Acinetobacter baumannii is a Gram-negative, strictly aerobic nonmotile bacterium with a DNA G-C content of 39% to 47%. A. baumannii is an important opportunistic nosocomial pathogen that causes pneumonia, bacteremia, urinary tract infections, meningitis, skin, and soft tissue infections. The major concern about A.baumannii is the emergence of resistant strains which necessitates the development of new prevention, control, and treatment methods. Recently the bacterial lipoproteins have attracted the attention of the researchers for the induction of protective immunity against infectious diseases. VacJ is a highly conserved outer membrane lipoprotein that exists in many A. baumannii strains that deserves research on its immunogenicity. The gene encoding mature vacJ of A. baumannii ATCC19606 was cloned and over-expressed in Escherichia coli as a fusion protein. The recombinant VacJ (rVacJ) was purified. 20 and 40 µg of the purified ∼31 kDa rVacJ were used for immunization of mice along with Freund’s or Alum adjuvants. Antibody titres raised against the recombinant protein were determined by indirect ELISA. Whole A.baumannii cell was detected at 1:200 serum dilution. Bacterial challenges of mice groups with varying doses of A. baumannii were performed in the active, passive, and intranasal forms. The bacterial load in the mice lungs was determined in both control and immunized groups. A high antibody titre was noted as a result of immunization with rVacJ. The rVacJ-Freund’s adjuvant elicited a higher antibody level compared to the rVacJ-Alum adjuvant. The mice groups challenged intraperitoneally with live A. baumannii did not survive while the intranasally challenged group exhibited a significant reduction of 600 fold of the bacterial load in the lungs. The findings are of significant value in the development of novel adjuvanted vaccines and precise routes of administrations in combat against the notorious multidrug-resistant A.bauamnnii.
acinetobacter baumannii, immunogenicity, outer membrane lipoprotein, VacJ
Acinetobacter baumannii is the common species of Acinetobacter identified as a notorious multidrug-resistant and an invasive pathogen in intensive care units. A. baumannii is the most important agent for nosocomial infections found in a wide variety of issues like blood, skin wounds, respiratory tract, and urinary tract, and is associated with high mortality. Among the healthcare-associated infections of A. baumannii, bloodstream infections, and ventilator-associated pneumonia are the most common, with mortalities ranging from 5% in general hospital wards to 54% in the intensive care unit (ICU) [1]. A. baumannii has been isolated from patients with severe wounds, trauma, or burns, osteomyelitis, and bone injuries from the soldiers injured in military operations during the 2003–2005 in Iraq or US military service members wounded in Afghanistan [2]. The bacterium has developed resistance to imipenem, an antibiotic of choice, and imipenem-resistant isolates that have shown better sensitivity to minocycline and tigecycline than sensitivity to levofloxacin, colistin, cefoperazone [3]. A 73.91% mortality was reported due to meningitis caused by A.baumannii [4]. Monotherapy seems to have failed to provide a good therapeutic effect in the treatment of A.baumannii infections. The clinical significance of A.baumannii has largely been driven by its remarkable ability to acquire or upregulate various resistance determinants, making it one of the most successful multidrug-resistant (MDR) organisms challenging the presently available antibiotic therapy [5]. Although the genetic and functional basis of multidrug resistance in clinical isolates of A.baumannii is under intense research, however, the bacterial pathogenicity is poorly understood [6]. Various approaches including infection model, phenotypic, and genomic analyses, significantly contributed to the identification of virulence factors signifying that A. baumannii devotes a considerable portion of its genes to pathogenesis [7]. Immunization is an effective strategy to prevent A. baumannii infections. As of today, there have been no vaccines against this pathogen. Immunogens eliciting antibodies against bacterial outer membrane proteins (Omps) are potential candidates due to their role in interacting with the host and the availability of those proteins for antibody neutralization due to their localization on the cell surface. Although inactivated or attenuated whole cells trigger antibodies against multiple proteins on the bacterial surface, however, there are safety concerns about whole-cell administration. Hence, there remains one approach to elicit antibodies against one or more of the major antigenic surface structure of the bacteria [8]. Outer membrane proteins of Gram-negative bacteria play important role in bacterial interaction with, and adaptation to the environmental conditions, and thus, are key elements in virulence. Virulence factors like capsules, porins, enzymes, biofilm, motility, and cell wall lipopolysaccharide influence pathogenesis in A. baumannii infections. These factors enable the pathogen to resist stressful environmental conditions and develop severe infections [9]. Outer membrane lipoproteins are involved in a variety of pathogenesis mechanisms of Gram-negative bacteria [10]. The virulence-associated chromosome locus J, VacJ, attributed to bacterial spreading, was first discovered in Shigella flexneri [11]. The gene, vacJ, is a widely distributed and highly conserved Omp, playing a major role in virulence of a great number of Gram-negative pathogens [12]. In its frequent association with Vps (Mla), VacJ forms the Vps-VacJ ABC transporter system that maintains outer membrane asymmetry. Mutations in vps-vacJ increased susceptibility of E.coli to Sodium dodecyl sulfate (SDS) [13]. The development of ceftriaxone resistance was reported as a result of the upregulation of VacJ in a putative outer membrane protein mutant of S. enterica serovar Typhimurium [14]. There was a 15 fold decrease in the pathogenicity of A.baumannii the mouse pneumonia model after deletion of vacJ gene [15]. Nakamura and co-workers [16] demonstrated the contribution of vacJ gene of Non-typeable Haemophilus influenzae to serum resistance and IgM binding that mediated bacterial escape from killing by complement-dependent. Maintenance of cellular integrity and stress tolerance of H. parasuis were shown to depend on VacJ. ΔvacJ mutant was more susceptible to SDS-EDTA, several antibiotics, osmotic and oxidation pressure than the wild type. The mutant strain also exhibited reduced biofilm formation, decreased adherence to PK-15 cell, and significantly low survival ratio from the serum and complement killing. These changes were attributed to the impairment of the bacterial outer membrane stability, thus, suggesting a putative role of VacJ lipoprotein in virulence regulation [17]. The recombinant VacJ of P. multocida elicited humoral immune response with a significant rise in antigen-specific titers of IgG, providing 50-66.7% protection at 8LD50 [18]. The recombinant VacJ of P. multocida elicited humoral immune response with a significant rise in antigen-specific titers of IgG, providing 50-66.7% protection at 8LD50 [18]. VacJ, is found in Pasteurellaceae members and many other Gram-negative bacterial species, however, to the best of our knowledge, the functional characterization in the pathogenicity of A.baumannii has not yet been determined. Understanding virulence, disease mechanisms, and resistance acquisition are central to the knowledge of disease caused by A. baumannii. The present study, for the first time, describes the immunogenicity and protective efficacy of Acinetobacter baumannii recombinant VacJ in a murine model.
Bacterial strains, plasmids, and primer design
Acinetobacter baumannii ATCC 19606, Shigella flexneri, and Haemophilus influenzae were used in this study. A pair of primers were designed for nucleotide sequences targeting the vacJ gene sequence (Nucleotide region –Nt 66 to end) encoding mature full-length VacJ (~31 KD) using the reference sequence at Gen Bank: CP010781.1. The primers sequences were Fwd5'-aataGGATCCATGCAAGAAAATCTTACTG-3' and Rvs5' aattGCGGCCGCTTATTTTTCGGTTTTATCAGT-3' with added restriction sites (underlined) for BamHI and NotI along with tags (small letter). DNA from A. baumannii ATCC 19606 genome was used as the template to amplify the selected gene. The PCR mixture consisted of 1 pM/µl of each primer. PCR reaction included 30 cycles of denaturation at 94°C for the 30s, annealing at 55°C for 30s, extension at 72°C for 50 s, and a final extension at 72°C for 5 min. The PCR amplified product was digested with BamHI and NotI, and ligated into pET28a vector digested with the same restriction enzymes. E. coli DH5α and E. coli BL21 (DE3) cells were successfully transformed with pET28a (vacJ). The recombinant clones were confirmed by a polymerase chain reaction, enzymatic digestion, and sequencing. Bacterial stocks were maintained in 30% glycerol at −80°C for long term storage.
Recombinant VacJ production and purification
E. coli BL21(DE3) cells harboring the recombinant plasmid were grown at 37°C in LB broth with Kanamycin and induced with 1 mM IPTG. After 5-6 h of IPTG induction, the cell pellet was re-suspended in denaturing buffer and was then sonicated 6 times for 10s with 1 min interval at 100w. The lysate was centrifuged at 10000×g for 20 min at 4 °C to remove the debris. The protein was purified under denaturing condition by affinity chromatography using Ni-NTA affinity column (Qiagen, USA). The recombinant protein expression was validated by western blotting with horseradish peroxidase (HRP)-conjugated anti-polyhistidine antibodies (1:10000 dilution) in which, 0.5 µg of each recombinant protein was loaded onto the SDS-PAGE.
Mice immunization
Female BALB/c mice weighing 20-25 grams, reared in a pathogen-free environment were procured from Pasteur Institute, Tehran, Iran. Mice were housed in clean standard and well-aerated conditions in the animal care facility at Shahed University and fed a standard antibiotic-free diet, and water ad libitum. The research was conducted in compliance with the principles stated in the Guide for the Care and Use of Laboratory Animals. The protocol pertaining to animal care was approved by the ethics committee of Shahed University. For immunization purposes, the mice groups (n=6/group) were divided into three tests and two control groups. Two test groups received 20 and 40 µg recombinant VacJ in 100 µl Freund’s adjuvant and the third group received 20 µg recombinant VacJ in 100 µl Alum adjuvant, while the control groups received PBS (100 µl) in 100 µl of each adjuvant. All groups were subcutaneously injected on days 0, 14, 28, and 42. The sera were assayed for the IgG titer raised against the recombinant proteins by ELISA. When required, the mice were anesthetized with a mixture of xylazine, ketamine, and PBS at 6:1:3 ratio by intraperitoneal injection.
Enzyme-linked immunosorbent assay (ELISA)
The IgG titer against the recombinant VacJ in the sera of each immunized mice was assessed by indirect ELISA. The protein concentration was estimated by the Bradford method. 100 µl of the recombinant VacJ (20 µg/ml) was coated in Micro titration polyvinyl ELISA plates and incubated at 4 °C overnight. The plate wells were washed 4 times with 200 µl PBST (PBS + 0.05% Tween 20) and non-specific sites were blocked with 100 µl of 5% skim milk solution in PBST at 37 °C for 1 h. 100 µl of each test sera were added in triplicate at 1:250 to 1:16000 dilutions followed by the addition of diluted (1:15000) anti-mouse HRP conjugate and kept for 1 h. After repeating the washing step, 100 μl of the substrate i.e. 3,3-5,5-tetramethylbenzidine (TMB) solution was added to each well. The plate was kept in dark at room temperature for 15 min to develop color. The reaction was stopped by addition of 100 μl of 3 M H2SO4 per well and the optical density was measured at 450 nm on an ELISA reader.
Whole-cell ELISA
A. baumannii and S. flexneri were grown in BHI, and H. influenzae was grown in BHI supplemented with blood. The bacterial cultures were grown at 37 °C to reach OD600 of 0.6. (108 cells/ml). 100 µl of the bacteria suspended in coating buffer (50 mM sodium carbonate buffer, pH 9.6) were coated in each well for 16-18 hours at 4°C. The plate was dried under a warm air blow to complete the bacterial attachment. The wells were then washed thrice with PBST. 100 μl/well of PBST plus 5% skimmed milk was added to the wells to block the reaction and incubated at 37°C for 1 h. After 3 times washing with PBST, serial dilutions (1:200 to 1:800) of mice sera were added and incubated at 37°C for 2 h. After washing the wells, 100 µl of anti-mouse IgG conjugated with HRP at 1:15000 dilution was added to each well and incubated at 37°C for 1 h. The washing step was repeated and 100 µl TMB was then added to each well and the plate was kept in dark at room temperature for 15 minutes to develop color. The reaction was stopped by adding 100 μl of 3 M H2SO4 into each well.
Mice sepsis model experiments
For the study of sepsis model and determination of 50% bacterial lethal dose (LD50), A. baumannii ATCC 19606 was grown for 18 h at 37°C in BHI broth and then adjusted to the appropriate concentrations of 106, 107, 108, and 109 CFU/ml in physiologic saline. Fifty percent lethal dose (LD50) values for the strains used in challenge studies were determined by infecting groups of 6 mice with 10-fold dilutions of bacteria and analyzing survival data using the Probit method [19]. Mice were infected by intraperitoneal injection with 200 µl of the bacterial suspension at 1×LD50, 2×LD50, and 5×LD50 concentrations and were carefully monitored for survival for 7 days [20]. For antibody treatment studies, mice were treated with 100 μl of sera from immunized mice or naïve serum by intravenous injection via the tail vein 1 h after infection with the above-indicated inocula. Postinfection tissue bacterial load was determined for immunized and control mice euthanized with an overdose of Xylazine:Ketamine 24 h after infection. Lungs were aseptically excised, weighed, and then homogenized in 2 ml of physiologic saline before plating of serial log dilutions on LB agar plates for bacterial quantification. For intranasal challenge, mice groups were anesthetized by intraperitoneal administration of Xylazine (20 mg.kg-1) + Ketamine (100 mg.kg-1). These mice were challenged intranasally with 10×LD50 of A. baumannii ATCC19606. The rate of bacterial colonization was determined per gram of mice lungs 24 hours after intranasal challenge.
Statistical Analysis, programs and websites
Quantitative data was analyzed with the One -way ANOVA test and One-sample t-test in the statistical program SPSS 22. Graphs were drawn with the Graph Pad Prism 7 program. Sequences analysis was done with Phyre 2, LipoP-5, NCBI BLAST.
VacJ sequence analysis and construction of recombinant VacJ clone
The VacJ lipoprotein whole gene is 900 bp long with ~41% GC. Full-length VacJ (MW 31180.84 Da, pI:4.86) was predicted with a signal peptide (1–22aa) using ProtParam and SignslP 4.1. There is a list of 3136 A. baumannii strains at the National Center for Biotechnology Information (NCBI) of which 2200 are clinical strains. 1312 clinical strains possess vacJ gene. Blast results of this gene with other strains showed 1103 strains with 100% identity, E value=0, query cover =100% and 128 strains with 99% identity, E value=0, query coverage=100%. The alignment of VacJ protein amongst the above strains with PRALINE showed 84% similarity. Blast study of A. baumannii vacJ in different bacterial species revealed this gene as the most similar to VacJ in S. flexneri and H. influenzae. The recombinant VacJ (rVacJ) was expressed in E. coli BL21 and confirmed by Western blot (Figure 1).

Figure 1. Western blot analysis of rVacJ
Immunogenicity of rVacJ
The titers of serum IgG against rVacJ (20 µg and 40 µg) with Freund’s adjuvant are shown in Figure 2. Indirect ELISA results indicate a significant difference (P˂0.05) between control and test groups. The elicitation of IgG titres against rVacJ (20 µg) with Alum and Freund’s adjuvants is shown in Figure 3. Significant (P<0.05) increase in antibody titres was noted in both test groups compared to the control group.
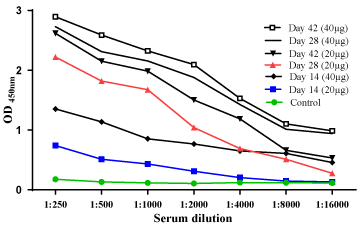
Figure 2. Immunized mice blood analysis for IgG titres against the 20µg and 40µg of immunizing protein. The Figure displays the absorbance values (OD450) at 2 fold serial dilutions of the sera and the absorbance value at the highest dilution tested (1:16000) at days 14, 28, and 42. Sera from mice in each immunization group (n=6) were assayed in triplicate at each timepoint. The adjuvant control group (n=6) were assayed at the last timepoint
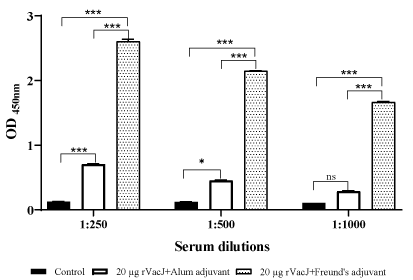
Figure 3. ELISA to detect IgG raised against rVacJ in sera of mice after administration of rVacJ along with alum or Freund's adjuvants Significance is displayed as: ns (non significant), *p<0.05, ** p≤ 0.01, and ***p<0.001
Whole-cell ELISA
The specificity of the antibodies produced was tested against A. baumannii ATCC19606, S. flexneri, and H. influenzae by whole-cell ELISA. A.baumannii was significantly (P<0.05) detected at 1:200 serum dilution. There was no significant difference (P>0.05) between the control and S. flexneri (sig = 0.64) or H. influenza (sig = 0.76).
Mice challenge studies
The immunized mice groups challenged intraperitoneally with three doses of A. baumannii viz., 1×LD50, 2×LD50, 5×LD50 showed no significant protection against A. baumannii. The mice groups challenged with similar doses of A.baumannii as in the active immunization did not survive. However, protection was noted in the intranasal active challenge with 10×LD50 leading to the survival of the mice monitored for a week. The bacterial colonization in the lungs of the immunized and control mice about 600 fold reduction in the bacterial load of the immunized group as compared to the control group (P<0.001). There was no significant difference between the rVacJ immunized group along with Freund’s or Alum adjuvants (Figure 4).
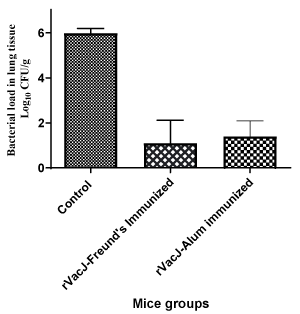
Figure 4. Bacterial colonization in the mice lungs of the intranasally challenged mice groups
Lipoproteins abound in the outer membranes of Gram-negative bacteria are potential target antigens for vaccine development [18]. VacJ is important for virulence in Burkholderia pseudomallei [21] and H. influenza [22] and, more specifically, for intercellular spread during infections caused by Shigella flexneri [11]. VacJ was first introduced as an outer membrane complex protein of A. baumannii [8], the deletion of its gene decreased bacterial virulence by 15 times [15]. Despite the wide distribution of VacJ-like proteins with varying lengths in many Gram-negative bacteria, its role in the elicitation of protective immunity has not yet been elucidated [18]. The role of VacJ in biofilm formation, binding, and invading the host cells have been shown in Actinobacillus [23], H. influenzae [17], and Burkholderia pseudomallei [24]. In the present study, vacJ sequence analysis from several A. baumannii strains revealed that this gene is highly conserved and widely distributed. This lipoprotein has a signal peptide that cleavage between aa 22 and aa 23. We successfully produced recombinant protein in E. coli and purified this under denaturing condition. Mice groups were immunized with various doses of rVacJ using Freund’s or alum adjuvants. The results showed that 40 µg rVacJ along with Freund’s adjuvant has better efficay to trigger immune response than 20 µg of rVacJ. The recombinant VacJ along with alum adjuvant did not increase the antibody titre. Shivachandra and co-workers [18] noticed no protection in mice immunized with rVacJ (30 mug) along with FCA followed by challenge with 100 LD50 of Pasteurella multocida. They reported 66.7% and 50% protection with higher rVacJ dose (75 mug) along with FCA and alum respectively, at 8 LD50. They concluded that a lipidated recombinant VacJ lipoprotein with suitable adjuvants could potentially act as candidate immunogen for vaccine development against pasteurellosis in livestock. Our results are in support of their findings with P. multocida. Acinetobacter baumannii rOmpA vaccine dose was reported to alter immune polarization and immunodominant epitopes suggesting that higher doses may be favorable for future development. These indicate that altering vaccine dosage can modify the breadth of epitope coverage, immunogenicity, and nature of the cytokine response to vaccines [25]. Vaccines often contain adjuvants to strengthen the response to the vaccine antigen. However, their efficacy at the administration site is poorly understood. We investigated the widely used adjuvants Freund’s and aluminum hydroxide (alum). Aluminum-based adjuvants activate the Nalp3 inflammasome to drive immune responses [26]. A 20 μg dose of rNT-DsrA administered with alum was reported to elicit antisera with comparable bacterial surface reactivity compared to that obtained with complete/incomplete Freund's adjuvant that elicits a humoral response [27]. It was shown that a 20 μg dose of rNT-DsrA administered with the alum adjuvant elicited high-quality antibodies with reactivity to the bacterial surface rendering protection against an H. ducreyi infection [28]. Production of type I interferon that inhibits inflammasome activation, was not shown to be stimulated by alum [29]. Complete Freund’s adjuvant (CFA) ensures prolonged local antigen availability by its depot function [30], and induce maturation of antigen-presenting cells (APC) and create a pro-inflammatory environment by its immunomodulatory molecules contents like trehalose-6,6′-dimycolate (TDM). This results in a chemokine-driven influx of innate cells [30,31]. The aluminum-containing adjuvants are thought to ensure effective antigen uptake and presentation by APC without the need for a depot and to create a local immunostimulatory environment [32]. The selection of adjuvant, route of immunization, or delivery system used for immunization can significantly influence the immune response elicited. In general, the use of alum as an adjuvant with pure protein induces a Th2-type response associated with the production of interleukin 4 (Il4), Il-5 and antibodies of the IgG1 isotype. On the other hand, Freund's complete adjuvant (FCA) can induce a Th1-type response, associated with production of IFNγ and Il-2 and antibodies of the IgG2a isotype [33]. A 600-fold decreased colonization was noted in the immunized mice lungs following active intranasal challenge with A. baumannii. These results are in agreement with Wang et al. [15] who indicated 15-fold reduction of ΔvacJ A. baumannii load in murine pneumonia infection model. These findings could lead to the development of novel adjuvanted vaccines and appropriate routes of administrations in a more rational manner.
All the experiments using animals were conducted in Compliance with Ethical Standards and animal care guidelines confirmed by Animal Care and Ethical Committee of Shahed University.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest.
The authors wish to thank the Molecular Microbiology Research Center of Shahed University, Tehran-Iran for supporting this work.
SM was carried out the wet lab experiments. SDA conducted microbiological tests. IR conceptualized and supervised the study. All authors were involved in the preparation of the manuscript.
- Bianco A, Quirino A, Giordano M, Marano V, Rizzo C, et al. (2016) Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in Southern Italy. BMC Infectious Diseases 16: 747.
- Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, et al. (2017) Clinical and pathophysiological overview of acinetobacter infections: A century of challenges. Clinical Microbiology Reviews 30: 409-447.
- Zhu W, Wang Y, Cao W, Cao S, Zhang J (2018) In vitro evaluation of antimicrobial combinations against imipenem-resistant Acinetobacter baumannii of different MICs. Journal of Infection and Public Health 11: 856-860.
- Schuertz KF, Tuon FF, Palmeiro JK, Conte D, Telles JPM, et al. (2018) Bacteremia and meningitis caused by OXA-23-producing Acinetobacter baumannii – molecular characterization and susceptibility testing for alternative antibiotics. Brazilian Journal of Microbiology 49: 199-204.
- Clark NM, Zhanel GG, Lynch JPI (2016) Emergence of antimicrobial resistance among Acinetobacter species: a global threat. Current Opinion in Critical Care 22: 491-499.
- Runci F, Gentile V, Frangipani E, Rampioni G, Leoni L, et al. (2019) Contribution of Active Iron Uptake to Acinetobacter baumannii Pathogenicity. Infection and Immunity 87: e00755-e00818.
- Antunes LC, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathogens and Disease 71: 292-301.
- McConnell MJ, Domínguez-Herrera J, Smani Y, López-Rojas R, Docobo-Pérez F, et al. (2011) Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant acinetobacter baumannii. Infection and Immunity 79: 518-526.
- Ayoub Moubareck C, Hammoudi Halat D (2020) Insights into Acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 9: 119.
- Sutcliffe IC, Harrington DJ, Hutchings MI (2012) A phylum level analysis reveals lipoprotein biosynthesis to be a fundamental property of bacteria. Protein Cell 3: 163-170.
- Suzuki T, Murai T, Fukuda I, Tobe T, Yoshikawa M, et al. (1994) Identification and characterization of a chromosomal virulence gene, vacJ, required for intercellular spreading of Shigella flexneri. Mol Microbiol 11: 31-41.
- Carpenter CD, Cooley BJ, Needham BD, Fisher CR, Trent MS, et al. (2014) The Vps/VacJ ABC transporter is required for intercellular spread of Shigella flexneri. Infect Immun 82: 660-669.
- Malinverni JC, Silhavy TJ (2009) An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proceedings of the National Academy of Sciences of the United States of America 106: 8009-8014.
- Hu WS, Lin JF, Lin YH, Chang HY (2009) Outer membrane protein STM3031 (Ail/OmpX-like protein) plays a key role in the ceftriaxone resistance of Salmonella enterica serovar Typhimurium. Antimicrobial Agents and Chemotherapy 53: 3248-3255.
- Wang N, Ozer EA, Mandel MJ, Hauser AR (2014) Genome-Wide identification of acinetobacter baumannii genes necessary for persistence in the lung. mBio 5: e01163-14.
- Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, et al. (2011) Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog 7: e1001247-e.
- Zhao L, Gao X, Liu C, Lv X, Jiang N, et al. (2017) Deletion of the vacJ gene affects the biology and virulence in Haemophilus parasuis serovar 5. Gene 603: 42-53.
- Shivachandra SB, Kumar A, Yogisharadhya R, Viswas KN (2014) Immunogenicity of highly conserved recombinant VacJ outer membrane lipoprotein of Pasteurella multocida. Vaccine 32: 290-296.
- Cornfield J, Haskell G (1950) The probit method. Science 111: 42-43.
- Harris G, KuoLee R, Xu HH, Chen W (2017) Mouse models of Acinetobacter baumannii infection. Current Protocols in Microbiology 46: 6G.3.1-6G.3.23.
- Cuccui J, Easton A, Chu K, Bancroft G, Oyston P, et al. (2007) Development of signature-tagged mutagenesis in Burkholderia pseudomallei to identify genes important in survival and pathogenesis. Infection and Immunity 75: 1186-1195.
- Gawronski JD, Wong SM, Giannoukos G, Ward DV, Akerley BJ (2009) Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung. Proceedings of the National Academy of Sciences106: 16422-16427.
- Xie F, Li G, Zhang W, Zhang Y, Zhou L, et al. (2016) Outer membrane lipoprotein VacJ is required for the membrane integrity, serum resistance and biofilm formation of Actinobacillus pleuropneumoniae. Veterinary Microbiology 183: 1-8.
- Lim J (2015) The characterization of the lipoprotein VacJ in Burkholderia pseudomallei and Burkholderia thailandensis: London School of Hygiene & Tropical Medicine.
- Lin L, Tan B, Pantapalangkoor P, Ho T, Hujer AM, et al. (2013) Acinetobacter baumannii rOmpA vaccine dose alters immune polarization and immunodominant epitopes. Vaccine 31: 313-318.
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453: 1122-1126.
- Fusco WG, Choudhary NR, Routh PA, Ventevogel MS, Smith VA, et al. (2014) The Haemophilus ducreyi trimeric autotransporter adhesin DsrA protects against an experimental infection in the swine model of chancroid. Vaccine 32: 3752-3758.
- Samo M, Choudhary NR, Riebe KJ, Shterev I, Staats HF, et al. (2016) Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface. Vaccine 34: 1193-1200.
- Riteau N, Sher A (2016) Chitosan: An adjuvant with an unanticipated STING. Immunity 44: 522-524.
- Billiau A, Matthys P (2011) Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukocyte Biol 70: 849-860.
- Seubert A, Calabro S, Santini L, Galli B, Genovese A, et al. (2011) Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proceedings of the National Academy of Sciences of the United States of America 108: 11169-11174.
- Kool M, Soullié T, Van Nimwegen M, Willart MAM, Muskens F, et al. (2008) Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. Journal of Experimental Medicine 205: 869-882.
- Mosmann TR, Coffman RL (1989) Heterogeneity of cytokine secretion patterns and functions of helper T cells. Advances in Immunology 46: 111-147.




