Objective: To determine the concordance between the results obtained by the premature infant pain profile (PIPP) with those of the newborn infant parasympathetic evaluation (NIPE) index.
Methods: This transversal study was conducted to assess and compare two diagnostic tests, the PIPP and the NIPE index. Using a prospective cohort composed of 142 records of very low birth weight infants, with gestational age 27-37 weeks. The data thus obtained were then subjected to an analysis of concordance to assess the value of the PIPP and the NIPE index as diagnostic tests.
Results: For the newborns aged 30 weeks or less, the mean values and the interquartile range were lower than for the more mature newborns, according to the NIPE index. The mean PIPP score was significantly higher for the newborns aged over 30 weeks than for those with a lower gestational age. The NIPE index was measured at baseline and at 5, 10, 15 and 20 minutes after the painful stimulus. These scores decreased during the first 5 minutes after the intervention. However, at 20 minutes, while the scores for the more mature newborns had returned to their baseline values, no such recovery was observed in the newborns aged less than 30 weeks.
Conclusions: The NIPE index is useful for assessing acute pain in the premature newborn. However, its reference values should be adapted to reflect the gestational age of the newborn. We think it is a useful tool in the care of the premature newborn.
Pain; Newborn; Premature infant; Newborn Infant Parasympathetic Evaluation; Premature Infant Pain Profile.
Recent multidisciplinary advances in studies of the pain experienced by newborns have greatly enhanced our understanding of the physiological and epidemiological mechanisms involved and of the impact thus produced on the development of the brain.
Newborn infants with a gestational age (GA) of less than 32 weeks, when subjected to procedures that are traditionally considered painful, may not present a visible behavioural response, due to certain neuroanatomical circumstances [1]. Thus, around week 30 of GA, the myelination of the pain-transmitting fibres between the brainstem and the thalamus concludes, but the descending, inhibitory and modulating pathways of pain intensity remain immature until about week 48. Although pain neurotransmitters (such as substance P and somatostatin) are abundantly present at birth, pain modulators such as dopamine and serotonin are much less present before week 40 of GA [2]. The anatomical and physiological immaturity of very low birthweight infants (VLBW) makes them more vulnerable to stressful and/or painful procedures, which is the case of most diagnostic or therapeutic procedures performed in a NICU. It has been estimated that the pain threshold of a newborn infant is 30-50% lower than that of an adult, and therefore newborns’ tolerance to pain is considerably lower [3].
After delivery, the brain of the premature newborn develops rapidly. However, this development may be negatively affected by pain and/or neonatal stress. Painful and tactile stimuli are known to provoke specific haemodynamic responses in the somatosensory cortex, which means that even very immature neonates have conscious sensory perceptions of pain, similar to that experienced by full-term infants [4]. In this respect, the long-term repercussions of repeated nociceptive stimuli might be extrapolated from data obtained from animal experimentation. Tests in rats have shown that repetitive neonatal pain causes neuronal death in cortical and subcortical areas, by an excitotoxicity mechanism, which suggests that pain may have a generalised effect on the developing brain [5]. Furthermore, an association has been observed between the number of skin punctures (by lancet) received by premature newborns and their restricted cognitive and motor development [6,7]. These studies have presented as neuroanatomical evidence a significant decrease in the white matter and in the maturation of the subcortical grey matter, aspects that have been related to an increased expression of excitatory NMDA receptors [8] in response to pain. Other studies have shown that the blockade of these glutamate-associated receptors can prevent neuronal death by excitotoxicity [2,9,10].
The present study analyses the values obtained by the Newborn Infant Parasympathetic Evaluation (NIPE) index of the newborn infant at different gestational ages in a non-stressful baseline situation and during nociceptive procedures, contrasting these results with the assessments of the PIPP scale in stressful situations arising from procedures commonly performed in a NICU. The fundamental study aim is to analyse the concordance of the NIPE index with other validated pain assessment scales when used for VLBW premature infants.
This transversal study was made of diagnostic tests performed on a prospective cohort of 142 records of VLBW premature infants admitted to the NICU at the San Cecilio University Hospital (Granada, Spain). In all cases, an uninterrupted electrocardiogram (ECG) record was obtained, and the NIPE index was registered from 1 February 2016 to 30 February 2019. The GA of the newborn infants was 27-37 weeks. The records were taken sequentially and included at least 24 hours of each postnatal week until discharge from the NICU.
It has been shown that both environmental noise and lighting influence the physiological constants of the newborn, inducing changes in heart rate, respiration, oxygenation, sleep phases and hormonal alterations, and producing desaturation and increased intracranial pressure in very unstable children. It is assumed that all external conditions and forces potentially influence developing organisms [11], which is why the vast majority of neonatal units ensure a meticulous control of noise levels and light exposure, using appropriate instruments to measure these parameters.
Newborn Infant Parasympathetic Evaluation (NIPE)
The analysis of heart rate variability is a non-invasive means of evaluating cardiac regulation by the autonomic nervous system [12] The spectral analysis of ECG data generates three components of clinical interest: a) low frequency component (LF: 0.04-0.15 Hz), mainly related to sympathetic activity; b) high frequency component (HF: 0.15-1 Hz), related to parasympathetic activity [13]; and c) the ratio between the two (LF/HF), which is proposed as an index of autonomic balance. In adults, at high frequencies (>0.15 Hz), pain, anxiety or fear are accompanied by a decrease in heart rate variability, which would indicate a decrease in parasympathetic tone during nociceptive stimulation and/or unpleasant emotions [14,15]. In newborns, although few studies in this regard have been conducted, some authors have reported a decrease in the influence of the parasympathetic tone during nociceptive stimuli, suggesting that this tone could be evaluated as an objective means of recording acute and chronic pain experienced by the newborn [16,17].
Measuring the newborn infant’s wellbeing via a NIPE monitor (Mdoloris Medical Systems, Loos, France) provides a continuous, normalised record of parasympathetic tone (pΣ). In cardiac activity, changes in the tone are reflected in the sinus node as variances in the intervals between successive R waves in the ECG. To facilitate analysis, therefore, the pΣ component is obtained, filtered and normalised. The NIPE index monitor is connected to the ECG monitor, which enables data to be obtained in a non-invasive way. The NIPE monitor performs a sampling of the RR series by measuring the area generated by the respiratory pattern. This approach is based on the understanding that the higher the parasympathetic tone, the greater the area generated by the ventilatory cycle. The NIPE index is expressed on a scale ranging from 0 to 100. It is an objective evaluation without interobserver variability. It reflects the activity of the parasympathetic system and gives a proportionate reading of the parasympathetic tone compared to that of the autonomic nervous system.
Premature Infant Pain Profile (PIPP)
In the neonate, responses to pain are associated with changes in behaviour, physiology and metabolism. Therefore, the pain experienced can be assessed by collecting information on each of these three facets. Newborns with a lower GA are less likely to demonstrate objective responses to pain, due to their incomplete neuroanatomical development. Facial expression in response to painful procedures has been widely studied and shown to be different from responses to other tactile stimuli [18]; therefore, it is considered the most reliable and consistent indicator of nociception, both for full-term term and for premature infants [19]. Facial expressions include a wide range of manifestations, such as grimaces, bulging and puckered eyebrows, tightly closed eyes, nasolabial wrinkles, open and pursed lips, hollowed tongue, trembling chin, or agitation. The majority of pain assessment instruments use facial activity as one of the main indicators of pain. Gross motor responses such as arm, leg and trunk movements, finger separation and movements of withdrawal from the painful stimulus have also been used to assess pain levels. However, infants with VLBW or who are critically ill may be unresponsive to a painful stimulus. Crying (usually assessed by its presence or absence) is another element that may be considered in the evaluation of pain, and changes in crying patterns have been related to pain intensity. On the other hand, up to 20% of premature newborns do not cry during or after nociceptive stimuli, and prior exposure to pain has been associated with altered behavioural responses and decreased autonomic reactivity to new painful stimuli, which leads to reduced PIPP scores being obtained.
Physiological responses to painful stimuli include increased heart rate, respiratory rate, blood pressure, intracranial pressure and palmar sweating. In addition, the pain is accompanied by a decrease in transcutaneous oxygen saturation, vagal tone and peripheral blood flow. Autonomic responses to pain include changes in skin colour, together with nausea, vomiting, hiccups, diaphoresis, palmar sweating and dilated pupils [20]. Nevertheless, physiological indicators alone cannot be used to determine pain levels, due to the lack of sensitivity and specificity of these indicators, especially for premature infants, whose physiological response is less apparent than in full-term infants.
Method
For this study, the PIPP scale was always scored by the same nurse, and the date and time noted, whenever a potentially painful or stressful manipulation (such as bladder or orogastric catheter, venipuncture, capillary puncture to determine gases or glycaemia, or ophthalmological examination) was performed. For our purposes, the PIPP scale is considered the “gold standard”, with a reported reliability of 93-96% for preterm infants [21,22]. All PIPP scores obtained were contrasted with those derived from the NIPE index at the same time.
The criteria applied for the newborns’ inclusion in the study group were haemodynamic stability and not having received vasoactive drugs or sedated analgesia. Exclusion criteria were the presence of seizures documented by amplitude integrated electroencephalography, necrotic enterocolitis, persistent ductus arteriosus or malformations.
Ethical questions
The newborns’ parents/guardians were informed that they could withdraw the newborn from the study at any time. The neonatologist responsible for the patient’s care was also authorised to do so. The study was approved by the Provincial Bioethics Committee and complied with accepted standards of good clinical practice.
Sampling and statistical analysis
In calculating the sample size, we assumed a maximum PIPP sensitivity of 96% in assessing pain in the premature infant, and that the NIPE index would achieve at least the same sensitivity. Therefore, for a confidence interval of 95% and an accuracy of 5%, at least 119 datasets would be needed.
The NIPE and PIPP results obtained were incorporated into an Excel file and then exported to a database in SPSS v.20.0. The median values and the interquartile range were determined, and Student’s t-test was performed. The concordance between the NIPE and the PIPP results in evaluating the pain experienced by the premature infant was analysed by the statistical method proposed by Passing and Bablok, which consists of performing a nonparametric estimation of the orthogonal regression slope (Lin’s concordance correlation coefficient). This method enabled us to determine whether there were constant or proportional differences between the two measurement methods, according to whether the 95% CI of the constant in the regression slope included the value 0 and whether the 95% CI of the slope did not include the value 1. The Lin coefficient was then used to determine the level of agreement between the two measurement methods. All statistical analyses were carried out using the SPSS 20.0 statistical package.
To analyse the baseline NIPE index, 142 24-hour records were collected, corresponding to newborns with 27-37 weeks cGA. These records were obtained at chronological ages of 1-40 days. Sixty newborns were excluded from the analysis, due to their need for vasoactive drugs, sedo-analgesia, intubation or invasive mechanical ventilation. Table 1 shows the characteristics of the newborns included in the analysis.
Table 1: Gestational and neonatal characteristics.
*Median (IQR);
A total of 142 painful procedures were performed on these infants: 96 (67.6%) capillary punctures with lancet for gases or glycaemia, 34 (23.9%) venipunctures, nine (6.3%) ophthalmological examinations, two (1.4%) bladder catheterisations and one (0.7) %) nasogastric intubation. In all cases, appropriate measures were taken to mitigate the pain, but neither anaesthetic creams nor oral sucrose were used in any case.
Figure 1 shows the distribution of percentiles for the NIPE index in newborns below and above 30 weeks corrected gestational age (cGA). The newborns with less than 30 weeks cGA presented lower mean values for the NIPE index and the corresponding IQR (Table 2). This finding is useful for inferring reference values above which the existence of pain should be suspected.
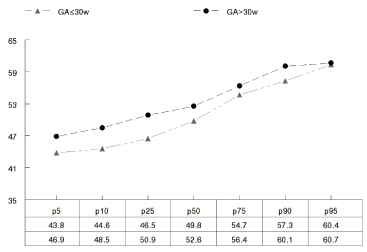
Figure 1. Distribution and percentiles of the NIPE index in newborns under and over 30 weeks corrected gestational age.
Table 2: Median and interquartile interval (IQR) of the NIPE index in baseline situation of newborns with corrected gestational age below and above 30 weeks.
*Median (IQR); cGA: Corrected gestational age
Our analysis of the potentially painful procedures to which the newborns were subjected indicates that skin punctures with lancet produced lower PIPP scores, and that there were no substantial differences in the PIPP score between newborns with less than or more than 30 weeks cGA. The mean PIPP score for ‘All procedures’ or specifically for venipunctures was significantly higher in the newborns older than 30 weeks cGA. Figure 2 shows the baseline NIPE index results, and those obtained at 5, 10, 15 and 20 minutes after the painful procedure was performed. The NIPE index scores were lower at 5 minutes, among both the younger and the older newborns. At 20 minutes, among those under 30 weeks cGA, the NIPE index scores had still not returned to the baseline values (Figure 2c). The mean PIPP score among the newborns over 30 weeks cGA after a painful procedure was greater than 8 points, a value at which the existence of moderate to severe pain is assumed [21,23] On the contrary, among the newborns under 30 weeks cGA, the same potentially painful procedures produced an average value of less than 8 on the PIPP scale (Figure 2c). These data contrast with the NIPE index values, which show, in both cGA groups, a proportional decrease at five minutes after the painful stimulus.
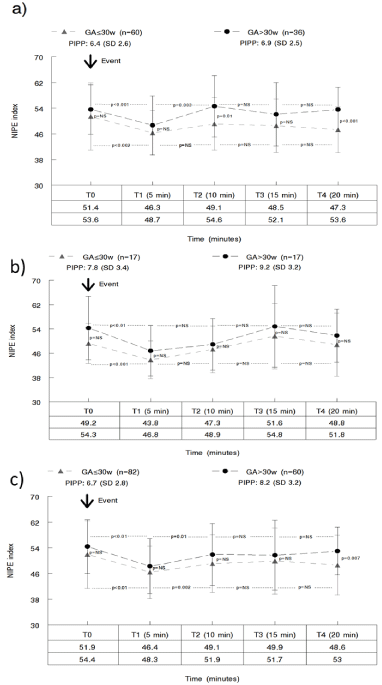
Figure 2. Evolution of the NIPE index from the baseline value and at 5, 10, 15 and 20 minutes after the painful stimulus: a) skin puncture, b) venipuncture, c) all procedures.
The analysis of concordance by the Lin coefficient (-0.037; p<0.01) showed there was no agreement between pain assessment according to the PIPP scale and the NIPE index scores. Comparison of the two measurement methods by the Passing and Bablok regression method obtained a constant value of -36 (95% CI: -83.0 to -19.6). Since this confidence interval does not include the value 0, we assume that the two measurement methods present constant differences. Furthermore, the regression slope has a value of 6 (95% CI: 3.6 to 13). This confidence interval does not include the value 1, and therefore we assume that the two methods present proportional differences.
Our results indicate that the sensitivity of the NIPE index to acute pain in VLBW newborns aged 30 weeks cGA or less is comparable to that observed in newborns with a higher cGA. In addition, the period of recovery after a nociceptive stimulus is longer for the newborns under 30 weeks cGA, and the baseline value of the NIPE index is also lower among this group of newborns.
Cremillieux et al. [24], studied a group of 29 premature newborns and reported that the NIPE index and the PIPP scale are non-concordant methods for assessing pain in preterm infants. This statement coincides with our findings. In contrast, Faye et al. [16], studied 28 newborns with at least 34 weeks GA, and observed concordance between the EDIN scale of neonatal pain and discomfort and the NIPE index in the assessment of postoperative pain.
Alexandre et al. [25] studied newborns with 33 weeks GA and observed good concordance between the comfort level of the newborn and the NIPE index. Our results show that the response of the VLBW newborn to nociceptive stimuli, in those under 30 weeks cGA, produced lower scores on the PIPP scale, with mean NIPE results after 20 minutes that were significantly lower than the baseline values. This observation suggests that newborns under 30 weeks cGA may experience a longer-lasting state of discomfort after being subjected to a nociceptive stimulus.
In the present study, the PIPP scale was taken as the gold standard because it is internationally validated for the assessment of neonatal pain [21,22]. Nevertheless, we cannot rule out the possibility that the lack of concordance between the PIPP and the NIPE index results may be because the former relies on a subjective individual evaluation, which we have tried to minimize by being a single evaluator who scores the scale PIPP. Bellieni et al. [26] detected interobserver variability in the scoring of pain assessment scales, and highlighted the difficulty of interpreting the facial response of newborns, and of VLBW infants in particular, to nociceptive stimuli.
The lack of concordance between an “objective” method of assessing parasympathetic tone, such as the NIPE index, and consequently the state of comfort of the newborn, and other “more subjective” methods of pain assessment such as the PIPP scale, led us to consider the importance of correctly determining pain intensity in order to understand its long-term repercussions. In recent years, there has been growing interest in addressing the problem of determining the pain experienced by newborns, especially those with VLBW; not only because of the unfavourable consequences of pain on neurological development, but also because it is ethically reprehensible not to employ all the means at our disposal to detect and mitigate any pain caused to the newborn.
The behavioural response to pain is related to GA and to postnatal age, while responses in preterm VLBW newborns are attenuated, in relation to those observed in full-term infants [27]. The latter aspect is particularly worrisome, since it suggests that repeated painful stimuli in VLBW newborns might overactivate groups of immature neurons, which are more susceptible to excitotoxic damage [8]. This hypothesis has led some authors to believe that painful procedures may have long-lasting effects on the future neurological and psychosocial development of immature newborns [28].
Buyuktiryaki et al. [29] recorded the NIPE index scores of 23 newborns aged 33-35 weeks of GA who had undergone pneumothorax drainage with thoracotomy. This technique routinely requires analgesia (with fentanyl) both during the procedure and while the chest tube remains inserted. NIPE monitoring enabled continuous assessment of the comfort status of the newborn during the analgesia process. During the 12 hours following the procedure, mean NIPE index scores of over 60 were recorded. As can be seen in Figure 1, a NIPE index score of 60 in newborns aged over 30 weeks GA corresponds to the 95th percentile of the distribution. Very immature newborns are often exposed to sedatives and/or anaesthetics. However, we believe, in line with other researchers in this field [23,30,31] that prolonged use of such analgesics might be associated with neurodegeneration of the immature brain. This neuroapoptosis induced by anaesthetics could be triggered by a decrease in the cerebral neurotrophic factor, a protein that is necessary for survival, growth and neuronal differentiation. It has been suggested that the activation of GABA or NMDA receptor agonists during periods of cerebral vulnerability may play a role in this regard. Certainly, neurodegenerative effects are closely related to the duration and dose of exposure [30]. In our opinion, the immediate knowledge of the newborn’s comfort status, as provided by use of the NIPE index, provides a yardstick with which to achieve appropriate dosing of analgesia and thus avoid or minimise its side effects.
Strikingly, for premature newborns of less than 30 weeks cGA, the baseline NIPE index scores were lower, while their responses to nociceptive stimuli were comparable to those observed in newborns of higher GA, and this difference in the NIPE index persisted after the pain stimulus was removed. This delay in regaining the baseline level of the NIPE index could indicate that VLBW infants suffer the effects of pain for longer than newborns of higher gestational age. Such a delay in the modulation of pain among infants of less GA was reported previously by Mainous et al. [1], who explained it as arising from the presence of neuroanatomical differences among newborns according to their degree of maturity.
The PIPP scale and the NIPE index are non-comparable methods of assessing the pain experienced by premature newborn infants. According to the NIPE index evaluation, following a nociceptive stimulus, the duration of repercussions on the parasympathetic tone is greater among newborns with a lower cGA. In our opinion, the NIPE index is useful for assessing acute pain in the premature newborn, but its reference values should be adapted to take into account the gestational age.
We thank the nursing staff at the Neonatal Unit for their invaluable assistance in the performance of this study.
J. Uberos designed the study protocol, performed the statistical analysis, interpretation of the data, and wrote the manuscript and critically reviewed and revised the manuscript. M. Molina-Oya was involved in the development of the registry, performed the statistical analysis and the interpretation of the
data.
Conflict of interest
The authors have no relevant conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with abovementioned standards and has been approved by the institutional ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
- Mainous RO, Looney S (2007) A pilot study of changes in cerebral blood flow velocity, resistance, and vital signs following a painful stimulus in the premature infant. Adv Neonatal Care 7: 88-104. [Crossref]
- Fitzgerald M (2005) The development of nociceptive circuits. Nat Rev Neurosci 6:507-520. [Crossref]
- Hatfield LA (2014) Neonatal pain: What's age got to do with it? Surg Neurol Int 5: S479-S489. [Crossref]
- Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ (2006) Pain activates cortical areas in the preterm newborn brain. Pain 122: 109-117. [Crossref]
- Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, et al. (2007) Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res 62: 283-290. [Crossref]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, et al. (2012) Procedural pain and brain development in premature newborns. Ann Neurol 71: 385-396. [Crossref]
- Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, et al. (2009) Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 143: 138-146. [Crossref]
- Chahal H, D'Souza SW, Barson AJ, Slater P (1998) Modulation by magnesium of N-methyl-D-aspartate receptors in developing human brain. Arch Dis Child Fetal Neonatal Ed 78: F116-F120. [Crossref]
- Manning SM, Talos DM, Zhou C, Selip DB, Park HK, et al. (2008) NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci 28: 6670-6678. [Crossref]
- Rabinowicz T, de Court, Petetot JM, Xi G, de los RE (1996) Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J Neuropathol Exp Neurol 55: 320-328. [Crossref]
- Marriner Tomey A (2003) Modelos y teorias de enfermería. Elsevier.
- Benarroch EE (2001) Pain-autonomic interactions: a selective review. Clin Auton Res 11: 343-349. [Crossref]
- Parati G, Mancia G, Di RM, Castiglioni P (2006) Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol (1985 ) 101: 676-678. [Crossref]
- Appelhans BM, Luecken LJ (2008) Heart rate variability and pain: associations of two interrelated homeostatic processes. Biol Psychol 77: 174-182. [Crossref]
- Miu AC, Heilman RM, Miclea M (2009) Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Auton Neurosci 145: 99-103. [Crossref]
- Faye PM, DE JJ, Logier R, Kuissi E, Jeanne M, et al. (2010) Newborn infant pain assessment using heart rate variability analysis. Clin J Pain 26: 777-782. [Crossref]
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP (2000) Biobehavioral pain responses in former extremely low birth weight infants at four months' corrected age. Pediatrics 105: e6. [Crossref]
- Bozzette M (1993) Observation of pain behavior in the NICU: an exploratory study. J Perinat Neonatal Nurs 7: 76-87. [Crossref]
- Schiavenato M, von Baeyer CL (2012) A Quantitative Examination of Extreme Facial Pain Expression in Neonates: The Primal Face of Pain across Time. Pain Res Treat 2012: 251625. [Crossref]
- Cong X, Ludington-Hoe SM, Walsh S (2011) Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol Res Nurs 13:204-216. [Crossref]
- Gibbins S, Stevens BJ, Yamada J, Dionne K, Campbell-Yeo M, et al. (2014) Validation of the Premature Infant Pain Profile-Revised (PIPP-R). Early Hum Dev 90: 189-193. [Crossref]
- Stevens B, Johnston C, Petryshen P, Taddio A (1996) Premature Infant Pain Profile: development and initial validation. Clin J Pain 12: 13-22. [Crossref]
- Tremblay S, Ranger M, Chau CMY, Ellegood J, Lerch JP, et al. (2017) Repeated exposure to sucrose for procedural pain in mouse pups leads to long-term widespread brain alterations. Pain 158: 1586-1598. [Crossref]
- Cremillieux C, Makhlouf A, Pichot V, Trombert B, Patural H (2018) Objective assessment of induced acute pain in neonatology with the Newborn Infant Parasympathetic Evaluation index. Eur J Pain 22: 1071-1079. [Crossref]
- Alexandre C, DE JJ, Rakza T, Mur S, Carette D, et al. (2013) Impact of cocooning and maternal voice on the autonomic nervous system activity in the premature newborn infant. Arch Pediatr 20: 963-968. [Crossref]
- Bellieni CV, Cordelli DM, Caliani C, Palazzi C, Franci N, et al. (2007) Inter-observer reliability of two pain scales for newborns. Early Hum Dev 83: 549-552. [Crossref]
- Cong X, McGrath JM, Cusson RM, Zhang D (2013) Pain assessment and measurement in neonates: an updated review. Adv Neonatal Care 13: 379-395. [Crossref]
- Olischar M, Davidson AJ, Lee KJ, Hunt RW (2012) Effects of morphine and midazolam on sleep-wake cycling in amplitude-integrated electroencephalography in post-surgical neonates >/= 32 weeks of gestational age. Neonatology 101: 293-300. [Crossref]
- Buyuktiryaki M, Uras N, Okur N, Oncel MY, Simsek GK, et al. (2018) Evaluation of prolonged pain in preterm infants with pneumothorax using heart rate variability analysis and EDIN (Echelle Douleur Inconfort Nouveau-Ne, neonatal pain and discomfort scale) scores. Korean J Pediatr 61: 322-326. [Crossref]
- Istaphanous GK, Loepke AW (2009) General anesthetics and the developing brain. Curr Opin Anaesthesiol 22: 368-373. [Crossref]
- Mancuso T, Burns J (2009) Ethical concerns in the management of pain in the neonate. Paediatr Anaesth 19: 953-957. [Crossref]


