Development of epithelial malignancies leads to well documented molecular and structural changes in the epithelium. Recently, it has been recognized that stromal biology is also altered significantly with preinvasive disease. Oral Squamous Cell Carcinoma comprises a bulk of all oral malignancies. In Oral Squamous Cell Carcinoma, cell invade the stroma in the form of islands, strands or sheets, which are embedded or surrounded by an extracellular matrix, thus producing reactive changes in the stroma. Reticulin fibers along with collagen fibers, is not only responsible for maintaining the functional integrity of tissues but is also thought to play an important role in pathogenesis and invasion in Oral Squamous Cell Carcinoma. In this study, reticulin fibers were studied histopathologically for the length, thickness, density of colour and uniformity of stain of in various grades of Oral Squamous Cell Carcinoma with Gomari silver reticulin stain. Thus, the present study helps in documentation of connective tissue changes in various grades of Oral Squamous Cell Carcinoma.
reticulin fiber, squamous cell carcinoma
Oral Squamous Cell Carcinoma is one of the most formidable health problems, in terms of morbidity and mortality, facing mankind today.
In Oral Squamous Cell Carcinoma, cells invade the stroma in the form of islands, strands or sheets, which are embedded or surrounded by an extracellular matrix, thus producing reactive changes in the stroma [1].
These changes may alter the biological aggressiveness of oral cancer.
Recently, it has been recognized that stromal biology is also altered signifcantly with preinvasive disease.
Reticulin fibers, along with collagen most abundant protein in the body, plays an important role in pathogenesis and invasion of Oral Squamous Cell Carcinoma [2].
Reticulin fibers (Type III) which are fine and delicate fibers that are found connected to coarser and stronger collagenous fibers ( Type I ).
Reticulin fibers are weakly birefringent, the weak reaction being attributed to there lack of physical size and masking effect of interfibrillar substance [3].
They had tendency to branch frequently and appears indistinct in H & E stained preparation.
Double Immuno electron microscopy shows that 20-25nm fibrils consits mainly of type I collagen whereas, the larger fibrils 30-35nm, label simulteneously for Type I and Type III collagens.
Most of the fibrils larger than 40nm in diameter label for Type III collagen [3].
Unlike the collagen fibers, the reticulin fibers are also thought to be responsible of producing significant changes in the connective tissue stroma.
Various studies are found on collagen fibers in the past literecture, but studies on reticulin fibers are also needed to find out the dual role of reticulin fibers in tumor invasion and metastasis.
Here, In this study, reticulin fibers were studied histopathologically for the length, thickness, density and staining intensity of reticulin fibres in various grades of Oral Squamous Cell Carcinoma with Gomori silver reticulin stain.
Reticular fiber demonstration technique are divided into those using dye as means of staining and metal impregnation methods [3].
Dye technique for reticular fiber demonstraction can not be considered completely reliable, as the density of stain being insufficient to resolve the fibers and staining technique do not redially differntiate between collagen and reticulin fibers. Metal impregnation technique, which provides better contrast and enabelling even finest fibers to be resolved and is the most widely used method for demonstration of reticulin fibers is used in this study [3].
The study is carried out with the following aims and objectives-
To study the reticulin fibers in various grades of Oral Squamous Cell Carcinoma. And To compare length, thickness, density and staining intensity of reticulin fibers in various grades of Oral Squamous Cell Carcinoma.
For the study, sections from 30 paraffin-embedded blocks of histopathologically diagnosed cases of Oral Squamous cell carcinoma (13 cases of Well diffentiated Squamous cell carcinoma, 12 cases of Moderately diffentiated squamous cell carcinoma, 5 cases of poorly differentiated Squamous cell carcinoma and 2 cases of normal mucosa as a control group) will be obtained from the archives of the Department of Oral Pathology and Microbiology of Sharad Pawar Dental College.
The sections will be stained subsequently with Gomori silver reticulin stain, which will be evaluated under the microscope.
Procedure of staining-
All paraffin embedded tissue blocks were sectioned at 5 u thickness with the help of semiautomatic microtome, the sections were taken on the slides and kept on hot plate for one hour to ensure adhesion of section to the slide. Sections were then deparaffinized in xylene and hydrated through decreasing grades of alcohol and taken to water. Consequently, the sections were stained with Gomori method for reticular fibers, using specific protocol for reticulin fibers staining.
Reticular fibers |
Black |
Nuclei |
Gray |
Other tissues |
Pale pink (Neutral fast red counter stain) |
Total 30 cases were evaluated in this study, which are histopathologically diagnosed as well differentiated Squamous cell carcinoma, moderately differentiated Squamous cell carcinoma and poorly differentiated Squamous cell carcinoma according to Brynes histological malignancy grading system.
For each case, 30 felids were taken into consideration.
In 13 cases of well differentiated Squamous cell carcinoma, 10 cases was showing long, thick, dense reticulin fibers which are dark in intensity of staining and maintaining their architecture was observed (Figure 1) and 3 cases was showing haphazardly arranged, thick and thin black colour reticulin fibers.
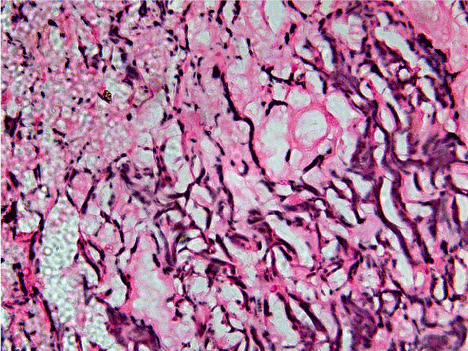
Figure 1. 40X view of moderately differentiated squamous cell carcinoma showing relatively short, thin but dense and fragmented, scattered reticulin fibers of varying degree from dark to light intensity of staining and haphazardly arranged throughout connective tissue stroma.
In 12 cases of moderately differentiated Squamous cell carcinoma, 8 cases was showing relatively short, thin but dense and fragmented, scattered reticulin fibers of varying degree from dark to light intensity of staining and haphazardly arranged throughout connective tissue stroma. At most of the places clumping of black reticulin fibers with loss of architecture are also appreciated (Figure 2). And 4 cases will shows long, thick, dark and light black colours reticulin fibers at some places only.
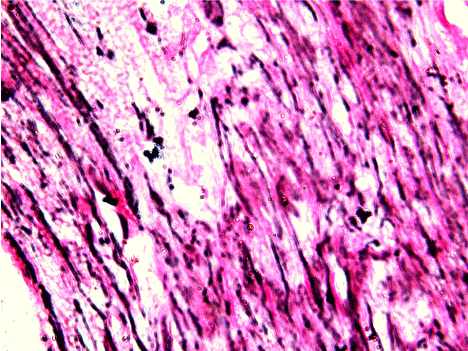
Figure 2. 40X view, diagram showing long, thick, dense, darkly staining reticulin fibers in well differentiated squamous cell carcinoma.
In 5 cases of poorly differentiated Squamous cell carcinoma, 4 cases shows very short, thin, almost fragmented reticulin fibers which was fading in intensity of staining seen. The fragmented reticulin fibers are dispersed throughout the connective tissue stoma (Figure 3). And only one case was showing long and thick reticulin fibers at many places (Graph).
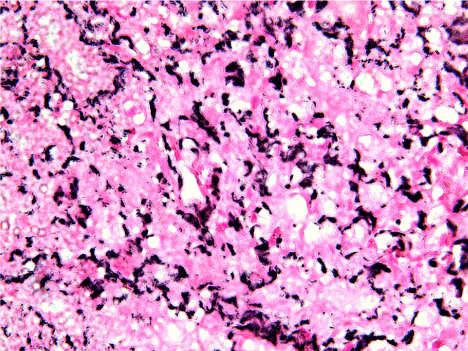
Figure 3. 40x view of poorly differentiated Squamous cell carcinoma shows very short, thin, almost fragmented reticulin fibers which was fading in intensity of staining seen. The fragmented reticulin fibers are dispersed throughout the connective tissue stoma.
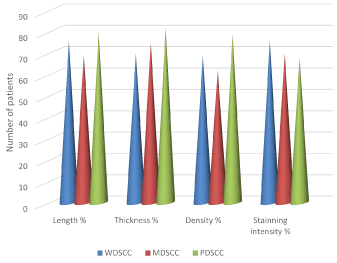
Graph: The percentage of length, thickness, density and staining intensity of reticulin fibers in well, moderate and poorly differentiated squamous cell carcinoma
When Comparing well differentiated Squamous cell carcinoma to moderately and poorly differentiated squamous cell carcinoma, the reticulin fibers in Well Differentiated Squamous Cell Carcinoma were long, thick having dense network of reticulin fibers with retained architecture particularly around the tumor island, which in Moderately Differentiated Squamous Cell Carcinoma converted to thin fragmentation and clumping of reticulin fibers with loss of architecture and in Poorly Differentiated Squamous Cell Carcinoma very thin, clump and totally fragmented but numerous reticulin fibers were observed.
Also, there is gradual decreased in intensity of staining from dark staining to light staining was observed from Well Differentiated Squamous Cell Carcinoma to Poorly Differentiated Squamous Cell Carcinoma.
In the present study, a significant change in staining pattern of reticulin fibers was observed from Well Differentiated Squamous Cell Carcinoma to Poorly Differentiated Squamous Cell carcinoma.
Among the fields observed in all the grades of Oral Squamous cell Carcinoma, most of the fields showed a weak staining of glycoprotein’s particularly around the tumor islands/nests (Figure 4).
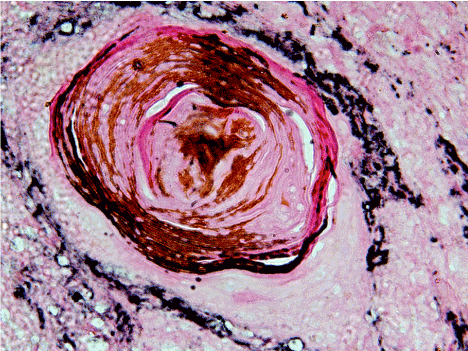
Figure 4. 40X view of well differentiated squamous cell carcinoma fields showing a weak staining of glycoprotein’s particularly around the tumor islands/nests.
Tumors are composed of two discrete interdependent components, the malignant cells and the extracellular matrix, which content ground substance and fibers. Fibers include collagen fibers, reticular fibers, elastic fibers and elaunin. Initially it was thought that stroma acts an active antagonist to tumor cell invasion. Recent evidences however suggest that tumor stroma is not just a passive structure but is associated with tumor progression [1]. The cancer cells, immune cells, fibroblast, myofibroblast, cytokines, vascular tissue and extracellular matrix constitute the tumor microenvironment which helps in tumor growth, invasion and metastasis [4-11].
In the present study, the relationship between connective tissue stroma of the host and the invading tumor cells, showed some observable changes in the host response in all the grades of squamous cell carcinoma when compared to the control stroma.
Among the fields observed in all the grades of SCC, most of the fields showed a weak staining of glycoproteins particularly around the tumor islands/nests. This may be a regressive change such as the lysis of stromal components, creating pathway for cell migration. There may be a change in stromal protein composition which is induced by mediators derived from tumor cells, supporting the in vitro studies of Iozzo [4]. Bernacki, et al. in their review described the presence of glycosidases in the interstitial fluid of various carcinomas, which support the observations made in this study [5].
The reticulin fibers in well differentiated Squamous cell carcinoma mostly encircling the tumor island and throughout the connective tissue stroma were showing dark intensity of staining as compaired to poorly differentiated Squamous cell carcinoma cases, this is probably because of more mature reticulin fibers are seen in well differentiated Squamous cell carcinoma and more immature reticulin fibers were seen in poorly differentiated Squamous cell carcinoma cases [2]. The reticulin fibers in the stroma are probably the products of benign fibroblasts or of the phenotypically altered tumor cells having a capacity to express stromal components, thereby localizing the tumor cells and preventing metastasis. These statements are supported by studies on cell lines which were derived from various neoplasms, to synthesize different types of collagen, predominantly the types I, III [2].
In a majority of connective tissues, the major type of collagen which is present is of type I, although some amount of type III and Type V are also present [2].
A majority of the fields in moderately differentiated and poorly differentiated squamous cell carcinoma in the present study, showed a gradual change in the length and thickness of reticulin fibers as well as density and intensity of staining particularly in the immediate vicinity of the invading tumour islands [2].
These could be due to Type III fibers are increasing as grades of malignancy increases. Furthermore, these differences in all grades of OSCC in the present study could be due to –
1. The action of enzymes such as collagenases or the metalloproteinases secreted by tumour cells.
2. An abnormal disintegration of the matrix by the tumour cells.
3. An uninhibited proliferation of the dedifferentiated tumour cells with the secretion of their abnormal matrix.
4. Disorganized or abortive stroma.
These all are responsible for the fragmentation, clumping, loss of architecture, gradual fading of reticulin fibers and loss of uniformity of stain and decreased staining intensity which is observed in Moderately Differentiated Squamous Cell Carcinoma and Poorly Differentiated Squamous Cell Carcinoma in present study.
The above results are further supported by the presence of a delicate meshwork (reticular) of Type III collagen at the invading front of the tumour islands in increasing gradations of skin cancer (Stenback, et al.) [6]. Few workers have also reported a qualitative change in Type I collagen with an embryonic phenotype adjacent to the tumour islands [7].
Observable changes were seen in the stroma, in all the three grades of Oral Squamous Cell Carcinoma’s. There was an increased stromal response in poorly differentiated carcinomas, when compared to the other grades.
Abundant reticulin fiber staining was visualized in the stroma particularly adjacent to the tumor islands/ nests in all the grades of Oral Squamous Cell Carcinoma; but it was comparatively less in poorly differentiated Oral Squamous Cell Carcinoma’s.
The above results are further supported by Brekken, et al, who stated that the extracellular matrix can influence tumour progression [8]. A study on human osteosarcomas by Junqueira, et al. [9] indicated the presence of both type I and III collagens in the fibroblastic areas of the tumour. Type III in the anaplastic areas and type II in the chondroblastic areas of osteosarcomas.
Kreig T, et al. [10] stated that a continuous cell line derived from human embryonal rhabdomyosarcoma synthesizes predominantly type III procollagen with a small amount of type IV.
Hence, the change in staining pattern from Well Differentiated Squamous Cell Carcinoma to Poorly Differentiated Squamous Cell Carcinoma is a radical change in the reticulin fibers length and thickness as well as density and staining intensity, which could be indicative of numerous enzymatic actions, which are taking place as a part of change in connective tissue which occurred during malignant transformation.
The observations of the present study indicated that the change in staining pattern of reticulin fibers in Oral Squamous Cell Carcinoma could acts as a prognostic marker which may be indicative of malignant transformation.
- George J, Narang RS, Rao NN (2012) Stromal response in different histological grades of oral squamous cell carcinoma: a histochemical study. Indian J Dent Res 23: 842. [Crossref]
- Aparna V, Charu S (2010) Evaluation of collagen in different grades of oral squamous cell carcinoma by using the Picrosirius red stain- a histochemical study. Journal of Clinical and Diagnostic Research 4: 3444-3449.
- Theory and practice of histological techniques. John D. Bancroft, Marilyn Gamble. Sixth edition. Elsevier 2008.
- Iozzo RV (1995) Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab Invest 73: 157-160. [Crossref]
- Bernacki RJ, Niedbala MJ, Korytnyk W (1985) Glycosidases in cancer and invasion. Cancer Metastasis Rev 4: 81-101. [Crossref]
- Stenback F, Makinen MJ, Jussila T, Kauppila S et al. (1999) The extracellular matrix in skin tumour development – a morphological study. J Cutaneous Pathol 26: 327-338.
- Bornstein P, Sage H (1980) Structurally distinct collagen types. Annu Rev Biochem 49: 957-1003. [Crossref]
- Rolf A. Brekken, Pauli Puolakkainen, David C. Graves, Gail Workman, Sharon R. Lubkin, E. Helene Sage (2003) Enhanced growth of tumours in SPARC null mice is associated with changes in the ECM. J Clin Invest 111: 487-495. [Crossref]
- Junqueira LC, Assis Figueiredo MT, Torloni H, Montes GS (1986) Differential histologic diagnosis of osteoid: a study on human osteosarcoma collagen by the histochemical picrosirius- polarization method. J Pathol 148: 189-196. [Crossref]
- Krieg T, Timpl R, Alitalo K, Kurkinen M, Vaheri A (1979) Type III procollagen is the major collagenous component produced by a continuous rhabdomyosarcoma cell line. FEBS Lett 104: 405-409.
- Astekar M, Metgud R, Sharma A, Soni A (2013) Hidden keys in stroma: Unlocking the tumor progression. J Oral Maxillofac Pathol 17: 82-88. [Crossref]





