Abstract
Peri-implantitis is characterized by persistent and progressive inflammation of soft tissue associated with infection and loss of supporting bone surrounding a dental implant. Onset can occur early after implantation and progress in a characteristically non-linear accelerating pattern. Clinically, peri-implantitis presents as chronic soft tissue inflammation with increasing probing pocket depths and radiographically visible bone loss compared with baselines. Surgical approaches for peri-implantitis are, of necessity, multifaceted and typically include restoration of the affected ridge via guided bone regeneration (GBR) using bone graft substitutes. Although there is currently no consensus on the most ideal graft type for the treatment of peri-implantitis, allograft bone has emerged as a safe and viable option. Here, we present a case describing the use of an allograft cortico-cancellous particulate mix combined with autogenous bone and allograft moldable demineralized bone fibers to facilitate GBR in the treatment of peri-implantitis. At 6 months postoperative, CT scans showed favorable bone density with mature characteristics and optimal thickness and height for adequate three-dimensional implant placement. Creation of a full-thickness flap confirmed good bone appearance with visible vascularization, rim thickness, and maturation immediately prior to successful implant and bridge placement, which was maintained at 24 months. This case further supports the clinical effectiveness of allograft mineralized particulate with allograft demineralized bone fibers resulting in radiographic bone gains and resolution of peri-implantitis.
Keywords
allograft, guided bone regeneration, moldable demineralized fibers, particulate, peri-implantitis
Introduction
Peri-implantitis is characterized by persistent and progressive inflammation of soft tissue associated with infection and loss of supporting bone surrounding a dental implant [1]. Onset can occur early after implantation and progress in a characteristically non-linear accelerating pattern. Clinically, peri-implantitis presents as chronic soft tissue inflammation with increasing probing pocket depths and radiographically visible bone loss compared with baselines. While treatment approaches may vary according to implant position, soft tissue characteristics, and defect configuration, among others, [2,3] clinical evidence suggests that surgical interventions generally provide more effective and predictable outcomes versus nonsurgical methods [4]. Surgical approaches for peri-implantitis are, of necessity, multifaceted and typically include restoration of the affected ridge via guided bone regeneration (GBR) using bone graft substitutes [3].
Although there is currently no consensus on the most ideal graft type for restoration of bone in peri-implantitis, bovine-derived xenografts and allografts derived from deceased consented human donors are among the most widely studied [3]. Autogenous bone is also often utilized due to its ability to supply all three essential components of bone remodeling: osteoconductivity, osteoinductivity, and osteogenicity [5]. However, autograft bone has limited availability, and its recovery requires an additional surgical site and procedure, thus increasing operative time, cost, and postoperative pain [6]. Further, the quality of autograft bone may be limited by comorbid patient-related factors, such as age, systemic conditions, and lifestyle risks [5]. Xenograft bone, while widely available, can only provide an osteoconductive matrix [3]. Thus, allograft bone has emerged as a safe and viable option and, depending on its processing, can provide from one to all three of the necessary components of bone healing [7].
Among these, the more traditional allograft cortical and cancellous particulates can provide an osteoconductive combination of open trabecular architecture and dense particles to allow healing through “creeping substitution”, [8], making them ideal for space maintenance or graft extension. Allograft demineralized bone matrices (DBMs) are another option, which increase the osteoinductive potential of the more traditional allografts by removing a portion of the mineral matrix to expose naturally-occurring osteoinductive proteins embedded within it and promote deposition of new bone [5]. While DBMs are available in many forms, older versions often employ a carrier such as glycerol, starch, or hyaluronic acid to improve handling [9]. However, a newer DBM has been introduced (OraGraft® Prime [F-DBM]; LifeNet Health, Virginia Beach VA USA) that is comprised of 100% interlocking allograft bone fibers to allow similar handling without the use of a carrier [9]. F-DBM has been shown to provide a biohospitable scaffold with a surface morphology that promotes cellular migration, attachment, and spreading to facilitate intercellular connections [9].
Here, we present a case describing the use of an allograft cortico-cancellous particulate mix (OraGraft MD 50/50 [C/C Mix}; LifeNet Health) combined with autogenous bone and F-DBM to facilitate GBR in the treatment of peri-implantitis.
Case presentation
This case report was written in accordance with the World Medical Association Declaration of Helsinki, as revised in 2013. Written informed consent was obtained for publication of case details. This case was performed by the first (AC) and second (SGM) authors at their office-based practice.
A female patient in her 40s presented with suppuration of increasing volume in Quadrant III and reported a 5-year pain-free progressive evolution. The patient was otherwise asymptomatic with no pertinent family or medical history. Clinical examination revealed association of the suppuration with a bridge on implants from Tooth 35 to 37 (Figure 1) and radiographic examination confirmed bone loss characteristic of moderate peri-implantitis (Figure 2). It was thus determined that the original implants would be removed, followed by GBR to facilitate proper three-dimensional placement of new implants.
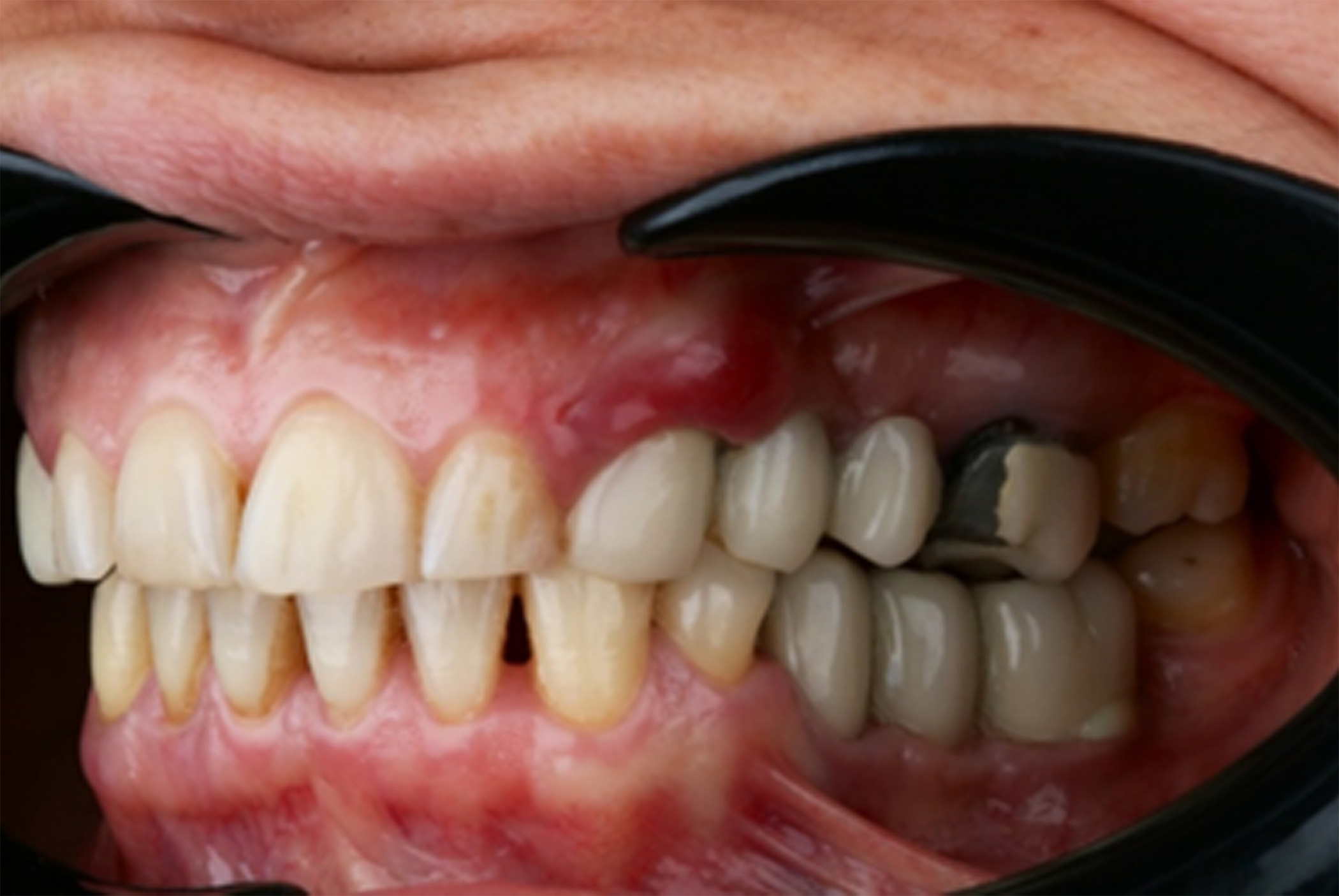
Figure 1. Presenting clinical photograph showing suppuration and increased volume in Quadrant III associated with a bridge on implants from Tooth 35 to 37
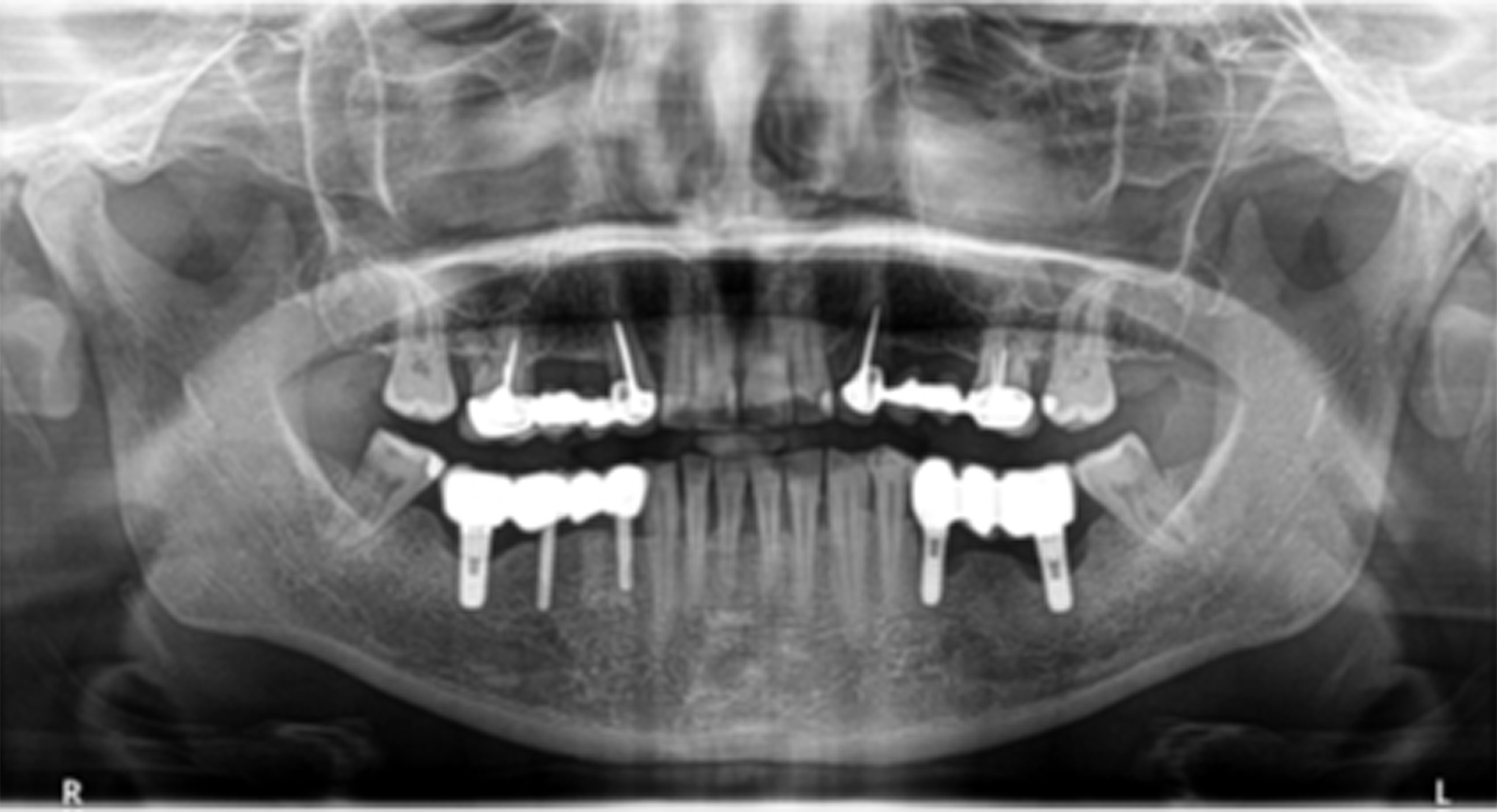
Figure 2. Presenting panoramic radiograph confirmed advanced bone loss characteristic of moderate peri-implantitis
Treatment
Following removal of the implant bridge and creation of a full-thickness flap, vertical and horizontal bone engagement around the original implants was confirmed with an active infection in progress (Figure 3). Both implants were explanted (Figure 4) with subsequent curettage of the bed and application of a local antibiotic (clindamycin).
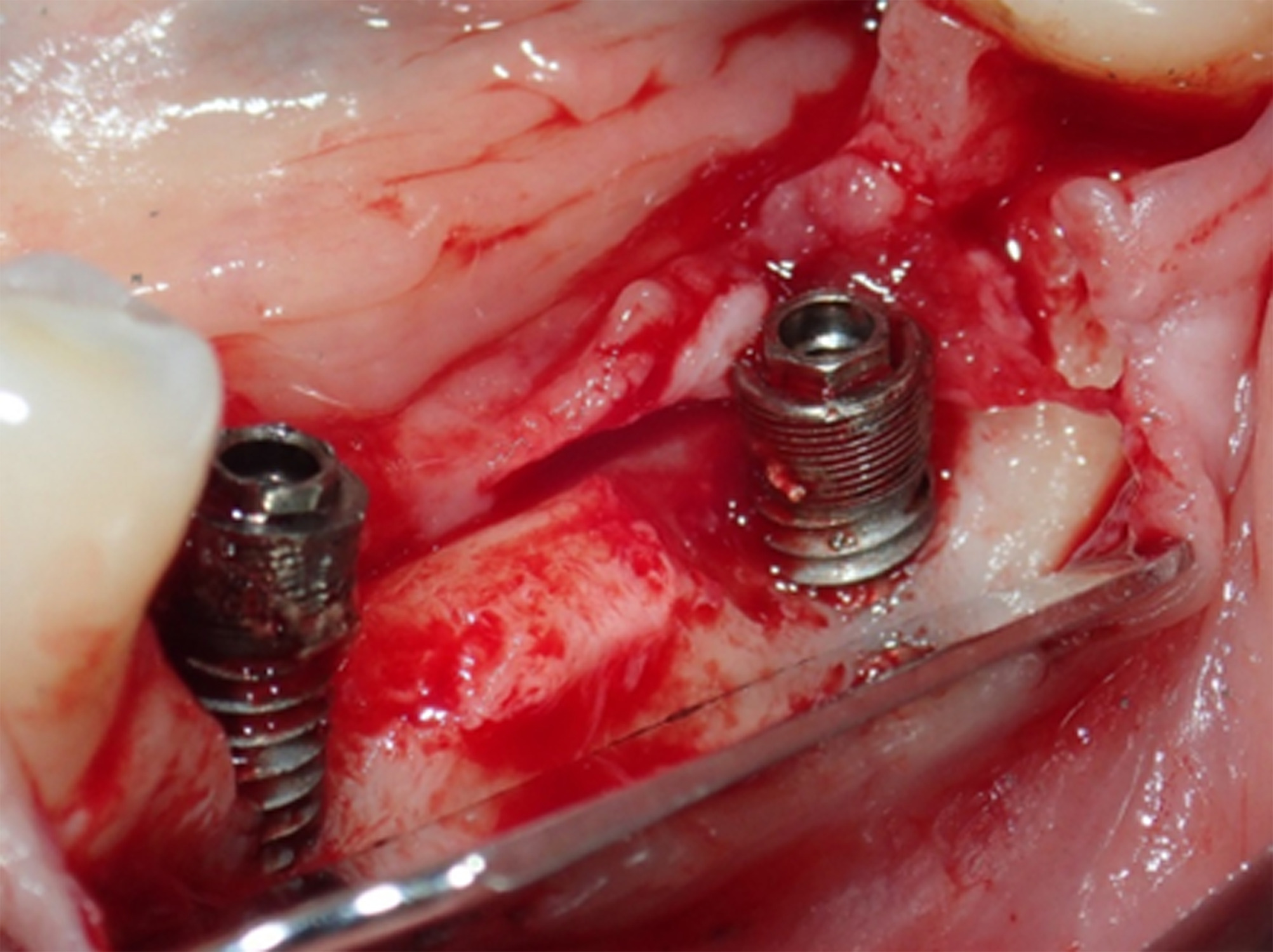
Figure 3. After removing the implant bridge and creating a full-thickness flap, vertical and horizontal bone engagement around both implants was confirmed with an active infection in progress

Figure 4. Due to the infection, both implants were explanted with subsequent curettage of the bed and application of a local antibiotic (clindamycin)
Next, buccal and lingual reaming was carried out to ensure bleeding and adequate irrigation of the bone graft (Figure 5). GBR was facilitated first through a combination of autograft obtained from the mandibular ramus using a SafeScraper® (META; Reggio Emilia, Italy) and C/C Mix (Figure 6). Then, an outer layer of F-DBM was added for extra volume and to contain the underlying particulate (Figure 7). To maintain stability of the bone graft, a Cytoplast™ dense titanium-reinforced PTFE membrane (Osteogenics; Lubbock TX, USA) was fixed with buccal and lingual pins (Figure 8). Finally, an outer layer of bovine pericardial membrane (Nobel Biocare, Kloten, Switzerland) was placed to provide a bilayer of interlaced, multidirectional Type 1 collagen fibers (Figure 9). A combination of horizontal mattress and simple sutures with nonresorbable Cytoplast™ PTFE 3.0 monofilament (Osteogenics) were then used to provide optimal closure of the flap without tension. Perioperative computed tomography (CT) scans confirmed pin and titanium-reinforced placement but, characteristic of regenerative materials, bone particulate and fibers were not radiopaque (Figure 10).
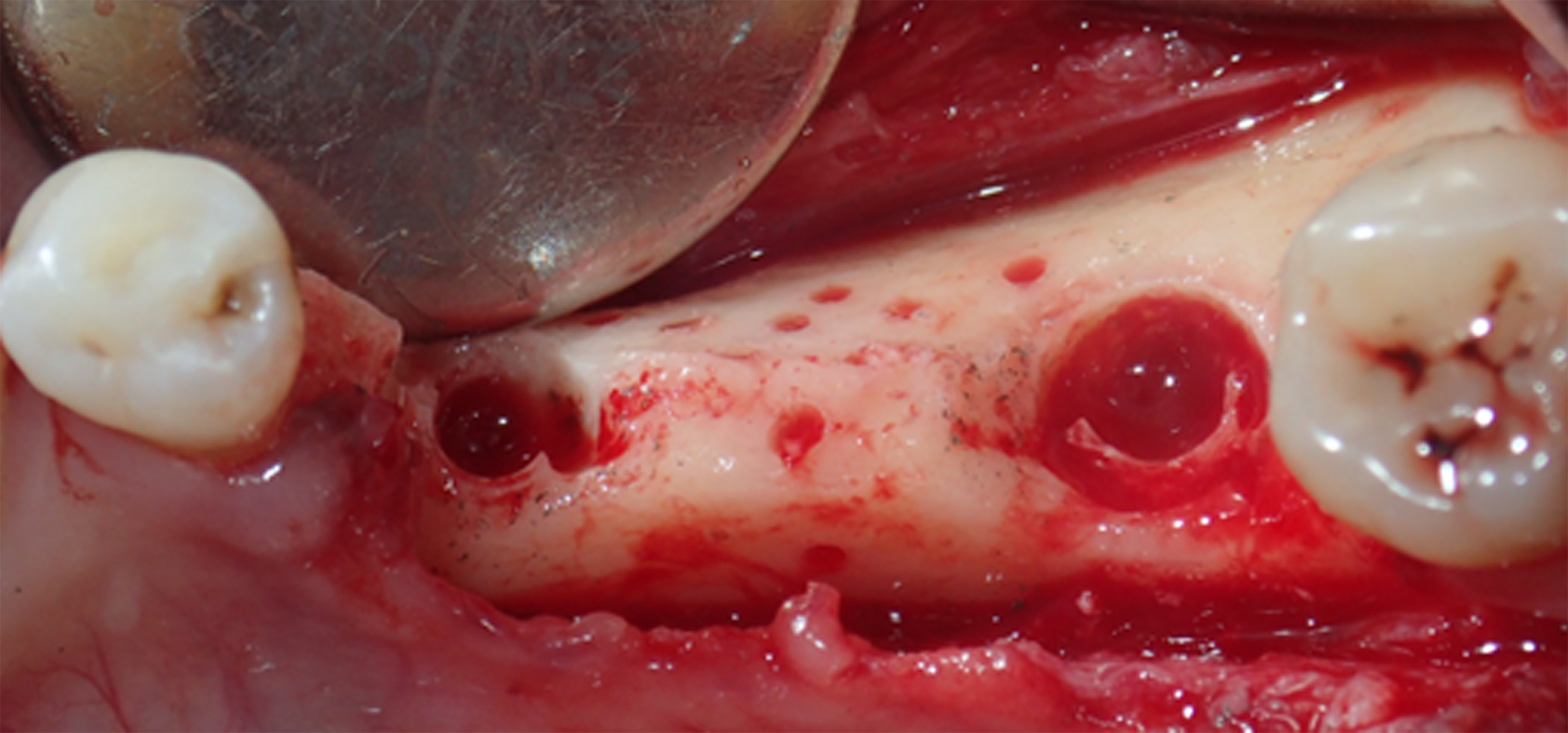
Figure 5. Buccal and lingual reaming was carried out to ensure bleeding and adequate irrigation of the bone graft

Figure 6. Guided bone regeneration was first facilitated through a combination of C/C Mix and autograft obtained from the mandibular ramus
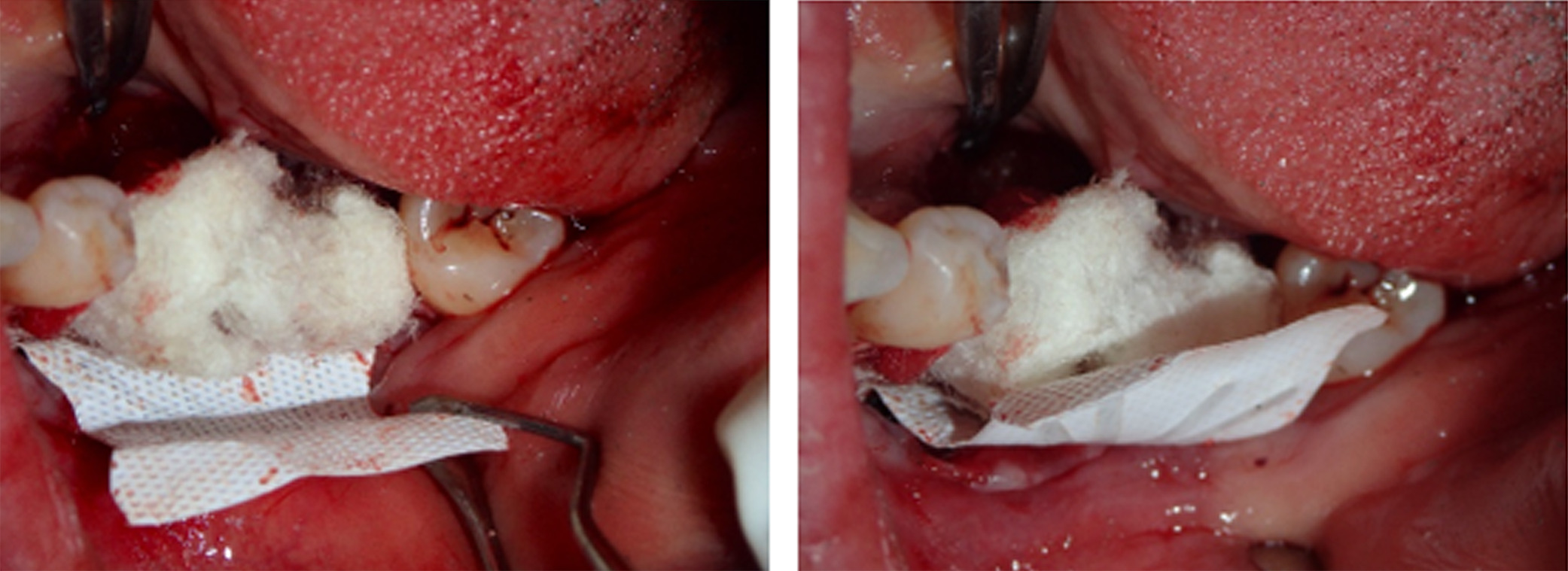
Figure 7. An outer layer of F-DBM was added for extra volume and to contain the underlying particulate
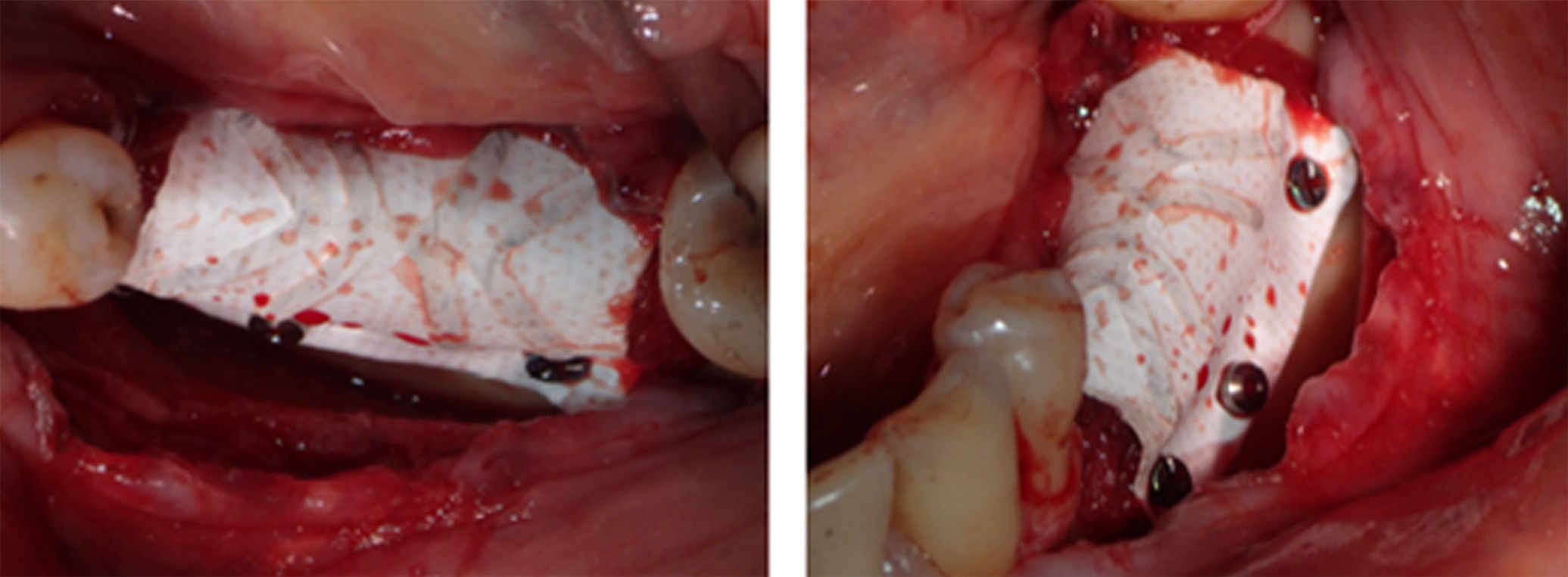
Figure 8. To maintain stability of the bone graft, a Cytoplast dense titanium-reinforced PTFE membrane was fixed with buccal and lingual pins
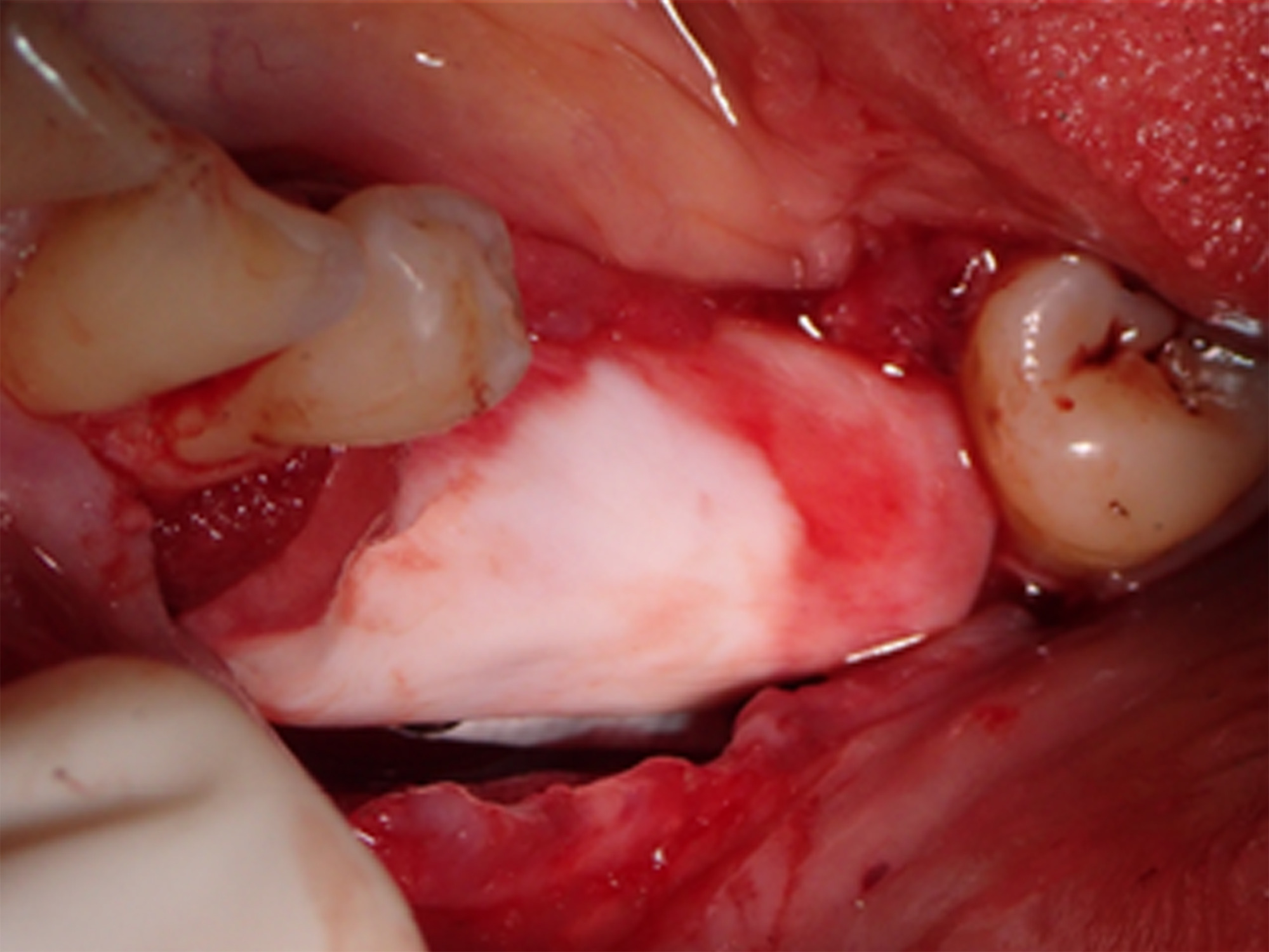
Figure 9. An outer layer of bovine pericardial membrane was placed to provide a bilayer of interlaced, multidirectional Type 1 collagen fibers
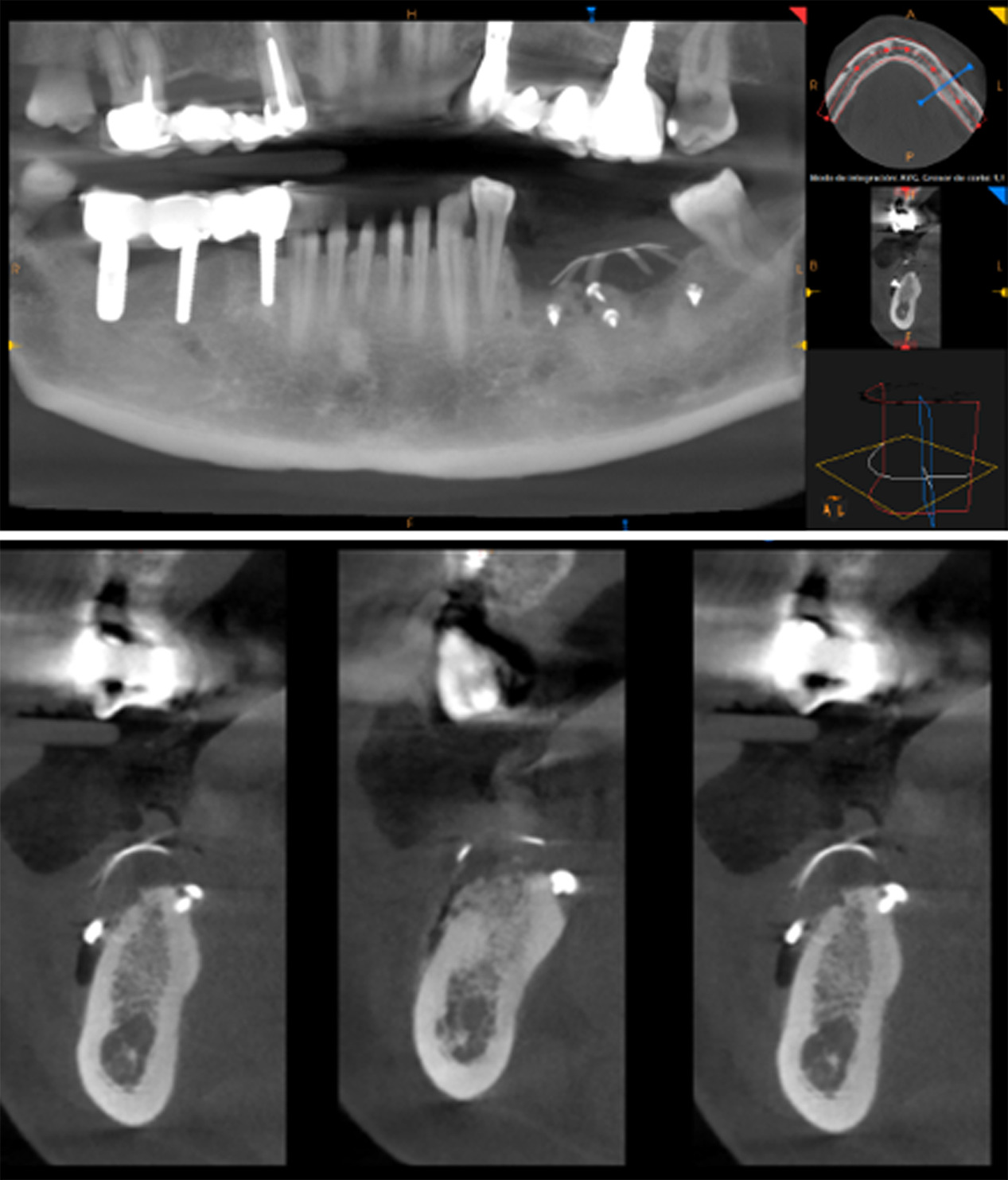
Figure 10. Perioperative CT scan illustrating that the pins were visible, but the grafting material was not. Characteristic of regenerative materials, bone particulate and fibers are not radiopaque
Results
At 3 months postoperative, the patient returned to the office presenting with an area of distal titanium-reinforced membrane exposure adjacent to Tooth 38 without pain, but with bleeding, swelling, and discoloration (Figure 11). The membrane was thus removed (Figure 12), revealing immature newly formed bone tissue with abundant blood supply, indicating that bone regeneration was not compromised by the membrane exposure (Figure 13).
At 6 months postoperative, CT scans showed optimal bone density with mature characteristics and optimal thickness/height for adequate three-dimensional implant placement (Figure 14). Removal of the titanium-reinforced membrane at 3 months postoperative did not appear to have a significant impact on the regenerative process. A full-thickness flap was created for implant placement, revealing a good bone appearance with visible vascularization, rim thickness, and maturation (Figure 15). The implants were placed, achieving favorable primary stability and optimal three-dimensional ridge placement due to the thickness/height achieved from the regenerative procedure (Figure 16). Periapical radiographs following placement of the healing screws confirmed favorable bone-implant interaction (Figure 17). A panoramic radiograph prior to crown placement showed evident hard tissue stability in the regenerated area (Figure 18). Finally, panoramic and periapical radiographs at 24 months postoperative showed healthy, stable bone regeneration and ideal placement of the implants and bridge (Figure 19).
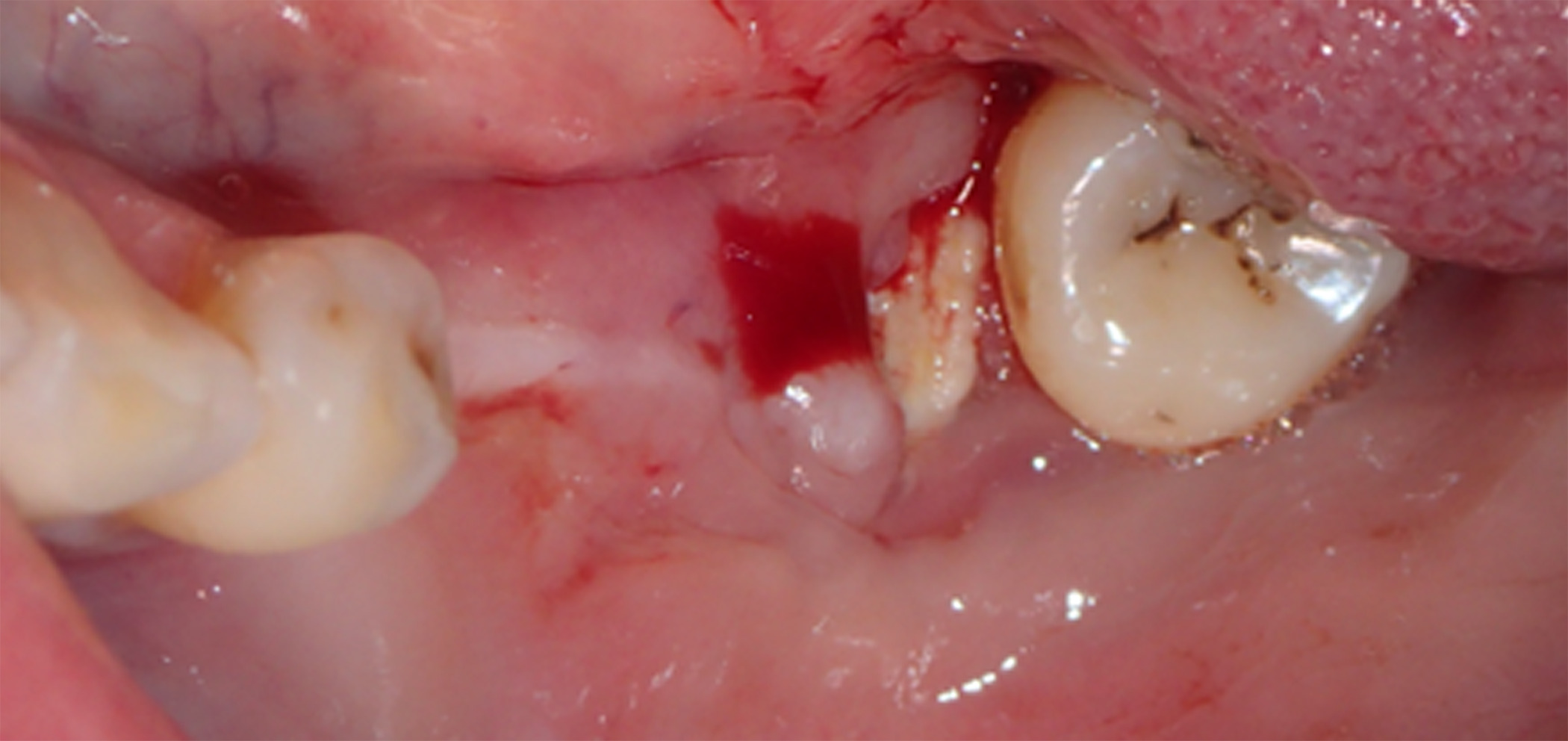
Figure 11. At 3 months postoperative, the patient returned to the office presenting with an area of distal titanium-reinforced membrane exposure adjacent to Tooth 38, without pain, but with bleeding, swelling, and discoloration
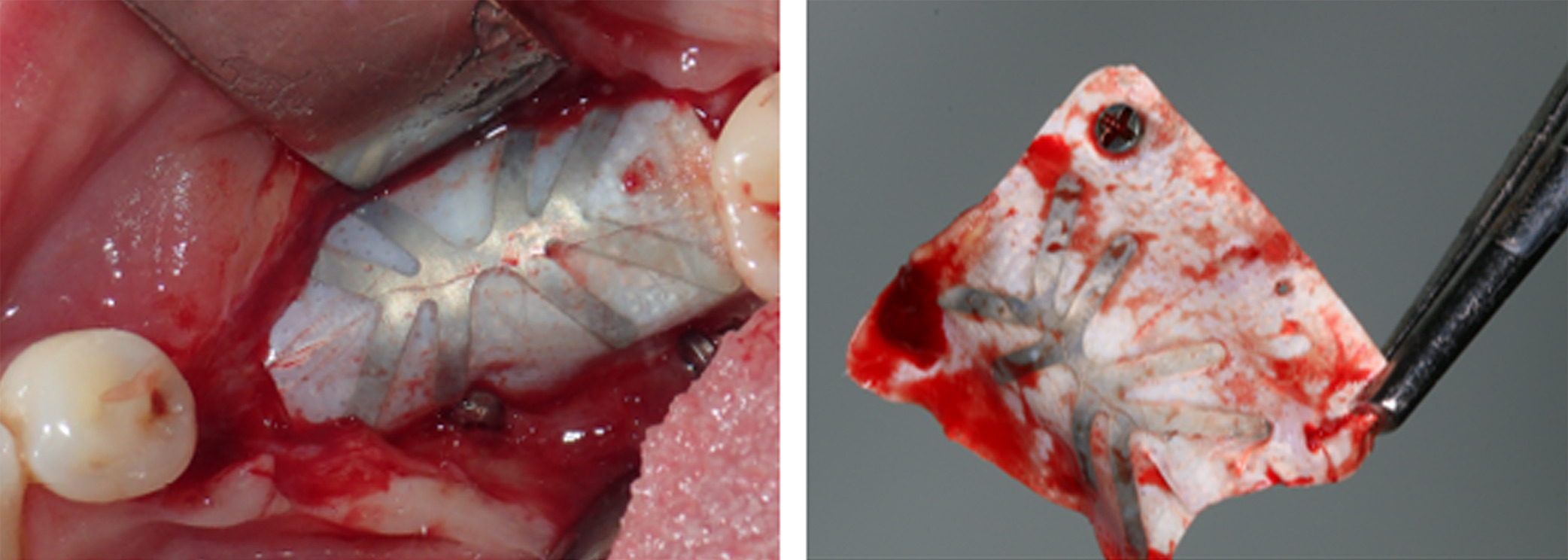
Figure 12. The membrane was thus removed at 3 months postoperative to prevent dissemination of the underlying graft
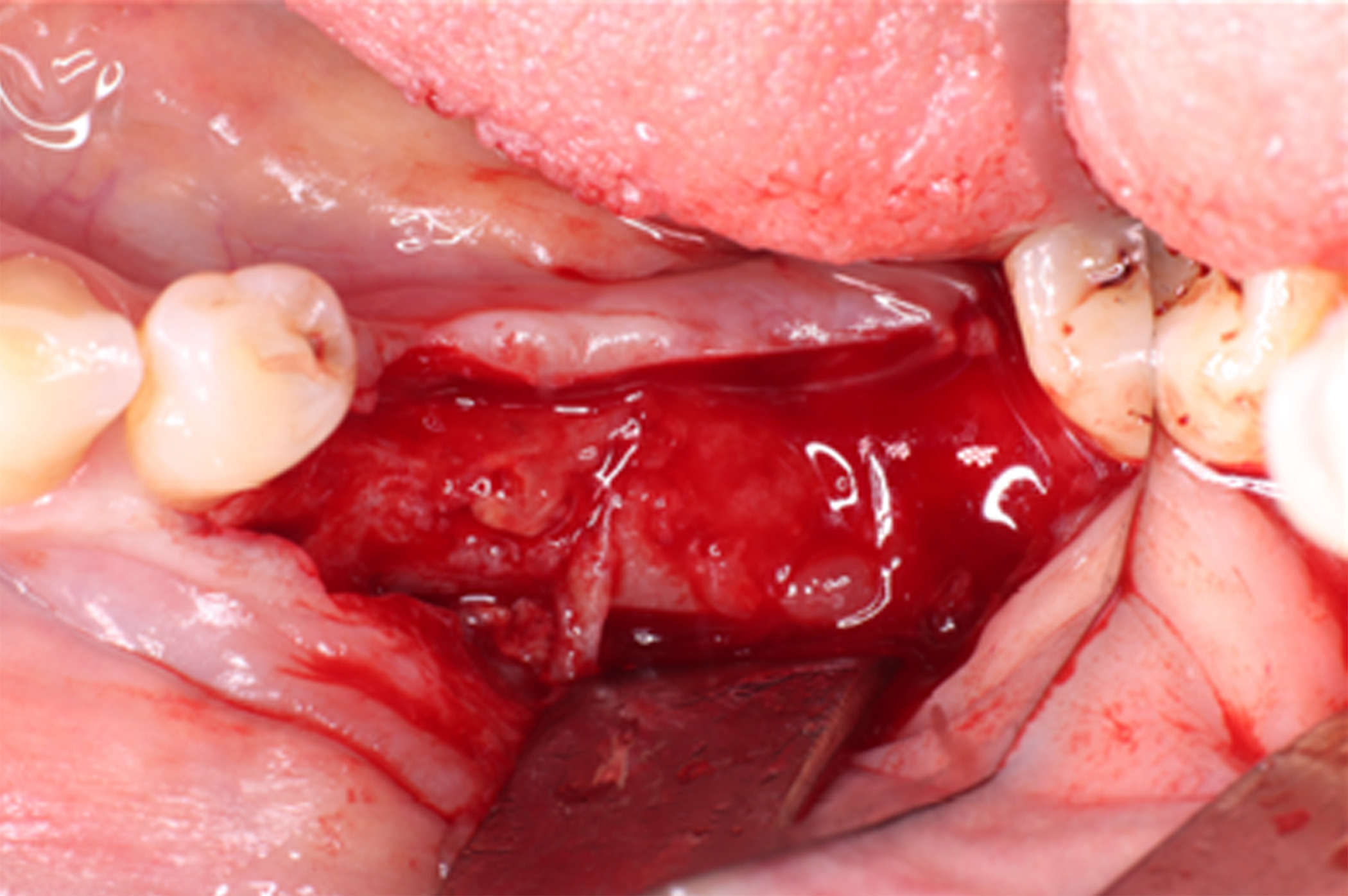
Figure 13. Removal of the membrane at 3 months postoperative revealed immature newly formed tissue with abundant blood supply, indicating that bone regeneration was not compromised by the membrane exposure
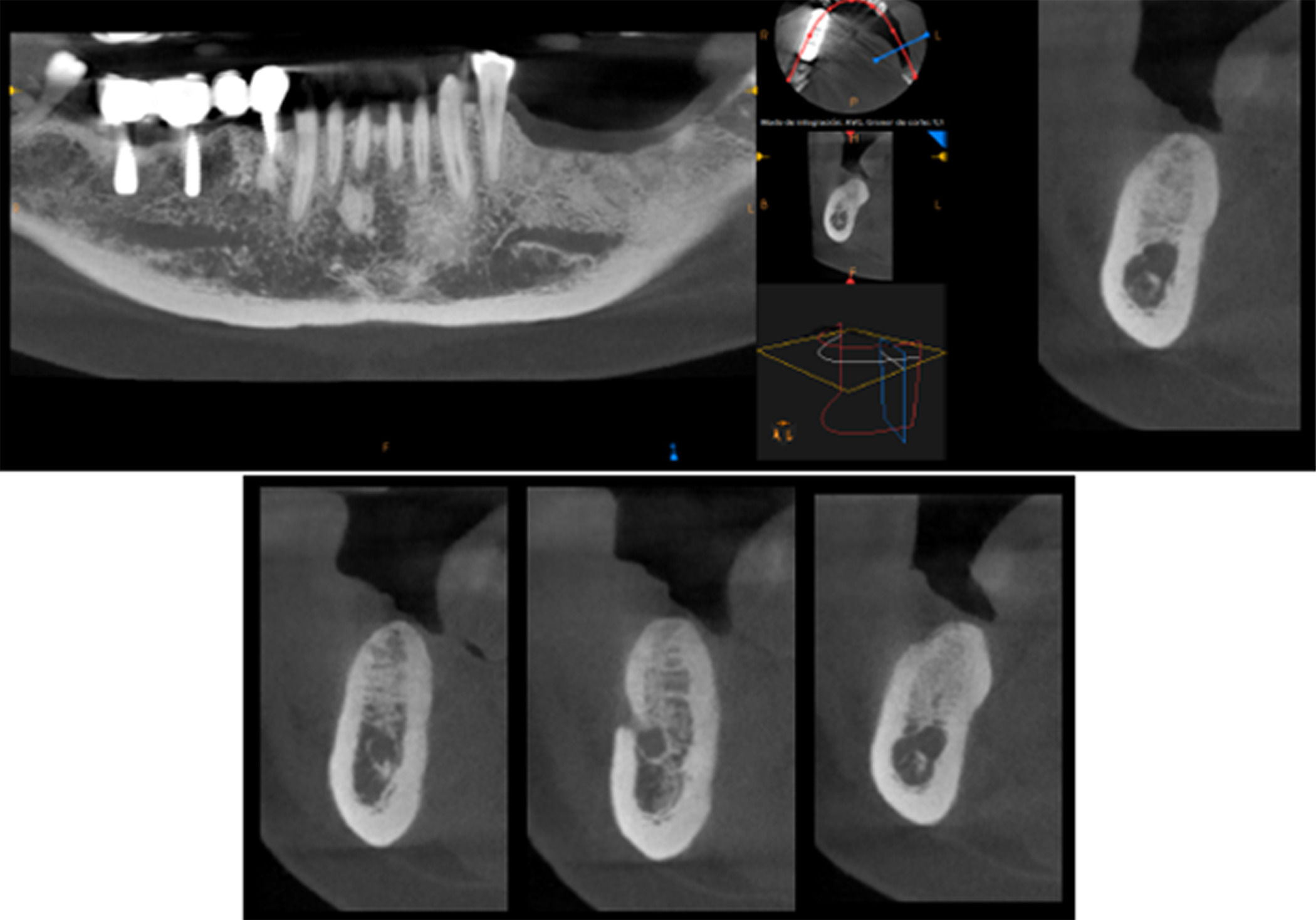
Figure 14. CT scan at 6 months postoperative showed optimal bone density with mature characteristics and optimal thickness/height for adequate three-dimensional implant placement
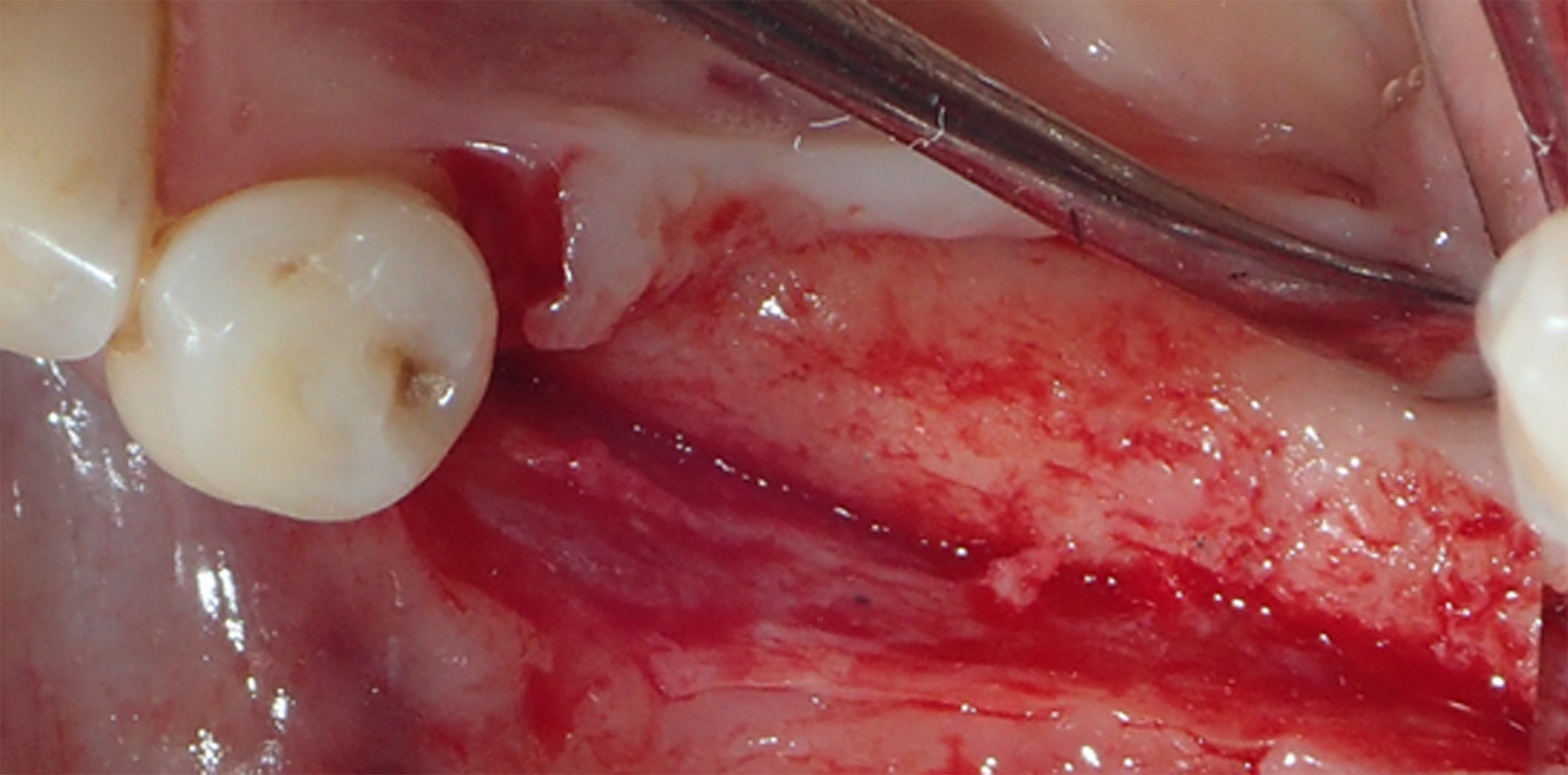
Figure 15. A full-thickness flap was created for implant placement, revealing good bone appearance with visible vascularization, rim thickness, and maturation
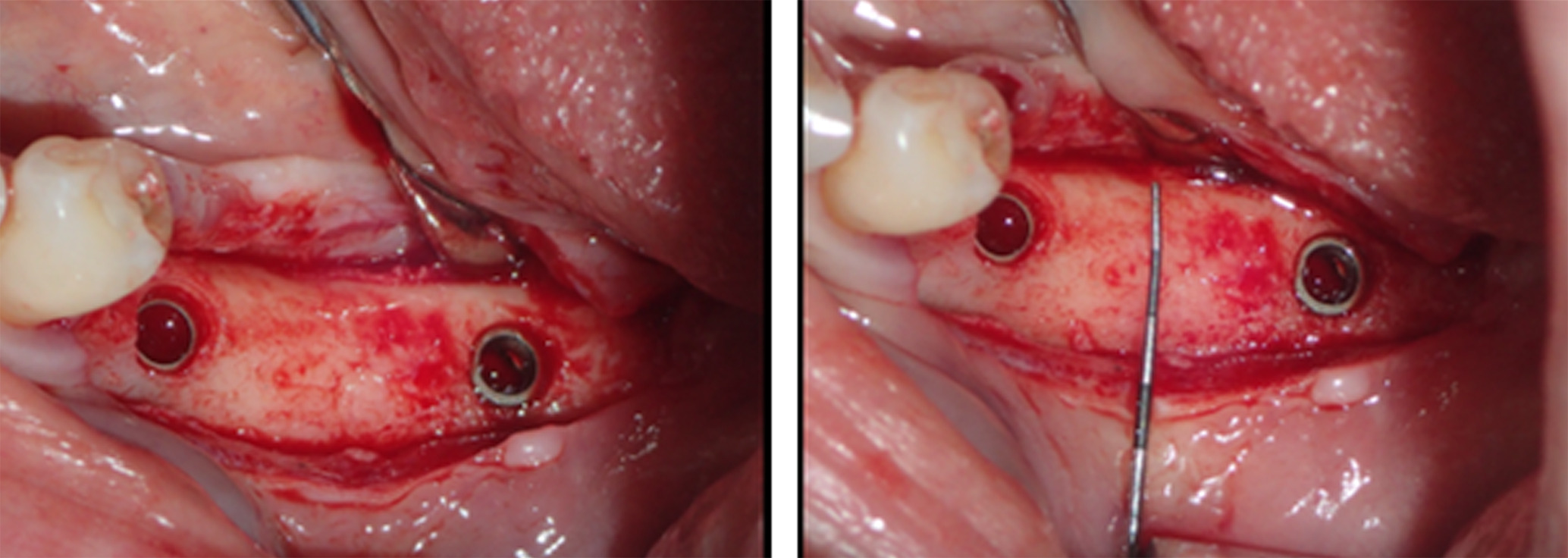
Figure 16. The implants were placed, achieving favorable primary stability and optimal three-dimensional ridge placement due to the thickness/height achieved from the regenerative procedure
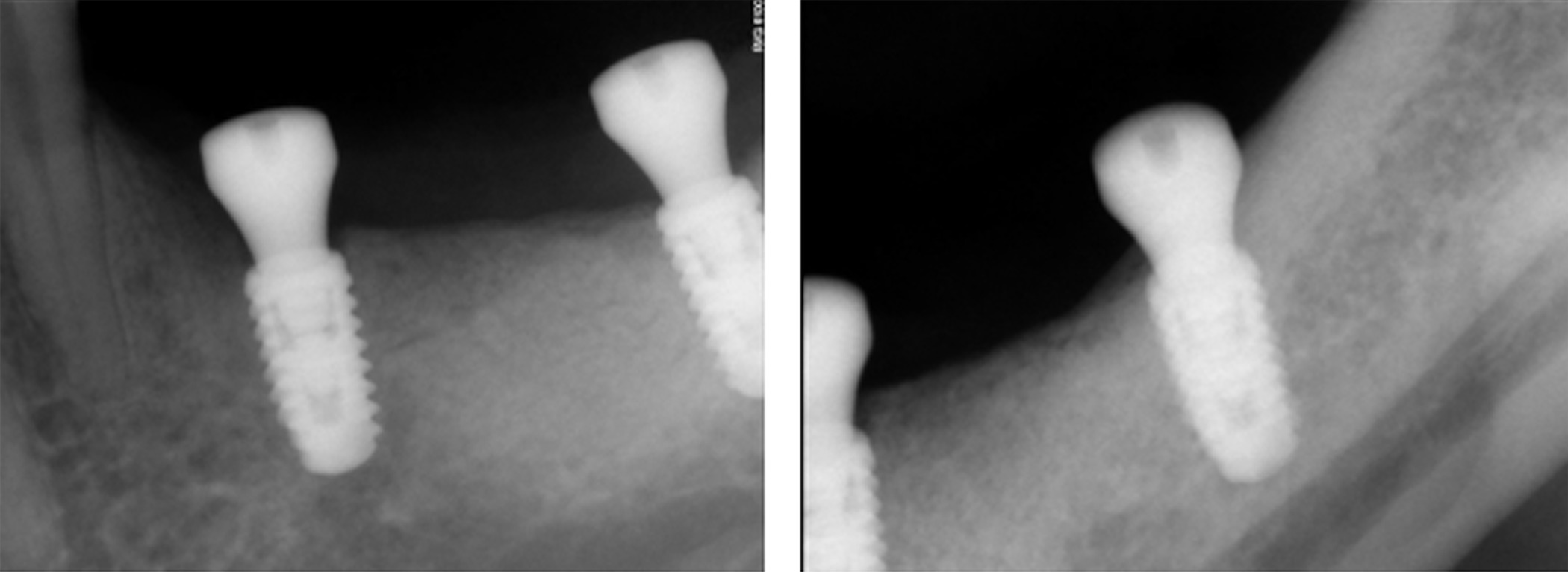
Figure 17. Periapical radiographs following placement of the healing screws showed excellent bone-implant interaction
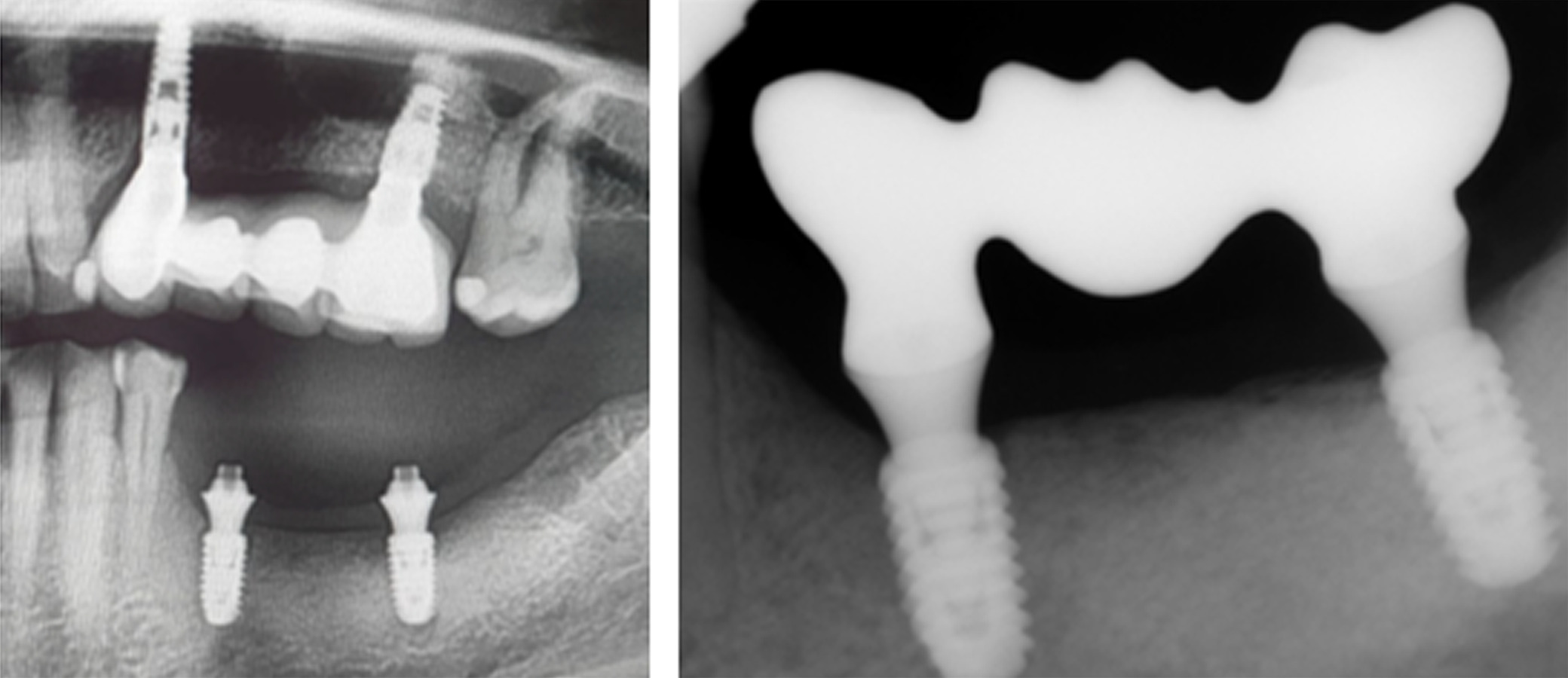
Figure 18. [Left] Panoramic radiograph prior to crown placement showing evident hard tissue stability in the regenerated area. [Right] Periapical radiograph showing bridge placement from Tooth 35 to 37
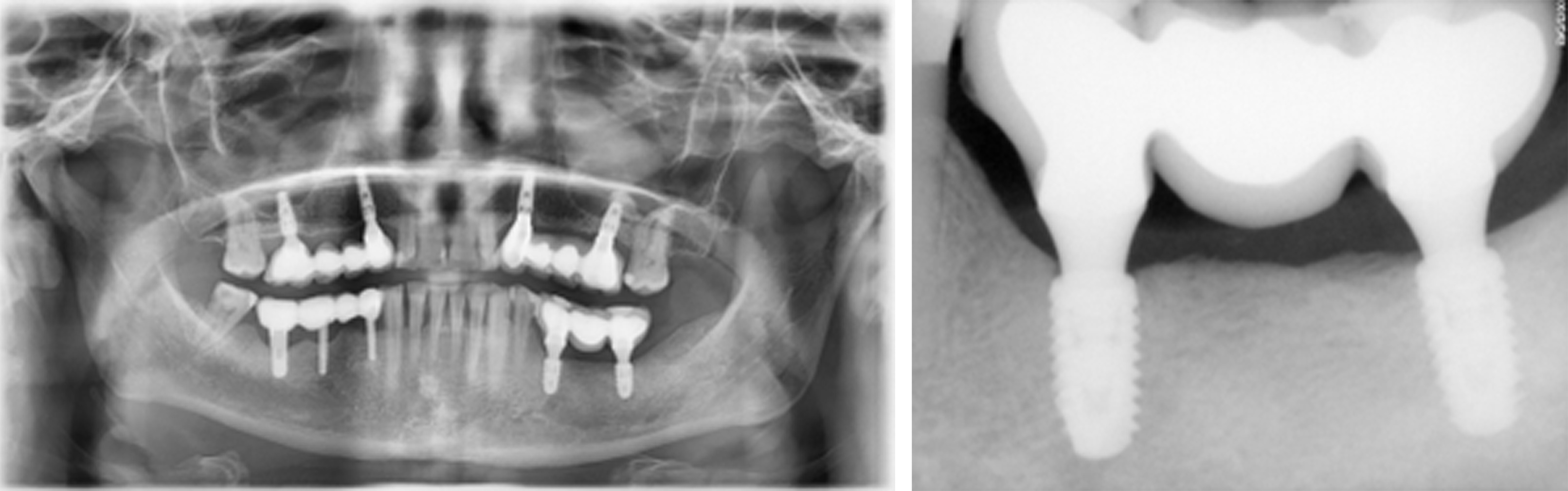
Figure 19. [Left] Panoramic and [Right] periapical radiographs at 24 months postoperative showing healthy bone regeneration and ideal placement of the implants and bridge from Tooth 35 to 37
Discussion
In this case of peri-implantitis associated with bridged implants from Tooth 35 to 37, treatment included GBR on the affected ridge using allograft C/C Mix combined with autogenous bone and an outer layer of allograft F-DBM. Subsequent exposure of the titanium-reinforced membrane at 3 months postoperative necessitated its removal, revealing healthy immature new bone tissue with abundant blood supply even at this early postoperative timepoint. Creation of a full-thickness flap at 6 months confirmed good bone appearance with visible vascularization, rim thickness, and maturation immediately prior to successful implant and bridge placement, which was maintained at 24 months.
While there is currently no consensus on the most ideal graft material for GBR in cases of peri-implantitis [3], successful use of allograft F-DBM with mineralized particulate has been reported previously in a study by Monje and colleagues involving 33 patients undergoing such treatment [2]. While the primary objective of that study was to assess the significance of barrier membranes, all patients received allograft F-DBM with mineralized particulate as the bone grafting material. The overall disease resolution rate at 12 months was reported at 77.1%, with significant radiographic bone gain from baseline measurements, regardless of the use of a barrier membrane. Similar outcomes were reported by Wen and colleagues using allograft DBM in a non-submerged reconstructive approach in the treatment of peri-implantitis [10].
Conclusion
This case highlights the successful use of a combination of allograft cortico-cancellous particulate, autogenous bone, and allograft fiber demineralized bone matrix (F-DBM) in guided bone regeneration (GBR) for the treatment of peri-implantitis. Taken together, the current case further supports the clinical effectiveness of allograft mineralized particulate with F-DBM in radiographic bone gains and resolution of peri-implantitis.
Conflicts of interest
AC and SGM declare that they have no conflicts of interest. BW is an employee of LifeNet Health®, the not-for-profit organization which processes OraGraft Prime and MD 50/50.
References
- Schwarz F, Derks J, Monje A, Wang HL (2018) Peri‐implantitis. J Clin Periodontol 45: S246-S266. [Crossref]
- Monje A, Pons R, Vilarrasa J, Nart J, Wang HL (2023) Significance of barrier membrane on the reconstructive therapy of peri‐implantitis: A randomized controlled trial. J Periodontol 94: 323-335. [Crossref]
- Monje A, Pons R, Nart J, Miron RJ, Schwarz F, et al. (2024) Selecting biomaterials in the reconstructive therapy of peri‐implantitis. Periodontol 2000 94: 192-212. [Crossref]
- Faggion Jr CM, Chambrone L, Listl S, Tu YK (2013) Network meta‐analysis for evaluating interventions in implant dentistry: The case of peri‐implantitis treatment. Clin Implant Dent Relat Res 15: 576-588. [Crossref]
- Khan SN, Cammisa Jr FP, Sandhu HS, Diwan AD, Girardi FP, et al. (2005) The biology of bone grafting. J Am Acad Orthop Surg 13: 77-86. [Crossref]
- Younger EM, Chapman MW (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3: 192-195. [Crossref]
- Gianulis E, Wetzell B, Scheunemann D, Gazzolo P, Sohoni P, et al. (2023) Characterization of an advanced viable bone allograft with preserved native bone-forming cells. Cell Tissue Bank 24: 417-434. [Crossref]
- Roberts TT, Rosenbaum AJ (2012) Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 8: 114-124. [Crossref]
- McLean JB, Carter N, Sohoni P, Moore MA (2019) Cell attachment and osteoinductive properties of tissue engineered, demineralized bone fibers for bone void filling applications. InClinical Implementation of Bone Regeneration and Maintenance. IntechOpen.
- Wen SC, Barootchi S, Wang HL, Huang WX (2022) Non‐submerged reconstructive approach for peri‐implantitis osseous defect with removal of implant crowns: One‐year outcomes of a prospective case series study. J Periodontol 93: 1250-1261. [Crossref]



















