc phosphate cement by solid state Nuclear Magnetic Resonance (ssNMR) spectroscopic NMR in collecting information about molecular organization. The analysis is conducted by solid state NMR spectroscopy, which can provide information at the molecular level on the zirconia /zinc phosphate interface. Specifically, NMR with magic angle spinning (MAS) of the phosphorus nuclei (31P) should be of particular interest in determining the chemical nature of the species at the interface and studying their own organization and mobility. Analysis of 31P nuclei is supplemented by NMR MAS studies on hydrogen atoms (1H ) and of 67Zn and 25Mg. The 1H and 31P NMR experiments show that phosphoric acid reacts with ZnO and MgO to form heterogeneous amorphous phases composed of zinc and magnesium phosphates. These phosphates are also very rich in hydroxyl groups and species such as Zn (HPO4).3H2O and Mg(HPO4).3H2O. The addition of zirconia powder changes the composition and the organization of the medium with the appearance of a new water-rich crystalline.
adhesion, zirconia ceramic, solid state NMR, cement, chemical organization
For many years, the use of dental ceramics has developed to treat losses of partial or even total tooth substances. There are three main reasons for this: their optical, biological properties and their adhesive potential for some of them. However, their mechanical properties vary widely, limiting their indications [1].
Zirconia has gained increased attention due to its proven biocompatibility, mechanical strength, and its use in Computer-Aided Design/Computer-Aided Machining (CAD/ CAM) technology. These are specifically indicated for the posterior areas of the mouth, where esthetics are not the primary focus and for cases with limited occlusal and palatal space and especially to make full crowns [2-3].
The adhesive potential of polycrystalline ceramic zirconia is structurally limited in fact by the hardness of the material and the almost total absence of glassy phase. It is relatively inert substrate with low surface energy and wettability. Discovering the best way to improve bonding and durability of zirconia restorations has been a common goal of researchers [4].
Many surface treatment protocols are proposed to compensate for this limited adhesive potential. However, either they complicate the realization of the prosthetic device by proposing to infiltrate the intrados with glass, or they risk weakening the structure by sandblasting the prosthetic intrados [5].
Preparation of the surface is decisive for the longevity of the prosthetic device [4,6,7]. The humidity conditions (sub-gingival preparations) contra-indicate any bonding technique and thus the use of resin cement. Clinically it is very difficult, given the situation of the cervical limit and sulcular fluid, to assemble by bonding in optimal conditions [2,8]. This sub-gingival situation also requires non-genotoxic biomaterials [9]. Based on this premise, it makes sense, in some clinical cases, to use a sealing cement when laying a zirconia prosthesis [2]. Zirconia has a toughness coefficient greater than 2 and can therefore be sealed.
Zinc phosphate cements have been used successfully for more than 20 years. In assembling prosthetic restorations with a metal infrastructure their performance shows no significant difference with resin cement [10]. When used to seal crowns in Zirconia, the survival rate of prostheses appears similar to bonding [11]. They are reference cements whose adhesion to the prosthetic part is called purely mechanical [12]. The aim of this study was therefore to investigate the interface between zirconia and zinc phosphate cement by solid state Nuclear Magnetic Resonance (ssNMR) spectroscopic NMR in collecting information about molecular organization.
The zirconia that was used in this study is a 3Y-TZP zirconia (this zirconia has 3% by molar weight of Yttrium oxide in order to stabilize the zirconia at room temperature. This percentage is equivalent to 4.5-6% by weight of Yttrium oxide.) The size of the grains is 0.50μm.
The sealing cement of the study is Crown and Bridge zinc phosphate cement (Dentsply Sirona), mineral matrix cement, medium grain (40μm) The powder is formed almost essentially of zinc oxide ZnO (88%) with small amounts of magnesium oxide MgO and silicon oxide SiO2. The liquid is formed by 66% phosphoric acid H3PO4 and 33% water.
The analysis is conducted by solid phase NMR spectroscopy. Solid phase NMR spectroscopy can provide information at the molecular level on the zirconia /zinc phosphate interface. In particular, the rotating NMR at the magic angle (MAS) of the phosphorus nuclei (31P) should be of specific interest in determining the chemical nature of the species at the interface and studying their own organization and mobility. Analysis of 31P nuclei is supplemented by RMN MAS studies on hydrogen atoms (1H ) and NMR of 67Zn and 25Mg .
Sample preparation
The following were analyzed: the powder of the cement, the powder-liquid mixture, and finally the mixture put in contact with the zirconia powder. For the powder-liquid mixture, 60 mg of cement powder was mixed with 40 mg of the phosphoric acid solution during 2 minutes until getting a smooth and creamy mix. Then half of the creamy mix was mixed with 50 mg of zirconia powder. The powder-liquid mixture with or without zirconia was left in the air to dry for 1 hour and then ground into a fine powder.
Solid state NMR
Solid state NMR experiments were recorded on a Bruker Avance 400 III HD or on a Bruker Avance 600 NEO spectrometer operating at 9.4 T and 14.1 T magnetic field. Samples were packed into 4 mm or 1.3 mm zirconia rotors. 1H and 31P NMR experiments have been performed at a spinning frequency of 60 kHz. 31P CP MAS (Cross-Polarization Magic Angle Spinning) were done with a recycle delay of 2 s and a contact time of 1 ms. 1H MAS performed with the DEPTH pulse sequence with a recycle delay of 5 s. 1H/31P double cross polarization MAS were performed with a first CP transfer of 6 ms or 3 ms, a second CP transfer of 2 ms or 0.5 ms and a recycle delay of 3 s. 1H/31P HETCOR experiment was carried out with a contact time of 0.3 ms and with frequency switched Lee-Goldburg homonuclear decoupling during 1H evolution. The 2D 1H-1H double-quantum–single quantum (DQMAS) were recorded with 2 periods recoupling using the back-to-back (BABA) sequence. 67Zn and 25Mg QCPMG were acquired with 50 echoes, a delay between train of 180° pulse of 20 rotor periods and a recycle delay of 2 s at 14.1 T (spinning frequency of 10 kHz). After registering the echo-trains, the echoes were summed up before processing. Chemical shifts were externally referenced to liquid TMS, 85% H3PO4, 1.1 M MgCl2, 1.0 M Zn(NO3)2 aqueous solutions for 1H, 13C, 31P, 25Mg and 67Zn, respectively.
31P NMR
The 31P CP MAS spectrum of cement obtained by mixing cement powder with the phosphoric acid solution is typical of an amorphous product with several contributions (Figure 1a). It can be deconvoluted with 2 signals centered at 1.2 ppm and -4.3 ppm (Figure 2). These signals are found in the spectral area where the species a,b-Zn3(PO4) (3 à 7 ppm) et g-Zn2P2O7 (-5 à -10 ppm) are observed. They can therefore be associated to an etching of ZnO particles by phosphoric acid, with a release of Zn2+ ions that recombine with the PO43- ions to give different zinc phosphate solid phases. These phases also most likely contain Mg2+ ions because the same etching is expected on MgO. The chemical shifts of Magnesium phosphates are also consistent with the signals observed [13,14]
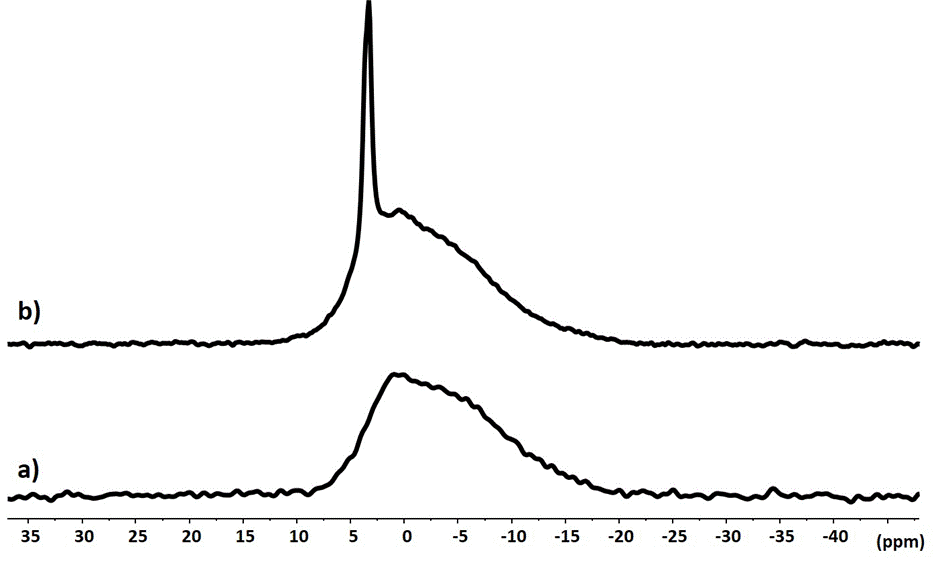
Figure 1. 31P CP MAS NMR spectra of cement powder after addition of the phosphoric acid solution in absence (a) and in presence (b) of zirconia
In the presence of zirconiae, there is a clear difference with a new sharp signal at 3.5 ppm (Figure 1b). This signal shows significant chemical shift anisotropy observed on the 3 kHz low-spinning frequency MAS experiment. The signal sharpness is therefore explained by the presence of a new crystalline phase in the presence of zirconia particles. It can be assumed that this phase forms on the surface of the zirconiae (Figure 3).
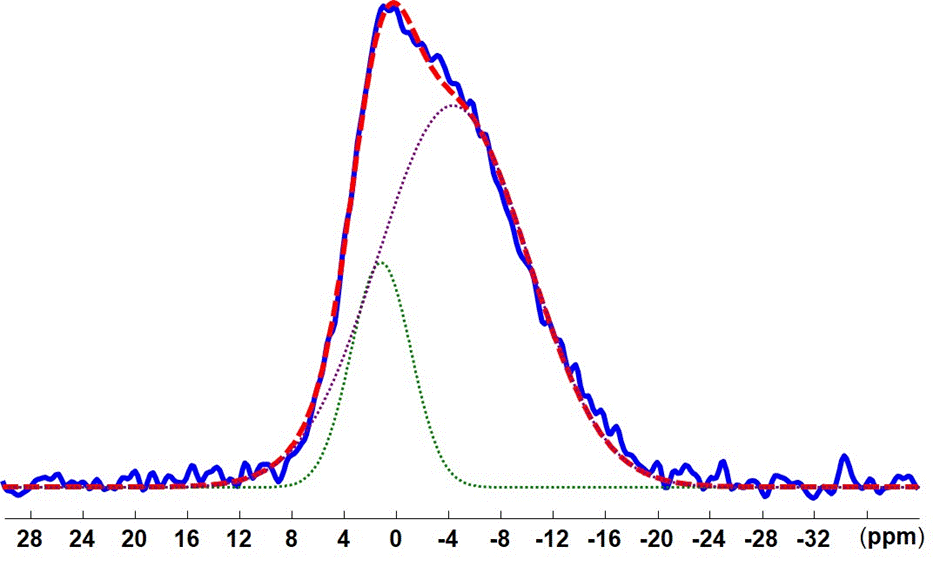
Figure 2. Spectral deconvolution of the 31P CP MAS spectrum of the cement powder after addition of the phosphoric acid solution in absence of zirconia
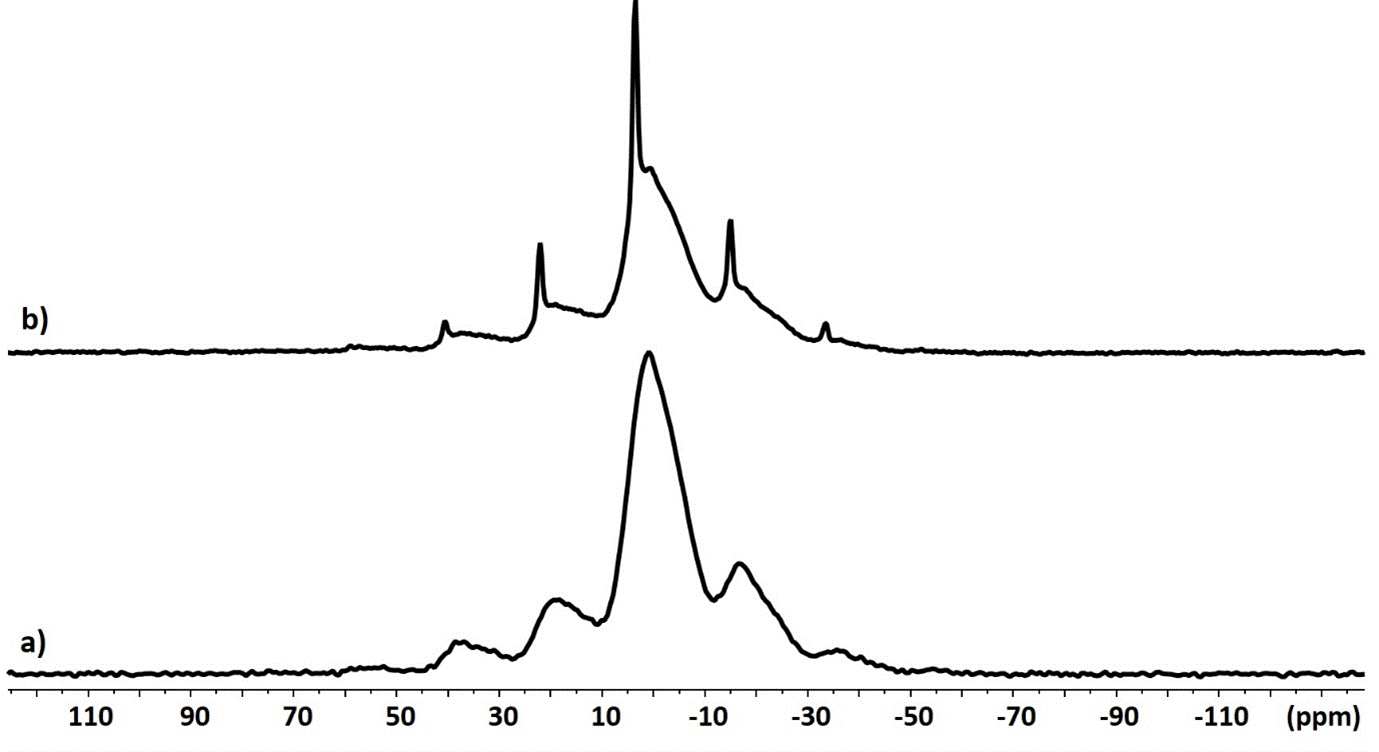
Figure 3. 31P CP MAS NMR spectra of cement powder after addition of the phosphoric acid solution in absence (a) and in presence (b) of zirconia at low-spinning frequency (3 kHz)
1H NMR
The 1H spectrum of cement powder prior to the addition of acid presents weak and broad signals in the 1-6 ppm area (Figure 4a). This indicates the presence in small amounts of physisorbed water molecules on the surface of oxides (around 4-5 ppm) as well as the presence of relatively isolated OH hydroxyl clusters (1 to 4 ppm) or hydroxyl groups forming hydrogen bonds (5-7 ppm). The same conclusion can be made for zirconiae powder alone with a main water signal (Figure 4b).
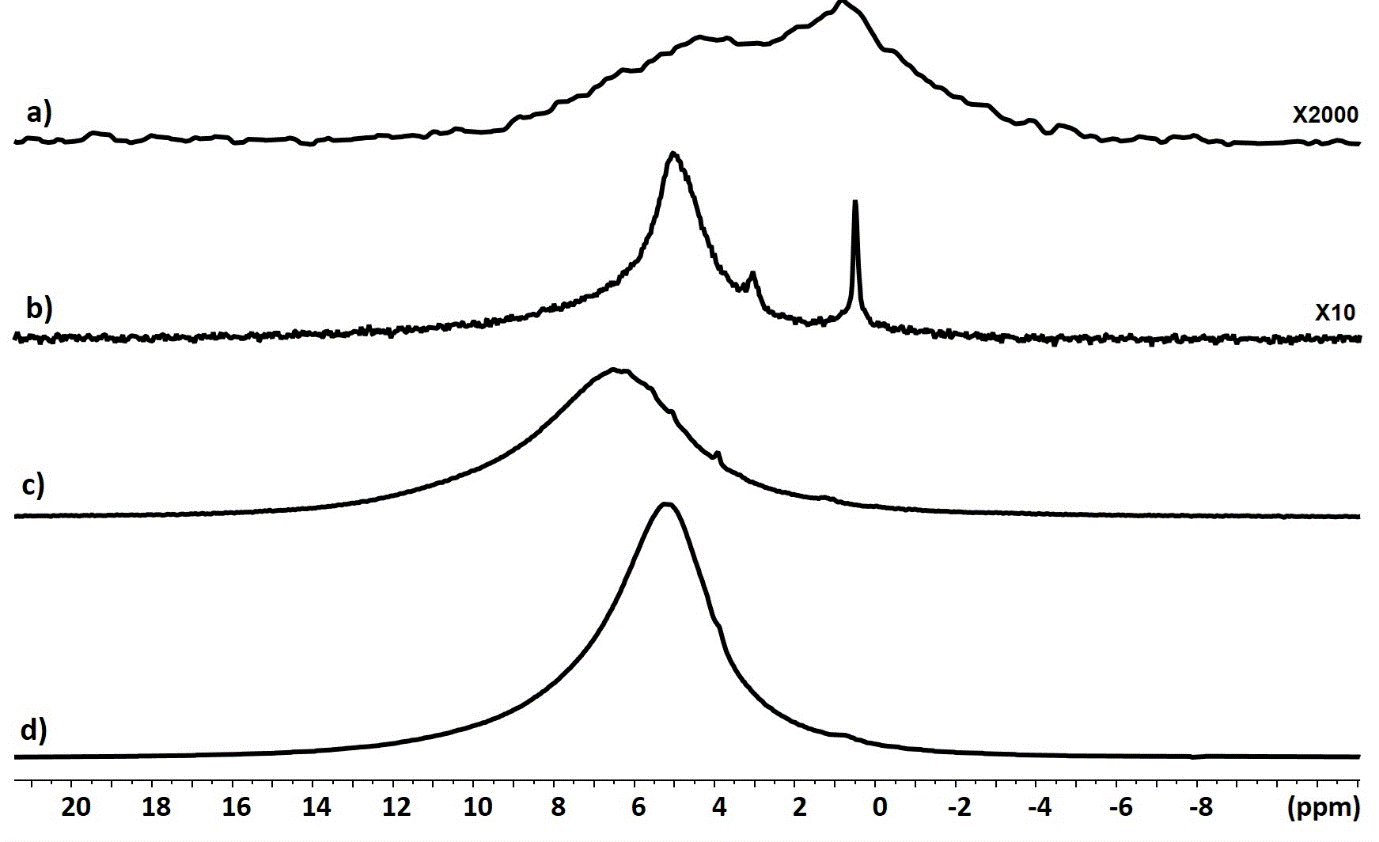
Figure 4. 1H MAS NMR spectra of cement powder (a), of zirconia particles (b) and of cement powder after addition of the phosphoric acid solution in absence (c) and in presence (d) of zirconia
These assignments are confirmed by 1H DQMAS experiment with strong DQ correlations for H2O molecules and OH groups involved in hydrogen binding (Figure 5a,b).
The 1H spectrum of cement powder after the addition of the phosphoric acid (Figure 4c) is much more intense and the signal shifts to the high frequency 6 - 13 ppm. This indicates the formation of an important network of acidic OH groups linked by hydrogen bonds. The DQMAS correlation with an ellipse form (Figure 5c) indicates that the distribution of these OH groups is heterogeneous in the material, i.e. spatial proximity is observed only for equivalent hydroxyl groups. The 1H spectrum of cement with zirconia has less shifted signals towards high frequencies 5 - 12 ppm, with a very intense signal around 5 ppm (Figure 4d). There is always the presence of a network of OH groups strongly linked by hydrogen bonding but the majority of the hydrogens are present either in OH groups in weak hydrogen bonding or in physisorbed water molecules. Two different signal areas centered at 5.2 and 7.0 ppm can be distinguished in the DQMAS experiment (Figure 5d) but their DQ correlations show they are spatially connected, which indicates an uniform distribution of hydroxyl groups in the sample. Globally, the 1H NMR experiments evidence a modification of the molecular organisation of the cement powder with or without the presence of zirconia particles.
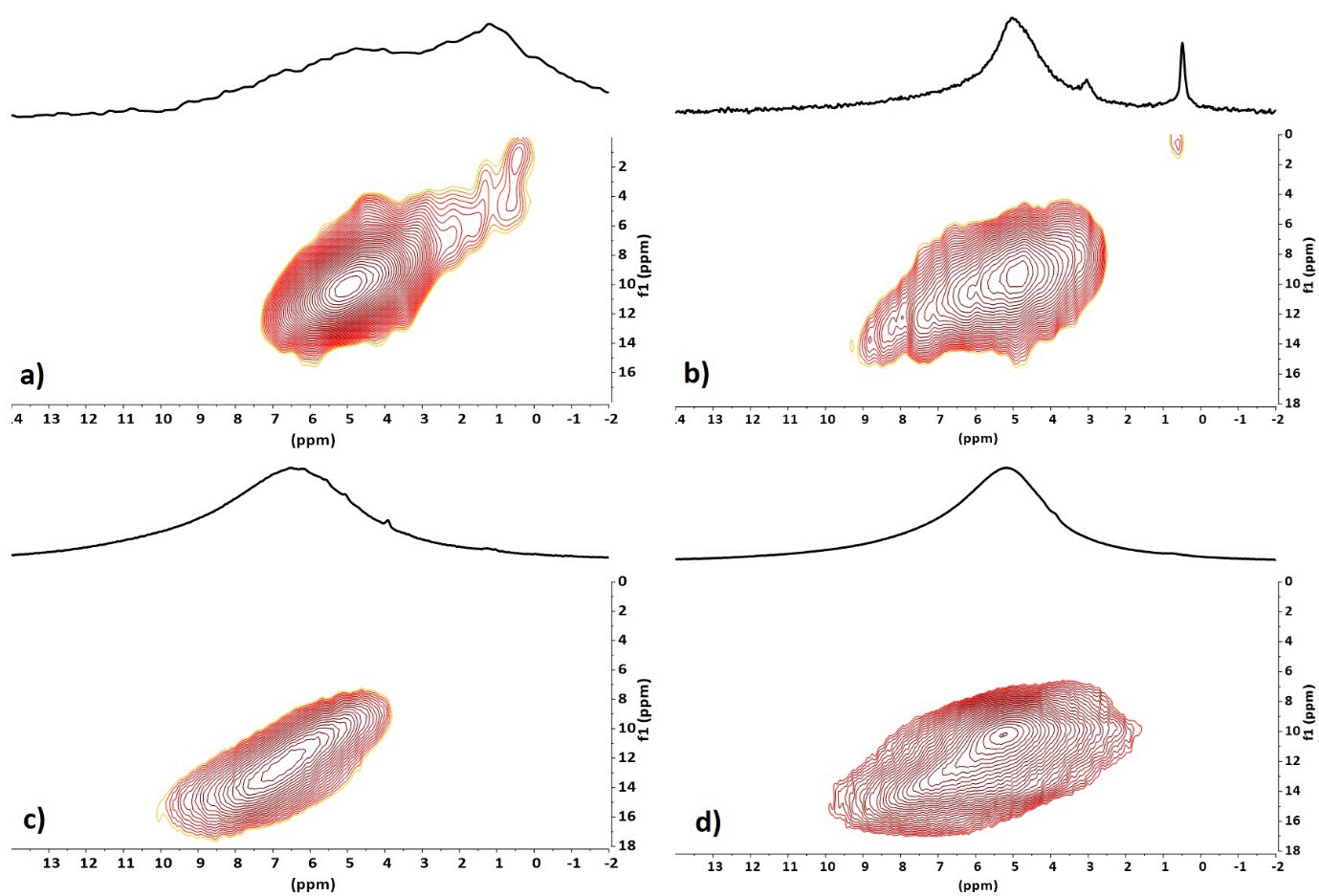
Figure 5. 2D 1H DQ-MAS NMR spectra of cement powder (a), of zirconia particles (b) and of cement powder after addition of the phosphoric acid solution in absence (c) and in presence (d) of zirconia
Differences between cement powder after the acid addition with or without zirconia were also analyzed through 1H/31P correlation experiments. The 1H edited 31P spectra show signals of hydrogen close to phosphorus atom (Figure 6). These experiments evidence that all the hydroxyl groups are close to 31P atoms even those around 5 ppm and are therefore in contact with phosphates. This experiment confirms the presence of two different types of OH groups with different populations in the presence or absence of zirconia
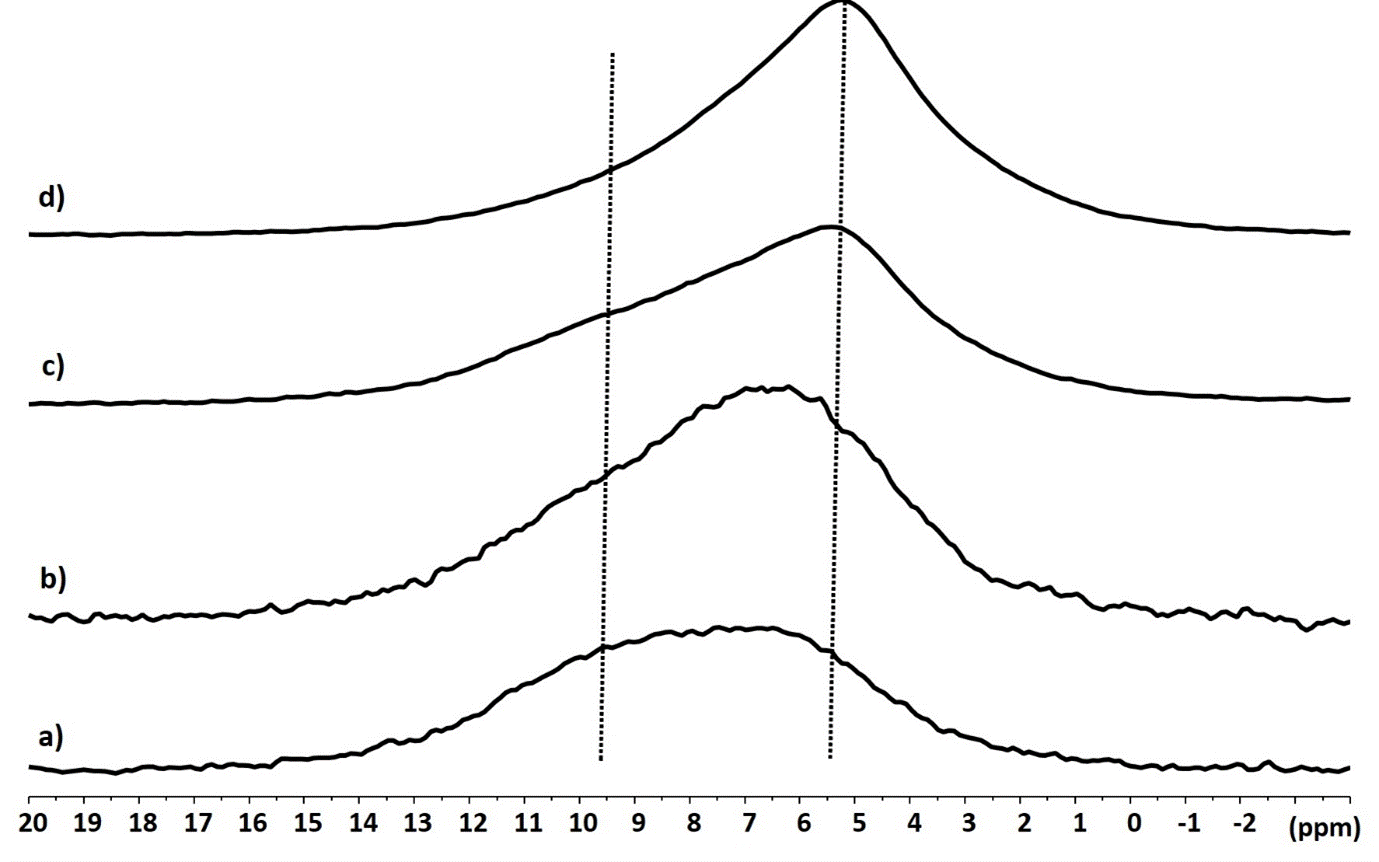
Figure 6. 1H/31P double cross polarization NMR spectra with short (3 ms - 0.5 ms, a,c) and long (6 ms - 2 ms, b,d) CP times of cement powder after addition of the phosphoric acid solution in absence (a,b) and in presence (c,d) of zirconia
The 2D 1H/31P HETCOR experiments (Figure 7) clearly show that the 31P signal at 3.5 ppm associated with the presence of zirconia correlates strongly with OH signals at 5.2 ppm. This confirms the appearance of a new crystalline phase in the presence of zirconia.
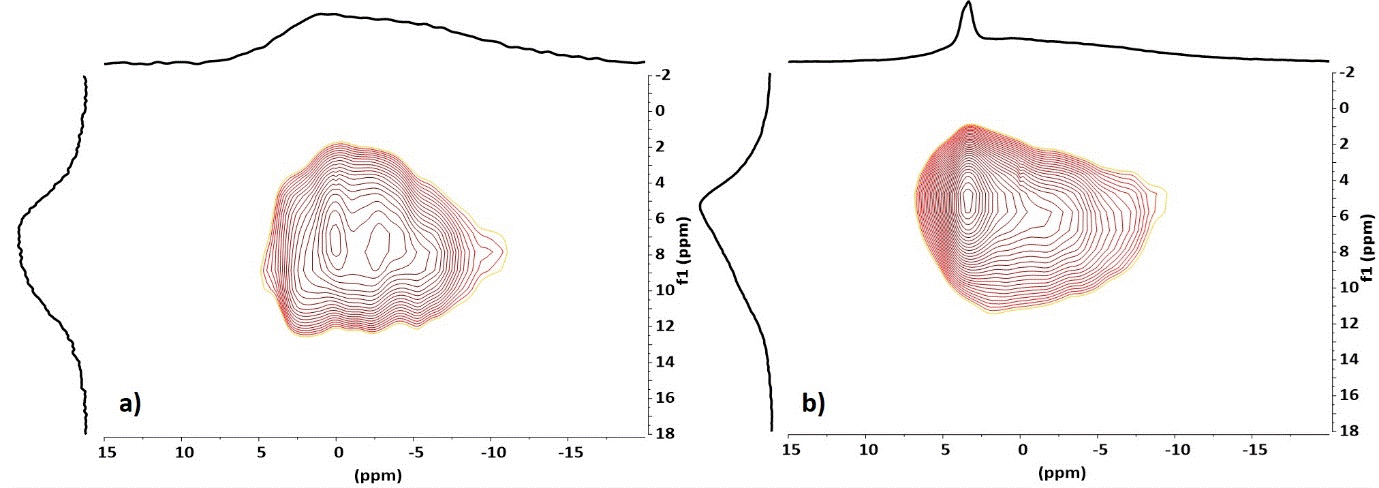
Figure 7. 2D 1H/31P HETCOR NMR spectra of cement powder after addition of the phosphoric acid solution in absence (a) and in presence (b) of zirconia
67Zn and 25Mg NMR
The 67Zn spectra vary little for the different samples (Figure 8 left). This indicates that the large ZnO particles present in the cement powder are mostly retained after the phosphoric acid etching. The amount of release Zn ions included in the different zinc phosphate phases is too small to be detected by 67Zn NMR. The same conclusions can be made for the 25Mg, where only the 25Mg signal of the large MgO particles is observed (Figure 8 right).
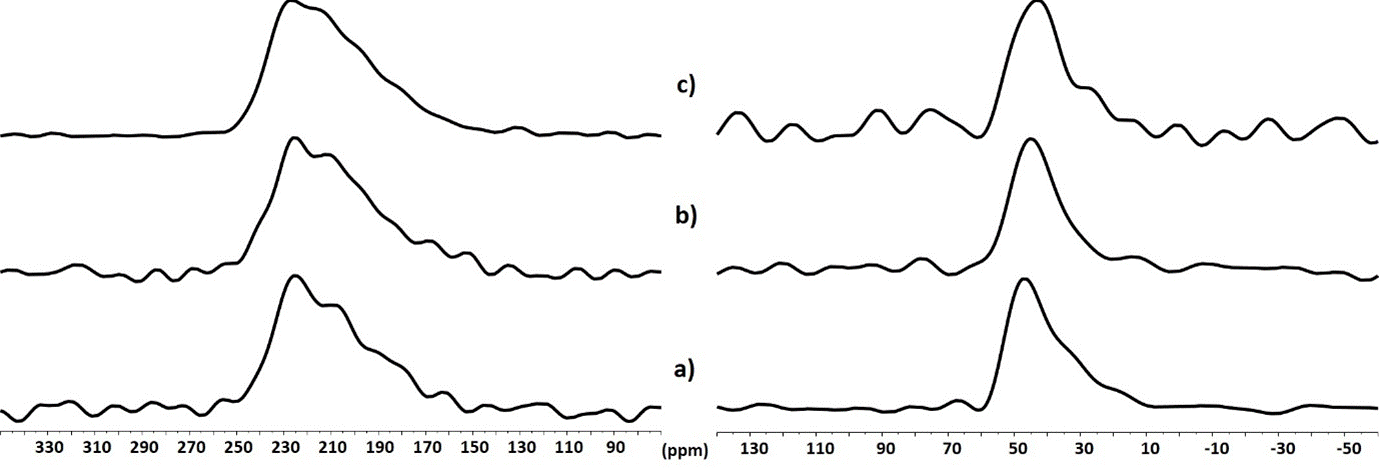
Figure 8. (left) 67Zn and (right) 25Mg QCPMG (echos summed) NMR spectra of cement powder (a), cement powder after addition of the phosphoric acid solution in absence (b) and in presence (c) of zirconia
Currently, no consensus exists regarding the best adhesion protocol for zirconia used in dentistry; this is important particularly for restorations where mechanical retention is deficient [15].
Zirconia has been commonly used as material for tooth-supported complete-coverage restorations. Adhesive and conventional cements have been suggested for cementation of these restorations. Zirconia tooth-supported crowns exhibited comparable survival rates and complication patterns after adhesive or conventional cementation and studies have shown that there are no significant differences in the resistance to rupture of zirconia crowns with adhesive or conventional cementation [10,16].
However, evidence on the effect of cement type on the clinical outcomes of teeth restored with zirconia restorations is unclear. Laboratory tests are important to determine some characteristics of the materials [17]. Although in vitro investigators point to an adhesive potential of zirconia, many practitioners prefer to seal for lack of scientific evidence especially given the multiple clinical factors that can influence adhesive potential [18]. The decision to use the bonding procedures is mainly associated with the preparation of the tooth, the situation of the preparation (anterior or posterior) and the use of glassy ceramics. Also the use of bonding procedures with resin cements represents only 38% [8,19].
Clinically, zinc phosphate cement has been used in multiple clinical situations by many practitioners for more than 100 years. It has been shown a change in its crystalline structure resulting in an expansion of cement when it is forced into a low thickness (10 to 30 μm). This expansion would explain the successfull results achieved with this cement.
Our study allows a chemical analysis of the reactivity of zinc oxyphosphate cement and zirconia at the interface. The 1H and 31P NMR experiments show that phosphoric acid reacts with ZnO and MgO to form heterogeneous amorphous phases composed of zinc and magnesium phosphates. These phosphates are also very rich in hydroxyl groups and species such as Zn (HPO4).3H2O et Mg(HPO4).3H2O are likely to be formed. The addition of zirconia powder changes the composition and the organization of the medium with the appearance of a new water-rich crystalline phase. The NMR 67Zn and 25Mg indicates that much of the initial ZnO and MgO remains present in cement. More generally it appears that the role of bonding efficacy in the fixed partial dentures (FPDs) survival rate is unknown [20]. A chemical analysis of the reactivity of the assembly material and the zirconia at the interface can contribute to the understanding of the efficiency of the assembly of dental ceramics.
Within the limitations of this in vitro study, the following conclusions can be drawn:
1 Mixing cement powder with the phosphoric in presence of zirconia confirms the appearance of a new crystalline phase.
2 It can be assumed that this phase forms on the surface of the zirconia.
The authors declare no conflict of interest.
The authors thank the manufacturer providing the materials used in the study.
- Tsoi James K (2019) Ceramic materials in dentistry. Advanced Dental Biomaterials. Advanced Dental Biomaterials 55-78.
- Sola-Ruiz MF, Agustin-Panadero R, Fons-Font A, Labaig-Rueda C (2015) A prospective evaluation of zirconia anterior partial fixed dental prostheses: Clinical results after seven years. J Prosthet Dent 113: 578-84. [Crossref]
- Tanis MÇ, Akay C, Karakis D (2015) Resin cementation of zirconia ceramics with different bonding agents. Biotechnol Biotechnol Equip 29: 363-67. [Crossref]
- Feitosa SA, Campos F, Yoshito W, Lazar DR, Ussui V, et al. (2018) Effect of the bonding strategy on the tensile retention of full-contour zirconia crowns. International Journal of Adhesion and Adhesives 85: 106-112.
- Liu D, Edmond HN Pow, Kit-Hon TJ, Matinlinna JP (2014) Evaluation of four surface coating treatments for resin to zirconia bonding. J Mech Behav Biomed Mater 32: 300-309. [Crossref]
- Ling Yin, Nakanishi Y, Alao AR, Song XF, Abduo J, et al. (2017) A review of engineered zirconia surfaces in biomedical applications. Procedia CIRP 65: 284-290. [Crossref]
- Inokoshi M, De Munck J, Minakuchi S, Van Meerbeek B, Inokoshi M, et al. (2014) Meta-analysis of bonding effectiveness to zirconia ceramics. J Dent Res 93: 329-334. [Crossref]
- Lawson NC, Litaker MS, Ferracane JL, Gordan VV, Atlas AM, et al. (2019) Choice of cement for single-unit crowns. J Am Dent Assoc 150: 522-530. [Crossref]
- Bandarra S, Neves J, Paraíso A, Mascarenhas P, Ribeiro AC, et al. (2021) Biocompatibility of self-adhesive resin cement with fibroblast cells. Journal of Prosthetic Dentistry 125: 705.
- Behr M, Rosentritt M, Wimmer J, Lang R, Kolbeck C, et al. (2009) Self-adhesive resin cement versus zinc phosphate luting material: A prospective clinical trial begun 2003. Dent Mater 25: 601-604. [Crossref]
- Maroulakos G, Thompson GA, Kontogiorgos ED (2019) Effect of cement type on the clinical performance and complications of zirconia and lithium disilicate tooth-supported crowns: A Report of the Committee on Research in Fixed Prosthodontics of the American Academy of Fixed Prosthodontics. J Prosthet Dent 121: 754-765. [Crossref]
- Kern M (2015) Bonding to oxyde ceramics-laboratory testing versus clinical outcome. Dent Mater 31: 8-14. [Crossref]
- Scrimgeour SN, Chudek JA, Cowper GA, Lloyd CH (2007) 31P solid-state MAS-NMR spectroscopy of the compounds that form in phosphate-bonded dental casting investment materials during setting. Dental Materials 23: 934-943.
- Roming (2008) Chem Matter.
- Özcan M, Bernasconi M (2015) Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J Adhes Dent 17: 7-26. [Crossref]
- Nakamura K, Mouhat M, Nergard JM, Lægreid SJ, Kanno T, et al. (2016) Effect of cements on fracture resistance of monolithic zirconia crowns. Acta Biomater Odontol Scand 2: 12-19. [Crossref]
- Özcan M, Vallittu PK (2003) Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent Mater 19: 725-731. [Crossref]
- Tzanakakis EG, Tzoutzas IG, Koidis PT (2016) Is there a potential for durable adhesion to zirconia restorations? A systematic review. J Prosthet Dent 115: 9-19. [Crossref]
- Liu Y, Hai-Yang Y (2009) Does dental zinc phosphate cement really shrink in clinical applications?. Med Hypotheses 73: 257-258. [Crossref]
- Quigley NP, Loo DSS, Choy C, Ha WN (2021) Clinical efficacy of methods for bonding to zirconia: a systematic review. J Prosthet Dent 125: 231-240. [Crossref]








