Inhaled drugs have been used since immemorial time by the human species. In the 19th century, as early as 1800, the British Army medical officers serving in India introduced anticholinergics into Western medicine for therapeutic purposes, however in many aboriginal cultures shamans (witch doctors) used drugs with hallucinogenic effects to invoke the gods tribal and have predictive visions (Figure 1). Humans have also used inhaled drugs with recreational effects. Respiratory diseases are leading causes of morbidity and mortality worldwide, and conventional smoking of tobacco, a major cause of them. The introduction of the electronic cigarette (EC) from the year 2007 in the USA opened the possibility of using it to significantly reduce conventional cigarette consumption, and reduce exposure to toxic inhalants in combustion smoke, such as reactive species of oxygen and carcinogens. Legalization opened a powerful market and was done without sufficient assessment of health risk and toxicity, as well as without sufficient marketing research and sales regulation. Over time, two unfortunate events have occurred with this tool - acute complications have appeared, some potentially fatal, such as the EVALI (Electronic-cigarette or Vaping-Associated Lung Injury) epidemic in the USA, oral ingestion poisoning (accidental or intentional) , explosion injuries of the devices, and foreseeable chronic complications similar to those of conventional cigarettes such as bronchial asthma, chronic bronchitis and emphysema. It has also been shown that the use of E-cigarette in young people predisposes in the future to the use of conventional cigarettes. The other unfortunate fact is that the tool has been implemented to inhale other drugs, other than nicotine, such as marijuana, cocaine, heroin, and solvents for inhalation whose toxicity are not known or insufficiently investigated. This was predictable to happen. The neoplastic toxicity of this tool is also unknown. This monograph, intend to update and present in a concise manner the recent information on these complications and that readers find such information in a single monograph. The work not only covers clinical and chemical aspects of vaping but also some reflections on economic, ethical and legal aspects of it.

Figure 1. Old use of hallucinogens
Toloache (a hallucinogenic plant) and Azteca that ingest their leaves. Codex Magliabecchi, Central National Library of Florence.
E-cigarette, ENDS (electronic nicotine delivery system), vaping, recreational drugs, toxicology
There are a number of known environmental agents that cause acute or subacute injury by inhalation of the lung. The manifestations depend on the characteristics (solubility and composition) of the toxic as well as the amount inhaled [1]. The common pathophysiological pathway includes inflammation, airway edema with epithelial sloughing, alveolar inflammation, and edema with hypoxemia [2]. The use of EC was promoted as a "safe" alternative for the delivery of nicotine with respect to conventional cigarette smoking since they are supposedly less toxic [3]. They are various devices operated by a battery that heats liquid nicotine and produces vapors or aerosols that are inhaled (Figure 2) [4]. This tool was developed in 2003 and entered the market of the United States of America in 2006-2007 [5]. Since its introduction, the devices have undergone substantial modifications in the control of the voltage and electrical power of the battery and the constituents of the liquid that is vaporized [6]. Currently the devices have evolved to the 4th generation with the JUUL EC device (see later). The electrical power of the batteries varies from 3.3-11.2 watts and their handling varies the aerodynamic diameter of the particles. Recent studies show that the deposit is greater in oropharynx (2-3 times) than in alveoli [7]. In addition to EC, very compact and ergonomic personal vaporizers are available in the market. These devices are grouped under the generic name of ENDS (electronic nicotine delivery systems) [8]. With these devices the peak concentrations of nicotine in blood are reached at 5 minutes, similar to the concentrations obtained with the conventional cigarette [9]. The term "vaping" is used colloquially to describe the inhalation of vapors or aerosols through the mouth, by a mouthpiece that has the device. The term also includes the concept that the liquid is heated to the vaporization temperature but not combustion.
Figure 2. Electronic-cigarette
Note the electronic device, the oily mixture for heating and the vapors that generated the colloquial term “vaping”.
Already in 2014 in the USA there were 466 brands of ENDS and 7764 flavorings to add to electronic cigarettes. Since its introduction, consumption has increased exponentially. There is a worrying increase in consumption in minors (12-17 years old). Marketing and ad campaigns show celebrities using them, as well as in popular campaigns, with evocative images and attractive flavorings (cotton candy). Propaganda on the Internet, TV, radio, malls and in print media is powerful. In 2013, 24 million minors were exposed to EC advertisements, which meant an increase of 256% compared to previous years [9]. Nicotine affects brain development in young people, increasing the risk of addiction, mood disorders, impulse control, and producing cognitive disorders that negatively impact learning, reading and attention [10]. Young vaping users are more likely in the future to use conventional cigarettes than those who do not vape [11]. A sample of 47,176 students enrolled in 17 public high school, reported 27% use of electronic devices, being more prevalent among Hispanics and whites than among blacks and Asians [12]. Students who vape, are more likely to use other illicit substances, and engage in sexual activities than those who do not. Drunkenness, opioids for pain, sexual risk, illicit substances, violent behavior and vaping have been associated [13,14]. In 2016 there were 10.8 million vapers in US and in 2018, 3.6 million were young and the use of EC had increased by 78% compared to 2017 [4]. In fact, since 2014 it is the most common form of tobacco use in young people in the USA. In contrast, 3.2% of adults reported use of EC in 2018.
Another additional problem is the use of EC to vape cannabis [4]. About 1 of 11 students started using cannabis in EC and currently 8% of high school students and 6-8% of young people, who use EC, use cannabis. Cannabis, in young people, reduces learning, memory and therefore academic achievement and education [15]. Within 13 years of having introduced the tool in the USA, and a decade in Canada, the deleterious effects on both acute and chronic health have already begun to appear or at least to be identified. This monograph describes and synthesizes these events.
EVALI
Published case reports have detailed a wide range of severe lung diseases in people who used EC with nicotine or cannabis extracts, but it is not until Layden's work that a series of a cluster (conglomerate, group, or outbreak) is reported of cases of acute pulmonary injury temporarily associated with the use of EC [16]. The outbreak is identified as of July 19, 2019 and included 28 cases from Wisconsin and 25 cases from Illinois. Both, the Wisconsin Department of Health Services (WDHS) and the Illinois Department of Public Health (IDPH) with epidemiological assistance from the CDC (Centers for Disease Control and Prevention) established a case-patient definition that were people who had reported the vaping and related products in the 90 days prior to the onset of symptoms and presented diffuse pulmonary infiltrates not attributable to infection. A syndromic definition for data capture was also designed [16]. In August 1st, the first case was reported to the CDC and by the end of September 908 cases had been identified in 50 states, the District of Columbia, and two U.S. territories (U.S. Virgin Islands and Puerto Rico). The rapid increase strongly suggested an epidemic character [17,18]. As of January 7, 2020, a total of 2602 cases of EVALI had been reported to the CDC, 57 deaths had been confirmed (2%) [19,20].
Overview
The clinical picture is presented days to weeks after the use of EC and related products, with acute respiratory symptoms in 95% of patients (non-productive cough, pleuritic pain, dyspnea), gastrointestinal symptoms in 77% of patients (nausea, vomiting, abdominal pain, diarrhea) and constitutional symptoms in 88% (fever, chills, asthenia, tachycardia). More frequent in men and 67% are <35 years old. Hypoxemia can be severe, even with supplemental oxygen and 47% require intensive care due to respiratory failure. Approximately one third qualify for intubation and mechanical ventilation [21,22]. The thoracic auscultation data appear to be irrelevant because, apart from tachycardia and tachypnea, there is an absence of auscultatory data of the airway and / or pulmonary parenchyma, contrasting with the clinical, gasometric and radiological picture [23]. The diagnosis is exclusion, as there is no specific diagnostic marker or test, and should be guided by clinical judgment. It is difficult to separate it from community-acquired pneumonia or influenza [24]. The differential diagnosis includes infectious, cardiac, gastrointestinal, rheumatologic, neoplastic, environmental and occupational diseases [22]. Some authors question whether this picture is a new clinical syndrome or there were already previous cases that had not been detected [18]. The cause of the 88% reduction in cases reported to the CDC is also unknown, from 214 people admitted during the week of September 15 (the apparent peak of the outbreak) to 26 admitted during the week of November 24, and if that represents a decrease in active case-finding or a true decrease in the incidence of cases. The NSSP (National Syndromic Surveillance Program) has allowed syndromic surveillance of EVALI in real time. This system includes the results of 70% of the USED (US Emergency Department) and the result is that the number of cases has decreased, from the peak in September, in the last 2 months (October and November), although not to the levels prior to June 2019, so monitoring and prevention are necessary [18].
The leukogram shows, in 87% of patients, leukocytosis (>11,000 per mm3), mainly with neutrophilia (>80%). The level of eosinophilia is usually <2%. The erythrocyte sedimentation rate was >30 mm per hour. CDC recommends measuring PCR and transaminase [23]. In the Layden series, 50% of the patients had mild and transient transaminitis, hyperkalemia and hyponatremia, also mild [16]. An extensive laboratory panel for respiratory viruses should be done to rule out virosis since viral pneumonia is part of the differential diagnosis, including influenza viruses, particularly at the time of the influenza peak. EC fluids may be contaminated with viruses, fungi and bacteria whereby studies of pneumococcus, legionella, mycoplasma pneumoniae and mycosis should be performed [25], especially that steroids are part of the EVALI treatment [24]. Procalcitonin may be helpful in discriminating bacterial infection. The problem is that reference levels vary between the hospitals; it is expensive and not easily available.
Radiological imaging
More than 90% of patients have a chest x-ray with parenchymal abnormalities, basically bilateral opacities and the CT scan is abnormal in 100% of cases. The CT scan decision is individual and depends on the clinical circumstance, but it should be considered whether EVALI enters into the differential diagnosis of the clinical picture and conventional chest radiography is normal, which should be requested in all suspicious patients [23]. Ground-glass opacities in both lungs are the universal finding, sometimes with sub-pleural sparing (Figure 3) [16]. Henry TS and collaborators reported a series of 34 patients with radiological and histological correlation: [8]. Basilar and ground-glass consolidation pattern is the most frequent. Acute lung injury was associated with acute eosinophilic pneumonia, and diffuse alveolar damage and had already been reported associated with vaping [5]. The pattern of hypersensitive pneumonitis has been described, although a specific antigen associated with vaping is not known. The other pattern is that of lipoid pneumonia with fatty attenuation in the lung, which is the characteristic of lipoid pneumonia (a negative value of attenuation between -150 and -30 Hounsfield Unit) [26]. Prior to the Layden series, cases of severe lung diseases associated with the use of nicotine or cannabis extract in EC had already been reported, with different radiological patterns [5,27-32]. Not all cases are acute. Also the pattern of pneumonia in organization and interstitial pneumonitis has developed but in a more chronic way, with exposure for up to 6 months to heavy metals in the ENDS. Diffuse alveolar hemorrhage, bronchiectasis and pleural effusion have been described associated with vaping [33], as well as respiratory bronchiolitis associated with interstitial lung disease, with the radiological pattern of “tree-in-bud” [34,35]. They should evaluate the possibility of community-acquired pneumonia according to clinical guidelines and radiological findings [36].
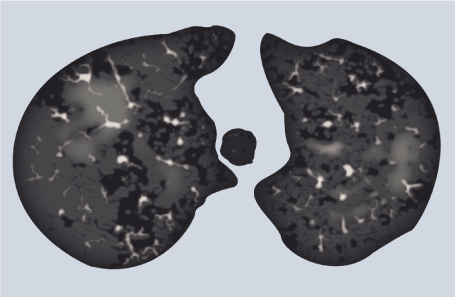
Figure 3. Thorax CT in a patient with EVALI
Ground-glass opacities (GGO pattern) in both lungs are the universal finding.
Bronchoscopy and BAL
Ex officio bronchoscopy has a debatable value in EVALI. Most reports have been made in patients who had already been manipulated with antibiotics, steroids or both. The most frequent finding is predominance of neutrophils and macrophages. While it is true there are reports of acute eosinophilic pneumonia (it has even been associated with the use of conventional cigarettes), the finding of images in the CT scan of acute pneumonia and >25% of eosinophils in the BAL (criteria for diagnosis of eosinophilic pneumonia) It is uncommon in EVALI [27], which is why it does not qualify to introduce the entity into pulmonary infiltrates with eosinophilia [37]. Also in patients with classical lipoid pneumonia as part of the EVALI presentation spectrum, the presence of lipid-laden macrophages (oil red O staining, or Sudan Black) and in the absence of infection, could direct the diagnosis towards EVALI [38]. The value of this finding in other presentation patterns is more debatable because it is not clear whether it can be a marker of toxicity or exposure, and therefore the value of both stains in BAL cells or in fresh lung biopsies remains unknown. If bronchial aspirate studies are required for bacteria, viruses and fungi as part of the differential diagnosis, then bronchoscopy may be indicated. Like chest CT, bronchoscopy and BAL should be evaluated on a case-by-case basis, especially that it can lead to substantial airway reactivity intra and postoperatively, to cough and prolonged hypoxemia. Postoperative ventilation availability is required or deferred until the stability of the clinical picture allows it [39].
Histological patterns
In the original Layden series there was little tissue obtaining either by transbronchial biopsy and / or open lung biopsy (3 patients). The histological findings were nonspecific. Mild and nonspecific inflammation, acute diffuse alveolar damage and foamy macrophages, and peribronchiolar and interstitial granulomatous pneumonitis were described, in the absence of a demonstrable infectious process [16]. Butt and colleagues reported histological findings from a series of 17 patients <35 years with EVALI. 71% used marijuana or cannabis oil. The tissue showed infiltration with neutrophils, few eosinophils and without granulomas. All cases showed the pattern of acute pulmonary injury such as acute fibrinous pneumonitis, diffuse alveolar damage or pneumonia in organization, usually bronchiocentric and accompanied by bronchiolitis. There was no exogenous lipoid pneumonia. No histological finding is specific but all the samples presented with foamy macrophages and pneumocyte vacuolization, so that finding in an appropriate clinical context may be a key suggestive of the diagnosis [40]. There is probably a chemical pneumonitis centered on the airway, rather than an exogenous lipoid pneumonia, as some authors have proposed, but the causal agent (s) are unknown to date [41]. The pneumonia in organization associated with vaping had already been described [42]. Other histological patterns have been described only in isolated patients and in anecdotal reports. The decision of a lung biopsy should be taken together with a pulmonology specialist and possibly only those patients whose evolution is inadequate or an alternate diagnosis is considered and in whom the biopsy result changes the treatment and / or the forecast.
Management
The patient must be admitted to a hospital if the oxygen saturation at ambient air is <95%, they have respiratory distress or comorbidities that compromise the pulmonary reserve (heart failure, COPD, OSA, DM, multiple comorbidities, and a poor family support network and / or social) since these patients are more likely to die at 3 days after discharge and higher risk of re-admission [43]. To discharge these patients in addition to clinical stability, it is required that there be no fluctuations of vital signs before discharge from the hospital and that there is the possibility of external evaluation at 48 hours (and not at 2 weeks as proposed at the beginning of the outbreak) [19]. External management can be considered if the patient is clinically stable, has less severe injury, oxygen saturation at room air is >95% (room air), possibility of reassessment at 24-48 hours, easy access to health care and adequate social support. Empirical antibiotics (including antivirals) are indicated. Patients should be instructed to seek medical help in case of worsening since this clinical evolution has been observed. In the Layden series, 71% of the patients admitted to the hospital were re-consultants [16].
Steroids in clearing the inflammatory response may be helpful in EVALI. In the Layden series and in another of the CDC the clinical improvement was between 82-92%, but this was a clinical assessment (of course of great observational value). The problem is that the natural evolution of this lesion is not known, and the improvement could have occurred without the need for steroids and / or for the suspension of vaping. This assessment becomes relevant when the diagnosis of infection, such as fungal pneumonia, strongly enters into the differential diagnosis, since it can worsen with steroids. In any case, since this is a diagnosis of exclusion, if the patient is critically ill, aggressive empirical steroid, antimicrobial and antiviral therapy is indicated [23]. The dose, duration and reduction of steroids should be done on a case-by-case basis and with the help of a pulmonologist. The duration (IV or oral) is at least 7 days, which is the average stay of these patients [44]. Empirically, and as a suggestion Methylprednisolone 125 mg IV every 6 hours for 48 hours and then change to 60 mg oral prednisone once a day for 6 days have been recommended, with the subsequent stepwise reduction as described [5,41]. Antimicrobial therapy should be chosen according to the guidelines of community-acquired pneumonia [36], and antiviral therapy, particularly in the influenza season, according to established regulations [20].
Monitoring, re-exposure and risk groups
The patient should be re-evaluated quickly, particularly if he had severe illness, was intubated or belonged to risk groups (24-48 hours), and if not at least 1-2 weeks after discharge. Clinical evaluation and oximetry of pulse should be performed and repeat chest x-ray according to medical criteria and 1-2 months later another clinical assessment, oximetry, ex-officio chest x-ray, spirometry and diffusion test [23]. The long-term effects of EVALI and risk of recurrence is unknown, and although many patients resolve their symptoms, there are clinical reports of exacerbations by reducing corticosteroids outside the hospital Some patients remain with persistent hypoxemia (with saturation <95%) requiring home oxygen and pulmonology control. Patients who received high doses of steroids may require evaluation with Endocrinology to monitor adrenal function [24].
It is unknown if patients with EVALI are at high risk for severe complications of influenza or other viral infections, particularly if they were infected simultaneously when they developed the event but the annual vaccine is recommended in all patients >6 months of age, including patients with EVALI. The pneumococcal vaccine should be administered according to current guidelines [45].
It is essential to avoid re-exposure to vaping, since restarting this practice runs the risk of restarting the symptoms and lung injury. Strategies for quitting tobacco as behavioral counseling and FDA-approved medication are effective tools. For patients addicted to cannabis or tobacco products there are several strategies and an assessment by addiction medicine services should be considered [46,47].
In addition to the aforementioned risk factors, pregnancy and older age are two other factors with a higher probability of poor outcomes. For example, patients >50 years have a higher percentage of endotracheal tube and mechanical ventilation and longer stays (15 days) than <50 years.
Vaping was promoted as a safe alternative to inhale nicotine from conventional cigarettes, however, smoking liquid nicotine (6%) for 30 minutes, 5 times a day produced a report of acute eosinophilic pneumonia described as early as 1989, and set out that EC increased IL-6 and IL-8 which are chemotactic for eosinophils (conventional smoking generates IL-5, IL-6, IL-17, and TNF, which attract eosinophils) [5]. The manufactures recommend after inhaling 16 puffs, suspend the vaping for 30 minutes, until the next delivery, to avoid excessive use and complications. This scheme has already been associated with bilateral pneumonia with pleural effusion [29]. Liquids containing nicotine have been associated (when vaping) with diffuse alveolar hemorrhage, exogenous lipoid pneumonia, acute interstitial lung disease (acute eosinophilic pneumonia, respiratory bronchiolitis) and hypersensitive pneumonitis [5,17,32-34]. Long before the introduction of the EC it was proposed (2001) that vaporizing cannabis by taking it to a vaporization point and not combustion could prevent the emission and exposure of users to benzene, toluene and naphthalene [48]. The temperature for vaporization should be >1800C but <2300C to prevent combustion [31]. It is obvious that neither the justifications nor the recommendations have avoided the danger and risks of the tool. EC fluids and the aerosols they generate contain a variety of chemical constituents that have adverse health effects [7]. The declared constituents of the EC, in addition to nicotine, are propylene glycol and glycerol (vegetable glycerin) (safe orally) [49]. Unlike traditional cigarettes, ENDS use these solvents to heat and aerosolize flavorings (known as “juices”). These solvents are aldehydes and alcohols, which when heated, mixed and aerosolized, generates new toxic compounds [50]. For example, formaldehyde is a degradation product of propylene glycol and can in turn react with propylene glycol and glycerol to produce hemiacetals (which contain formaldehyde and have been used as biocides on an industrial level). Inhaled formaldehyde is carcinogenic. These hemiacetals have been detected in studies in EC using NMR (nuclear magnetic resonance spectroscopy), and have been shown to deposit more in the respiratory tract than gaseous formaldehyde increasing the risk of cancer [51].
The use of flavorings, in addition to making the inhalation of vapors more comfortable, reduces the odors of classic smoking and cannabis, making it difficult to detect them in public places, which favors “second-hand vaping” [6]. The presence of diacetyl and 2,3-pentanedione as flavorings has been detected in EC fluids [52]. Diacetyl has been used to flavor popcorn for microwaves, and in exposed workers a pathology known as "popcorn lung" due to inhalation of diacetyl has been described. In severe bronchiolitis with the radiological pattern of “tree-in-bud” requiring ECMO for respiratory failure and evolving to a restrictive disorder 4 months after the event, diacetyl has been described in relation to EC fluids [35]. The 2 flavorings mentioned disturb the genetic expression of the processes related to the physiology of the cilia and the cytoskeleton in normal bronchial epithelial cells [53].
ENDS have an increasing use to deliver concentrates and cannabis oil [8]. In the Layden series, 84% of patients reported the use of tetrahydrocannabinol (THC). However, 17% of patients reported only the use of nicotine products and 44% reported the use of both. In the Butt series, 71% used THC, and the majority of high school students who vape basically use nicotine and THC, in cartridges or in liquids [16,20,40]. FDA has collected more than 440 devices and products for vaping in 18 US states and reported THC concentrations range between 14-76% [40]. In ages ranging from 18-44 years of age, the exclusive use of THC or cannabidiol (CBD) obtained from illegal distributors, off the street, or from friends or informally is frequent, not knowing all the compounds or ingredients of the product or frequency and dose habits. If it is known that patients who developed EVALI by exclusive use of THC used it more than 5 times a day [54]. Marijuana is defined by the FDA as the original parts (flowers and leaves) or derivatives of the Cannabis sativa plant [55]. In 2009, due to the high potential for abuse of its main psychoactive ingredient (α-9-THC), marijuana was categorized as hallucinogenic and was assigned to Schedule1 of controlled substances, which also includes opioids and its derivatives, and the evaluation of this categorization given the legalization of consumption in many states keep going [55]. Significant reports of pulmonary injury from cannabis use in the form of (conventional) combustion include: alveolar hemorrhage, pneumonia in organization, pneumothorax [56], chronic cough and dyspnea [57], chronic bronchitis due to pyrolytic agents such as tar, ammonium, and nitrogen oxide [58], and severe acute pulmonary injury [59-61]. Emphysema and cancer are controversial) [62]. This led to the use of vaporized cannabis with the idea of minimizing the generation of deleterious products from its combustion. Similarly, vaping cannabis oil (cartridges pre-filled with concentrated marijuana oil), avoiding vaporizing raw marijuana was believed to be safe [6]. Obviously this is not true.
THC activates 2 receptors, CB1 (SNC and peripheral) and CB2 (immune system). The activation of CB1 inhibits the release of neurotransmitters such as acetylcholine, norepinephrine, GABA, dopamine, serotonin and prostaglandins. Hence, it’s relaxing and anxiolytic effects. Toxic dose of cannabis by inhalation is 7.5 mg / m2 of body surface. Synthetic cannabinoids (>160) have exquisite affinity for CB1, so, initially relatively low doses are required to obtain the psychotropic effects. Over time the tolerance phenomenon is created, the receptors are easily saturated and increasingly high doses are required to obtain the same effects, potentiating the occurrence of dangerous side effects [6].
An absolutely controversial aspect is its beneficial effects on human health. In neuropathic pain and painful cramps the results are varied. There is insufficient evidence of a role in cancer-associated pain and rheumatic pain. The improvement in anxiety is maximized with the THC-CBD mixture, but it is ineffective in depression and psychosis and in ADHD (attention-deficit hyperactivity disorder). There is really little evidence of the use of cannabis in mental disorders and more evidence-based interventions should be promoted. Cannabis oral solution (Epidiolexr) is used by pediatric neurologists as a second-line drug in refractory epilepsy at the infant level, and its use is approved in Lennon-Gastaut syndrome [63]. FDA has approved highly purified CBD studies for Lennox-Gastaut syndrome and Dravet syndrome. It is ineffective for focal seizures. Artisanal cannabis products have a low level of scientific credibility. If there seems to be evidence that it may work in convulsions associated with tuberous sclerosis [64].
Another modality of inhaling cannabis is to use butane hash oil or BHO (“dabs or dabbing” [rubbing]) that has been associated with severe pneumonitis [4,30,65]. In a report by Perrine, et al. more than 90% of patients hospitalized with EVALI, with an average age of 23 years, had used this modality [20]. Basically it is marijuana extracted with butane. It has become very popular, and is among the most illicit forms of use in the US. Its history dates back to the Vietnam War. The soldiers extracted the THC and concentrated it in a liquid that used solvents such as acetone and petrol. It can be used in liquid form or a kind of spreadable wax. Consumers smeared the product on rolled paper or saturated the tobacco with the liquid concentrate [66]. Currently the extraction is done by passing liquid butane through the raw vegetable and hydrophobic products (THC) dissolve in butane. The mixture is filtered and in regulated production environments the butane is removed under vacuum, but in home production the butane is purged with heat. It is done in garages and only a steel tube, filter paper and a pyrex (“blasting”) are required. In blasting, the non-purged butane accumulates in enclosed spaces and can explode due to sparks or static electricity affecting the personnel that manufactures it [67]. In addition to the impurities contained in unpurged butane (for example, terpenes), consumers are exposed to high concentrations of THC. This modality offers up to 75% THC compared to 5-20% of traditional consumption. Terpenes when heated to 9780F are degraded to methacrolein and benzene, which has been shown, in experimental animals that induces pulmonary edema [30]. Butane inhaled by users (which could not be extracted in home production) produces acute health problems (seizures, cardiac arrhythmias, respiratory depression, vagal inhibition and anoxia) and chronic health problems (cardiomyopathies, kidney damage, liver damage, and psychiatric syndromes). The illicit production of BHO is increasing [67].
Other substances with adverse effects present in EC are ultra-fine matter particles, volatile organic compounds, polycyclic aromatic hydrocarbons, nitrosamine vapors (tobacco-specific) and inorganic chemicals [68]. Lipopolysaccharides (endotoxins) are constituents of the outer membrane of gram-negative bacteria. (1-3) -β-D-glucan is a common component in the cell wall of many fungi, certain bacteria and algae. Both are frequent pollutants of EC [69]. Within the inorganic chemicals are metals, which are elevated in the liquid of the EC such as, Cr, Ni, Pb, Zn and Mn. The coils of the devices when heated can release these metals [70]. Cobalt exposure has been reported as a cause of interstitial giant cell pneumonia after 6 months of vaping [71]. The "cobalt lung" is considered at work level as pneumoconiosis due to chronic exposure to cobalt.
Vitamin E (E) acetate has been considered as another of those allegedly responsible for the outbreak. Among the FDA collected products from 18 states, some had a mixture of THC and E (31-88%) (40). E is recently introduced in EC liquids. In a small study in Minnesota, all 20 products containing THC analyzed in 2019, contained E, in contrast to none of 10 products collected in 2018 [72]. Laboratory tests of the BAL liquid samples from 51 patients (isotopic dilution mass spectrometry) detected E in all samples (in addition to THC), but was not identified in any of the control group samples [21]. Vitamin E acetate is the ester of vitamin E (α-tocopherol) and acetic acid and can cause respiratory dysfunction because when heated they generate ketenosis that can be toxic irritants. E is a thickening agent, which is used in products that contain illicit THC. It is inexpensive, good-looking, desirable aroma and has the same viscosity as THC and is used to "cut" THC. Studies are being conducted exposing animals to aerosolized E to determine if it can cause lung injury. However, the evidence is insufficient to rule out the contribution of other chemicals to the development of EVALI [73].
An additional problem with vaping is that it is used to dispense illicit drugs. It is a route that offers a rapid onset of drug action and almost all illicit drugs have been used. Synthetic cannabinoids, synthetic cathinones, cocaine, gamma hydroxybutyric acid (GHB), heroin, fentanyl and amphetamines are used by this route. This use was predictable when the tool was introduced and can be used for continuous or acute administration, increasing use in addicted youth, increasing toxicity and with accidental pediatric exposure. Thus, the use of illicit drugs in public places is favored, and detection is not easy, which raises how debatable the indication of EC as a tool to reduce nicotine consumption.
Another aspect in which vaping has evolved is in the devices. In addition to conventional EC (also called vapes), there are e-hookahs, vape pens, tank systems, mods and ENDS. “Pod Mod” is a small equipment (similar to a pod), with refillable cartridges with liquid solutions containing nicotine, flavorings and other well-encapsulated substances. JUUL is a popular brand of Pod Mod that resembles a USB device, easy to use, transport, and mask (Figure 4). Traditionally, EC use solutions with free-base nicotine. JUUL and other pod mod use nicotine salts obtained from loose-leaf-tobacco. It contains 2-10 times higher concentration of nicotine than EC (59 mg / ml) which is equivalent to using 20 conventional cigarettes, making them very addictive [74].

Figure 4. JUUL Pod Mod
Juul’s pod mod cartridge on the left. USB drive on the right. Notice the similarity.
Several US states have legalized medical marijuana (for example, Pennsylvania since 2016) but the doses of active ingredients (THC and CBD) are not standardized, product concentrations are not regulated, and prescription practices vary. The typical recreational dose of THC for people who did not previously use marijuana is 10 mg. There are products for medicinal, oral use, containing in 0.1 ml, 50 mg of THC, that is, 10-20 times the recommended dose for therapeutic use [75]. Intentional or accidental poisoning is easy to obtain. Neurotoxic effects include anxiety, paranoia, and delirium, more common in adults, and depression, more common in children [76]. Numerous cases have been reported in pediatric literature, but only a few cases in adults [77-79]. In the same way, intentional or accidental, intake of the liquid to vape can occur. Nausea, vomiting, headache and dizziness, usually mild [80], but lethal cases have been described [81]. In children, the intake of 10-20 ml of the EC fluid can be fatal [82].
Overheating of the internal battery may occur; caught fire and explodes (thermal injury) Already in 2016, Brownson collected reports of 25 accidental cases (2009-2014), and reported a casuistry of 15 more cases, dividing the lesions into three types: chemical burn, flame burn and explosion injury [83]. Katz's recent report of a 17-year-old male patient with extensive lacerations in the mouth, interrupted lower incisors, and bone incongruities of his left jaw due to device explosion exposes how dramatic this situation can be [84].
A recent report that followed 32,000 American patients for 3 years showed that consumers of EC have the same risk of developing chronic lung diseases as consumers of conventional cigarettes. The study shows that vapers are 30% more likely to develop chronic bronchitis, bronchial asthma and emphysema than controls that do not vape. The risk is tripled again if EC and conventional cigarettes are consumed, a practice that is not uncommon. The study comes from the PATH (Population Assessment of Tobacco and Health) source and is a longitudinal study based on a population [85]. The finding that the risk of using EC is independent of conventional smoking comes from the different proteins expressed in human lung epithelial cells obtained from conventional smokers and vapers [86]. Other previous studies had raised this finding, that is, the risk of COPD due to the use of EC, independent of conventional cigarettes [87]. Studies in experimental models of mice have also shown that exposure to EC alters the mechanisms of aniviral and antibacterial defenses and that chronic exposure induces COPD changes in a nicotine-dependent manner [88,89]. Nicotine of EC causes disruption of the airway barrier and induces systemic inflammation and multiorgan fibrosis in mice and acrolein in humans impacts the function of lung cells favoring the development of COPD [90,91].
Proponents of vaping argue that the use of EC is more effective than other systems (such as nicotine replacement) for quitting smoking, with only some side effects such as pain and pharyngeal irritation [92]. However, the information is controversial at this point. First, vaping is not only used to dispense nicotine. Second, although some authors believe that the most likely cause of EVALI is the use of a specific type of refillable cartridges, usually illicit containing cannabis and toxic ingredients such as vitamin E acetate [85], it is very likely that there are other compounds involved, as the varied histological response and clinical presentation to pulmonary injury suggest it. There are patients with EVALI who only vaped nicotine [16]. The use of EC will remain at risk, even if a specific cause of EVALI is identified [93]. Third, the practice of using vaping and conventional cigarettes is more frequent than suspending the conventional cigarette when switching to vaping and young people who start vaping quite frequently then move on to the conventional cigarette in adulthood [11]. In Bhatta's work the use of EC was associated with low efficacy to stop smoking [85,94]. Reliable data on the effectiveness of smoking cessation are limited (9). Fourth, the recommendations of the literature are clear in the sense that EC should never be used in young people, young adults, adults who are going to start consumption and pregnant women. It is precisely towards many of these segments of the population that propaganda is directed towards EC [20,22].
Already in 2016 in the US, concern was described about the ascending escalation of the use of EC in minors. The scientific community had been impacted by regulatory and legal aspects and divided in its response. Some approved the policy of "harm reduction" and others defended a campaign of concern whose philosophy is to avoid the adoption of a new product whose long-term effects are unknown [9]. The economic aspect had an influence on decision making. In 2017, the sale of EC generated $10 billion [95]. In July 2017, the FDA commissioner announced a new and comprehensive plan to regulate the use of nicotine and tobacco consumption, and intended to delay the regulation of EC until August 2022, with the idea of allowing scientific deliberation and product innovation. FDA's claims and comments recognize "harm reduction" as an appropriate public health strategy to reduce morbidity and mortality associated with tobacco combustion. There is no federal regulation of the EC in the US and the products have a wide availability and give the feeling that the concern is, not "damage reduction" but consumer protection [96]. It will be interesting and worrying as the outbreak of EVALI, other acute pathologies, and the possibility of chronic complications from the use of EC will influence the policy of "harm reduction" and the policy of tolerance with delivery systems of smoked supposedly less toxic.
In Canada in May 2018, federal legislation legalized nicotine EC (which was already a thriving illegal market), and in October 2019 the sale of EC with cannabis also became legal (which was already used by many Canadians). This happened despite the epidemic of EVALI in the US. In Canada there are about 272,000 vapers between 15-24 years [93], but EVALI cases are already described not only in Canada but also in Mexico, Argentina, Ecuador and Brazil. Another country where vaping is increasing is in China (incidentally 90% of vaping devices come from China). Conventional smoking in China reaches the figure of 300 million (the approximate total population of US). Ten million young Chinese vape. We do not know the data of the economic gains in China but they must be juicy
The EC industry has taken a distance from EVALI claiming that the fault lies in the illegal cannabis products (before conclusive evidence), as if they were not responsible for popularizing a new methodology for inhaling drugs and claim to reassure consumers by telling them they should not worry if they adhere to known brands. But JUULU was already sued for selling capsules of contaminated liquid, with dates close to expiration and for misrepresenting the nicotine contents of its products [97]. The US government has taken action against JUUL for marketing that attracts young people and for legal health claims regarding “harm reduction” [98]., Health JUUL has withdrawn its products from the US market, but not from Canada. On the contrary Canada has considered allowing EC companies to make these “harm reduction” claims [99]. According to Canadian authors, the logical and responsible action to take against toxic and harmful products is to withdraw and prohibit them. EC distributors (which are owned by the tobacco industry) should not be allowed to manipulate the public and the government by making them believe that without the EC smokers will return to conventional tobacco, as a lever for an appropriate but beneficial regulatory action. This is a false statement, based on the claim that there are no alternatives to reduce the consumption of tobacco by combustion. And thus keep millions of nicotine addicts. EVALI led the US government to government action. Federal and state governments have proposed implementing bans on flavored EC that companies are defending in court [93].
Will politicians and administrators be willing to prioritize health over juicy economic income and political interests?
- The legalization of EC to dispense nicotine and cannabis was premature since the health and toxicity risks of drugs, solvents, and additives, was not adequately assessed; nor was an adequate assessment of the necessary regulations for marketing and sales
- EVALI and other acute and chronic pathologies exemplify the risk of legalizing tools that are not well studied, standardized or well controlled.
- In addition, and this was expected to occur, vaping has been used to dispense any illegal drug that can be inhaled.
- These pathologies affect the health of a very vulnerable age group such as minors and young people who are or will be the productive forces of society.
- Governments have initiated some legal actions, without even knowing if they intend to have a concerted and honest action to curb the problem or it is a fictitious policy while the epidemic and the cries subside.
- Will the political administration of the countries most involved, particularly in the first World, be willing to place the health of their minors and young people (who are the future of the species) above economic interests? Seeing what happens with the business of war, the poor distribution of poverty, and excessive aggression to the planet, we inhabit it is painfully honest to think not.
None.
No.
This work was only carried out by the author. Author AA contributed in the planning, data collection, data analysis, writing and critical review. AA read and approved the final manuscript.
- Kales SN, Christiani DC (2004) Acute chemical emergencies. N Engl J Med 350: 800-808. [Crossref]
- 2. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JM, et al (2019). Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18-18.
- National Academics of Sciences, Engineering, and Medicine (2018) Public health consequences of e-cigarettes. National Academies Press, Washington, DC.
- https://e.cigarettes.surgeongeneral.gov/documents/surgeon-generals.advisory-on-e-cigarette-use-among-youth-2018.pdf
- Arter ZL, Wiggins A, Hudspath C, Kisling A, Hostlller DC (2019). Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep 27: 100825-100825.
- Breithbarth AK, Morgan J, Jones AL (2018) E-cigarette: unintended illicit drug delivery system. Drug Alcohol Depend 192: 98-111.
- Floyd EL, Queimado L, Wang J, Regens JL, Johnson DL (2018). Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoSOne 13: e0210147-e0210147.
- Tameyasu T (1992) Unloaded shortening after a quick release of a contracting, single fibre from crayfish slow muscle. J Muscle Res Cell Motil 13: 619-629. [Crossref]
- Dinakar C, O'Connor GT (2016) The Health Effects of Electronic Cigarettes. N Engl J Med 375: 1372-1381. [Crossref]
- E-cigarette use among youth and young adults: a report of the Surgeon General-executive summary (2016) Atlanta: Department of Health and Human Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- https://www.nap.edu/resource/24952/012318cigaretteConclusionsbyEvidence.pdf
- Chiou WL, Nation RL, Peng GW, Huang SM (1978) Improved micro-scale high-pressure liquid-chromatographic assay of gentamicin in plasma. Clin Chem 24: 1846-1847. [Crossref]
- Demissie Z, Everett Jones S, Clayton HB, King BA (2017) Adolescent risk behaviors and use of electronic vapor products and cigarettes. Pediatrics 139: e20162921-20162921.
- Giorgio A (1979) [New classification of bacterium (author's transl)]. Ann Sclavo 21: 743-769. [Crossref]
- https://www.cdc.gov/nchs/nhis/data-questionnaires-doicumentation.htm
- Layden JE, Chinai I, Pray I, Kimball A, Layer MC, et al (2019). Pulmonary illness related to E-cigarette use in Illinois and Wisconsin-Preliminary report. N Engl J Med.
- Hswen Y, Brownstein JS (2019) Real time digital surveillance of vaping-induced pulmonary disease. N Engl J Med 381: 1778-1780.
- Kumar KL, Bordiuk JD (1991) Hemicrania continua: a therapeutic dilemma. Headache 31: 345. [Crossref]
- Evans ME, Twentyman E, Click ES, Goodman AB, Weissman DM, et al (2019) Update: Interim guidance for healthcare professional evaluating and caring for patients with suspected E-cigarette, or vaping, product use-associated lung injury and reducing the risk for rehospitalization and death following hospital discharge-Unites States, MMWR.
- Perrine CG, Pickens CM, Boehmer TK, King BA, Jones CM, et al (2019). Characteristics of a multistate outbreak of lung injury associated with E-cigarette use on vaping-United States. MMWR 68: 860-864.
- Blount BC, Kornowski MP, Shield PG, Morel-Espinoza M, Valentin-Blasinil, et al (2019). Vitamin E acetate in bronchoalveolar-lavage fluid associate with EVALI. N Engl J Med.
- Schier JG, Meiman JG, Layden J, Mikosa CA, VanFrank B, et al (2019). Several pulmonary diseases associated with electronic-cigarette-products use-interim guidance. MMWR 68: 787-790.
- Siegel DA, Jatlaoui TC, Koumans EH, Kiernan EA, Layer M, et al. (2019) Update: Interim Guidance for Health Care Providers Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use Associated Lung Injury - United States, October 2019. MMWR Morb Mortal Wkly Rep 68: 919-927. [Crossref]
- Jatlaoui TC, Witlz J, Kabbani S, Siegel DA, Koppaka R, et al (2019). Update: Interim guidance for health care providers for managing patients with suspected use-associated lung injury-United States. MMWR 68: 1081-1086.
- Lee MS, Allen PG, Christiani DC (2019) Endotoxin and (1-3)-β-D-Glucan contamination in electronic cigarette products sold in the United States. Environ Health Perspect 127: 47008-47008.
- Alvarado A, Arce I (2016) Metabolic functions of the lung, disorders and associated pathologies. J Clin Med Res 8: 689-700.
- Sommerfeld CD, Weiner DJ, Nowalk A, Larkin A (2018). Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics 141: e20163927-e20163927.
- Thota D, Latham E (2014) Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med 47: 15-17.
- Moore K, Young H, Ryan MF (2015) Bilateral pneumonia and pleural effusions subsequent to electronic cigarette use. Open J Emerg Med 3: 18-22.
- Anderson RP, Zeckar K (2019). Lung injury from inhaling butane has oil mimics pneumonia. Respir Med Case Rep. 26: 171-173.
- He T, Oks M, Esposito M, Steinberg H, Makaryus M (2017) "Tree-in-Bloom": Severe Acute Lung Injury Induced by Vaping Cannabis Oil. Ann Am Thorac Soc 14: 468-470. [Crossref]
- McCaulen L, Markin C, Hosmer O (2012) An unexpected consequence of electronic cigarette use. Chest 141: 1110-1113.
- Augusti M, Yamamoto M, Cabrera F, Eusebio R (2018) Diffuse alveolar hemorrhage induced vaping. Case Rep Pulmonol 2018: 9724530-9724530.
- Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, et al (2017). Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep 5: eoo230-e00230.
- Landman ST, Dhalinhai I, Mackenzie CA, Martin T, Slede A, et al (2019). Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. CMAJ 191: E1321-E1321.
- Metlay JP, Waterer GW, Long AC, Anzueto A. Brozek J, et al. (2019). Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med 68: e1-e47.
- Alvarado A (2020) The eosinophil: Physiology and Pathology. Clin Res Trials 6: 1-11.
- Gordon DA, Lowey S (1992) Distribution of developmental myosin isoforms in isolated A-segments. J Muscle Res Cell Motil 13: 654-667. [Crossref]
- Romano M, Razandi M, Ivey KJ (1991) Role of sulphydryl compounds in the defense of rat gastric epithelial cells against oxygen reactive metabolite-induced damage. Ital J Gastroenterol 23: 55-59. [Crossref]
- Ivessa NE, De Lemos-Chiarandini C, Tsao YS, Takatsuki A, Adesnik M, et al. (1992) O-glycosylation of intact and truncated ribophorins in brefeldin A-treated cells: newly synthesized intact ribophorins are only transiently accessible to the relocated glycosyltransferases. J Cell Biol 117: 949-958. [Crossref]
- Dadvison K, Brancato A, Heetderks P, Mansour W, Matheis E, et al (2019). Outbreak of electronic-cigarette-associated acute lipoid pneumonia-North Carolina, July-August 2019. MMWR 68: 784-786.
- Khan MS, Khateeb F, Akhtar J, Khan Z, Lal A, et al (2018). Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin Respir J 12: 1295-1299.
- Mikosz CA, Danielson MA, Anderson KN, Pollack LA, Currie DW, et al (2019). Characteristics of patients experiencing re-hospitalization or death after hospital discharge in a nation-wide outbreak of E-cigarette or vaping product use-associated lung injury-United States 2019 MMWR.
- Kalinsky A, Bach CT, Nacca NE, Ginsber G, Marraffa J, et al (2019). E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Resp Med 7: 1017-1026.
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, et al (2014). Use of 13-valent Pneumococcal Conjugate Vaccines and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged >65 year: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 63: 822-825.
- Budney AJ, Moore BA, Rocha HK, Higgins ST (2006). Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependency in outpatient treatment. J Consult Clin Psychol 74: 307-316.
- Diadmond G, Panichelli-Mindel SM, Shera D, Dennis M, Tims Fm et al (2006). Psychiatric syndromes in adolescents with marijuana abuse and dependency in outpatient treatment. J Child Adolesc Subst Abuse 15: 37-54.
- Gieringer OH (2001) Cannabis “vaporization”: a promising strategy for smoke’ harm reduction. J Cannabis Ther 1: 153-170.
- Burstyn I (2014) Peering through the mist: systematic review of contaminants in electronic cigarettes, tell us about health risks. BMC Public Health 14: 18-18.
- Jensen RP, Strongin RM, Peyton OH (2017) Solvent chemistry in the electronic cigarette reaction vessel. Sci Rep 7: 42549.
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH (2015) Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372: 392-394. [Crossref]
- Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, et al (2016). Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanediones, and acetoin in a simple of 51 products, including fruit, Candy, and cocktail-flavored e-cigarettes. Environ Health Perspect 124: 733-739.
- Christiani DC (2019) Vaping-induced lung injury. N Engl J Med.
- [Crossref] Navon L, Jones CM, Ghinai I, King BA, Briss PA, et al. (2019) Risk Factors for E-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) Among Adults Who Use E-Cigarette, or Vaping, Products - Illinois, July-October 2019. MMWR Morb Mortal Wkly Rep 68: 1034-1039.
- http://www.fda.gov/regulatoryinformation/legislation/ucm148726.htm
- Beshay M, Kaiser H, Niedhart D, Reymond MA, Schmid RA (2007) Emphysema and secondary pneumothorax in young adults smoking cannabis. Eur J Cardiothorac Surg 32: 834-838.
- Tashkin DP (2013) Effects of marijuana smoking on the lung. Ann Am Thorac Soc 10: 239-247. [Crossref]
- Moir D, Rickert WS, Lavasseur G, Larose Y, Maertsen R, et al (2008). A comparison of mainstream and side-stream marijuana and tobacco cigarette smoke produced under two machine smoking condition. Chem Res Toxicol 21: 494-502.
- Hashmi HR, Duncalf R, Khaja M (2016) A case report of cannabis induced hemoptysis. Medicine (Baltimore) 95: e3232.
- Gilbert CR, Baram M, Cavarocchi NC (2013) “Smoking wet”: respiratory failure related to smoking tainted marijuana cigarettes. Tex Heart Inst 40: 64-67.
- Monfort M, Larakeb A, Gouraud F (2013) [Hemoptysis in a young man smoking cannabis]. Arch Pediatr 20: 637-639. [Crossref]
- Joshi M, Joshi A, Bartter T (2014) Marijuana and lung diseases. Curr Opin Pulm Med 20: 173-179. [Crossref]
- VanStraten AF, Ng YT (2012) Treatment of Lennox-Gastaut Syndrome. Pediatr Neurol 47: 151-161.
- Pamplona FA, da Silva LR, Coan AC (2018) Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis. Front Neurol 9: 759. [Crossref]
- McMahon MJ, Bhatt NA, Stahlmann CG, Phillip AI (2016) Severe pneumonitis after inhalation of butane hash oil. Ann Am Thorac Soc 13: 991-992.
- Booth M (2015) Cannabis: a History. Picador 2005 ebook Macmillan, New York.
- Al-Zouabi I, Stonger JM, Lane ES (2018) Butane hash oil and dabbing: insights into use, amateur production techniques, and potential harm mitigation. Subst Abuse Rehabil 9: 91-101.
- Pisinger C, Dossing M (2014) A systematic review of health effects of electronic cigarettes. Prev Med 69: 248-260.
- Schmidt S (2019) Microbial toxins in E-liquid: a potential, new vaping related exposure to explore. Environ Health Perspect 127: 094001-094001.
- Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul F, et al (2018). Metal concentration in e-cigarettes liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect 126: 027010-027010.
- Ellioth DRF, Shan R, Hess LA, Elucker B, Henry TS, et al (2019). Giant cell interstitial pneumonia secondary to cobalt exposure from e-cigarette use. Eur Respir J.
- Taylor J, Wiens T, Peterson J, Saravia S, Lunda M, et al. (2019) Characteristics of E-cigarette, or Vaping, Products Used by Patients with Associated Lung Injury and Products Seized by Law Enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep 68: 1096-1100. [Crossref]
- Outbreak lf lung injury associated with the use of e-cigarette, or vaping, products (2019) Atlanta: Centers for Disease Control and Prevention 68: 1013-1019.
- Bach FH, Jarrett-Toth E, Benike CJ, Sheehy MJ, Sondel PM, et al. (1976) Three HLA-D region antigens defined by primed LD typing. J Exp Med 144: 549-554. [Crossref]
- Lee MA (2018) CBD & cannabis dosage guide. Project CBD.
- Mohamed Abdallah ZM (1978) Value of the renal cortical index in hiliosinuscystosis. Actas Urol Esp 2: 63-68. [Crossref]
- Vo KT, Horng H, Li K, Ho RH, Wu AHB, et al (2018). Cannabis intoxication case series: the dangers of edibles containing tetrahydrocannabinol. Ann Emerg Med 71: 306-313.
- Bosch RJ, Benard F, Aboseif SR, Stief CG, Lue TF, et al. (1991) Penile detumescence: characterization of three phases. J Urol 146: 867-871. [Crossref]
- Garg SK, Makkar HP, Nagal KB, Sharma SK, Wadhwa DR, et al. (1992) Oak (Quercus incana) leaf poisoning in cattle. Vet Hum Toxicol 34: 161-164. [Crossref]
- Vakklanka JP, Hardison LS Jr, Holstege CP (2014) Epidemiological trends in electronic cigarette exposures reported to US poison centers. Clin Toxicol (Phila) 52: 542-548.
- Kim JW, Baum CR (2015) Liquid Nicotine Toxicity. Pediatr Emerg Care 31: 517-521. [Crossref]
- Chen BC, Bright SB, Trivedi AR, Valento M (2015) Death following intentional ingestion of e-liquid. Clin Toxicol (Phila) 53: 914-916.
- Brownson EG, Thompson CM, Goldsberry S, Chong HJ, Friedrich JB, et al. (2016) Explosion Injuries from E-Cigarettes. N Engl J Med 375: 1400-1402. [Crossref]
- Zhenchevskii RA (1975) Postoperative hernias and adhesions in the abdominal cavity. Vestn Khir Im I I Grek 115: 45-49. [Crossref]
- Bhatta DN, Glantz SA (2020) Association of E-cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med 58: 182-190.
- Ghosh A, Coakley RC, Mascenik T (2018) Chronic e-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med 198: 67-76.
- Bowler RP, Hansel NN, Jacobson S, Graham Barr R (2017) Electronic Cigarette Use in US Adults at Risk for or with COPD: Analysis from Two Observational Cohorts. J Gen Intern Med 32: 1315-1322. [Crossref]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim J-H, et al (2015). Exposure to electronic cigarettes impairs pulmonary anti-bacterial and antiviral defenses in a mouse model. PLOSE ONE 10e0116861.
- García-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, et al (2016). Chronic electronic cigarette exposure in mice induced features of COPD in a nicotine-dependent manner. Thorax 71: 1119-1129.
- Crotty Alexander LE, Drummond CA, Hepokoski M, Anuja V, Malhotra A, et al (2018). Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 314: R834-R847.
- Moretto N, Volpi G, Patore F, Facchinetti F (2012) Acrolein effects in pulmonary cells: relevance to chronic obstructive pulmonary disease. Ann NY Acad Sci 1259: 39-46.
- Hajek P, Phillips-Walker A, Przulj I, Pesola F, Smith KM, et al (2019). A randomized trial of E-cigarettes versus nicotine replacement therapy. N Engl J Med 380: 629-637.
- Stanbrook MB (2019) Vaping-associated lung illnesses, highlights risk to all user of electronic cigarettes. CMAJ 191: E1319-E1320.
- Halper SD, Harbay MP, Saulsigiver K, Brophy C, Thoxel AB, et al (2018). A pragmatic trial of E-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med 378: 2302-2310.
- Wahba P (2014) US. E-cigarette sales seen rising 24.2% per year through 2018. Fortune.
- Fairchild AL, Lee JS, Bayer R, Curran J (2018) E-Cigarettes and the Harm-Reduction Continuum. N Engl J Med 378: 216-219. [Crossref]
- Belluz J (2019) Juul allegedly 1 million contaminated vaping products. Vox Media 2019 October 30.
- www.fda.gov/news-events/press-announcements/fda-warns-juul-labs-marketing-unauthorized-modified-risk-tobacco-products-including-outreach-youth
- Weeks C (2020) Health Canada considers allowing e-cigarette companies to promote harm-reduction- benefits. Globe and Mail (Toronto).




