Abstract
Acne is one of the most common inflammatory dermatoses among adolescents and adults, frequently leaving scars. Improving acne scar appearance represents an important challenge of cosmetic dermatology. This study sought to evaluate cosmetic outcomes of onion-extract-based Mederma®Advanced Scar Gel on acne scars.
Thirty subjects were included in this single-center, investigator-blinded, randomized, intra-individual comparison versus control study. At screening, mean age of subjects was 32.3 years and 73.3% of subjects were skin phototype III. The Investigator reported overall appearance of targeted test-scar was significantly improved after the 12-week application period, starting from Day 29 ± 2 days. Each individual parameter, redness, texture and softness of scar after gel was applied, was significantly improved at Days 15 ± 2 days, 29 ± 2 days and 57 ± 2 days respectively. Likewise, 83.0% of subjects found overall test-scar appearance slightly better or much better than control-scar from Day 57 ± 2 days and reported statistically significant improvements of individual appearance parameters and particularly scar visibility reduction and smoothness of the test-scar. The product’s cosmetic acceptability was well-appreciated by subjects and local tolerability was excellent. In conclusion, under the study conditions, Mederma®Advanced Scar Gel improved acne scar appearance after only 14 days of once-daily application and efficacy was optimal after 2 months of use.
Keywords
atrophicacne scarring, scar appearance, onion extract, cosmetic outcomes
Introduction
Acne vulgarisis one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults [1,2], many of whom may experience facial scarring [3]. Some forms of facial scarring have been reported to occur in up to 95.0% of acne patients, 30.0% of whom may be affected by severe scarring [3]. Scarring results from an altered wound healing response to cutaneous inflammation, with inflammatory cell infiltrates found in 77.0% of atrophic scars [4]. Among the different categories of acne scars, atrophic scars are characterized by overall localized reduction in collagen content [5,6]. They are more common than other types of scars and can be further categorized into three subtypes, based on morphologic criteria such as size and depth: ice-pick, boxcar and rolling [5]. Acne scars are often a major source of aesthetic and psychological concerns [7] for the affected subjects and reducing/improving the appearance of these scars represents an important challenge of cosmetic dermatology. Such scars usually need cosmetic care and will not go away completely on their own. Many invasive treatment options are available for improving acne scar appearance, including dermabrasion, laser treatment, punch techniques, fat transplantation, other tissue augmenting agents, needling and combined therapy [8]. However, their high cost and problematic side effects (e.g. erythema, oedema, post-treatment scabbing…) restrict their applications. Topical botanical agents are commercially available and have visible cosmetic outcomes on scars, such as surgical scars [9-11]. The concentrated onion extract with Allantoin aqueous gel, Mederma®Advanced Scar Gel (Laboratoire HRA Pharma) first developed and commercialized in USA, has shown to improve post acne scarring appearance [12] and is marketed in many countries worldwide.
Materials and Methods
Study Design and subjects
This was a single-center, investigator-blinded, randomized, intra-individual comparison versus control study. It was conducted in a single investigational center (Centre Pharmacologie Clinique Appliqué à la Dermatologie, CPCAD) located in the Public University Hospital of Nice, France.
Eligible subjects were aged 18 to 50 years old with skin type I to VI according to Fitzpatrick classification [13] and with a past history of mild to moderate acne, and at least one mild to moderate atrophic acne scar on each side of the face. The Investigator selected the target scar on each side of the face, so that at baseline, target scars of included subjects were comparable. Subjects understood the full nature and purpose of the study and were willing to sign a written consent to participate in the study. Female subjects of childbearing potential had to use one of the reliable methods of contraception during the investigation and agreed not to change it during the study. Excluded from the study were, among others: subjects who were pregnant, breast-feeding or intending to get pregnant; subjects having used within 3 months before inclusion, Retin A or other Rx/OTC Retinyl A, or having planned to use these treatments during the study; having carried out within 6 months before inclusion cosmetic care of acne scarring, including chemical peeling, dermabrasion, laser treatment, punch techniques, fat transplantation, other tissue augmenting agents, needling, or combined therapy, or having planned to use these procedures during the study; subjects with known allergies or sensitivities to ingredients contained in the investigational product (IP); suffering from a serious or progressive disease that could compromise their participation in the study according to the Investigator (e.g., diabetes, cardiac pathologies, hepatic disorder, renal disorder, pulmonary disease, cancer, neurological or psychological disease, inflammatory/immunosuppressive disease).
The maximum study duration participation per subject was 14 weeks: a 2-week screening period followed by a 12-week product application period.
Investigational product
The IP was Mederma®Advanced Scar Gel, manufactured, formulated and provided to the Investigator under the responsibility of HRA Pharma. Mederma®Advanced Scar Gel formulation contains several ingredients like allium cepa, an onion extract and Allantoin. Mederma®Advanced Scar Gelhas been previously demonstrated to improve the appearance of scars, to reduce complaints about the scar appearance in the scar areas and to provide functional improvement of scarred areas [10,12]. Mederma® Advanced Scar Gel was applied once daily, preferably in the morning.
Ethical aspects
The study protocol was reviewed by the French Ethics Committee (EC) Sud Est VI prior to inclusion of subjects. According to the French Health Authorities and based on French legislation (article L1121-1 of the Public Health Code): as the cosmetic IP is already commercialised in the European Union, its standard use during the study guaranteed its safety of use; interventions were considered to have no risk to the subjects involved. EC’s approval was, therefore, not mandatory. The study was conducted in accordance with the protocol, the Declaration of Helsinki, and in compliance with the applicable local regulatory requirements.
Study procedures and Randomization
Subjects attended 6 visits at the study center: a screening visit (between Day-14 and Day 1), a baseline visit at Week 1/Day 1 ± 2 days during which the IP was dispensed to subjects and 4 tolerance and efficacy evaluation visits (Week 2/Day 15 ± 2 days; Week 4/Day 29 ± 2 days; Week 8/Day 57 ± 2 days and Week 12/Day 85 ± 2 days). Subjects were to apply the IP once daily (preferably in the morning) for 12 weeks.
At baseline visit, each subject who fulfilled all inclusion/non-inclusion criteria was assigned a randomization number. This randomization number was computer-generated and dispensed in thechronological order of his/her randomization in the trial and no number should be omitted or skipped. The date and time of randomization defined this number, independently of the Site Identification Number that was initially assigned at the screening visit. The randomization list allocated for each subject the side of the face where the IP was to be applied, only on the targeted scar. The control-scar did not receive any product. The study was single-blinded: the randomization list was kept out of the sight of the Investigator by the biomedical research assistant in charge of IP dispensation, therefore, the investigator did not know on which target scar (left side or right side of face) the IP was applied.
All collected data (participant outcome reports and investigator evaluations) were recorded in the electronic Case Report Form (e-CRF) by the Investigator or designated person.
Study outcomes
The main cosmetic outcome was target scars’ overall appearance, as rated by the Investigator after 12 weeks (Day 85) using a 100-point Visual Analogue Scale (VAS) (0 being the worst imaginable case and 100 being the best imaginable case); and by subjects, at each evaluation visit, using a 5-point ordinal scale (appearance on left side: much better / slightly better / no difference / slightly worse / much worse than right side).
Other cosmetic outcomes were: Investigator’s ratings of scar overall appearance, at each evaluation visit, using the 100-point VAS; Investigator’s ratings of individual appearance parameters: softness, redness and texture (roughness) using a 11-point ordinal scale (0 = not soft at all/no redness at all/no roughness at all, 10 = maximum softness/redness/roughness); subjects’ ratings of softness, redness, texture and discomfort using a 11-point ordinal scale (0 = not soft at all/no redness at all/no roughness at all/no discomfort at all, 10 = maximum softness/redness/roughness/discomfort); subjects’ reported outcomes (itching, reduction in scar visibility, improvement in scars thickness and smoothness); first visible effects of the tested product (subject’s assessment); subjects’ cosmetic acceptability (18 questions on subject’s perception/acceptability of the product).
Statistical analysis
All statistical analyses were performed using R software version 4.0.2. Data were presented as mean ± standard deviation (SD), number (n) and percentages (%), where appropriate. For each study endpoint, statistical comparisons between product application and non-application sites were performed using a paired Student t-test or a Wilcoxon matched-pairs signed-rank test, at all post-baseline visits and changes from baseline were calculated. Multiplicity was controlled using the Benjamini-Hochberg procedure. The answers to subjects’ overall appearance assessment were analysed and presented after consideration of randomisation results, i.e. which target scar (test or control) on which side of the face. The answers to the cosmetic acceptability questionnaire were summarized in a frequency histogram by category. AEs were presented descriptively. Statistical significance was identified where P value was less than 0.05 with a 2-tailed test.
Results
Subject’s disposition and baseline characteristics
From September 2021 to December 2021, 30 subjects were screened, randomised and completed the study as planned in the protocol. Most subjects were female (n=24; 80.0%) and mean age (± SD) was 32.3 (8.7) years. Among included subjects, 22 (73.3%) had type III skin (Table 1). Physical examination results of subjects by the Investigator at screening were within normal ranges.
Table 1. Subjects’ baseline characteristics
|
|
N=30 |
Age (years) |
|
32.3 ± 8.7 |
Gender |
|
|
|
Female |
24 (80.0) |
|
Male |
6 (20.0) |
Skin type |
|
|
|
Type II |
4 (13.3) |
|
Type III |
22 (73.3) |
|
Type I |
4 (13.3) |
Values are presented as mean ± SD or numbers (%).
Main cosmetic outcome
The Investigator rated the target scar’s overall appearance at Day 85, after 12 weeks of IP daily application, using a specific VAS (Figure 1). At Day 1 (baseline), mean scores (± CI) of overall appearance of test-scar and control-scar were comparable (43.47 ± 8.46 and 45.90 ± 6.95 respectively). At Day 85, a statistically significant difference (p<0.001) was observed between the overall appearance scores of the test-scar (58.80 ± 6.02) compared to the control-scar (50.30 ± 4.95). At Day 85, overall appearance of the test-scar was statistically significantly improved (p<0.001) as compared to Day 1. No significant change in overall appearance was observed in the control-scar, between Day 1 and Day 85 (Figure 1). The Investigator also rated the target scar’s overall appearance at each evaluation visit, from Day 15 to Day 57. Overall appearance of target test-scar was statistically significantly improved from Day 29 (55.80 ± 6.65) to Day 57 (61.13 ± 5.68; p<0.001); and statistically significant improvement from baseline was reported for test-scar compared to control-scar at Day 29 (55.80 ± 6.65 vs 53.14 ± 5.75; p=0.050) and Day 57 (61.13 ± 5.68 vs 55.48 ± 5.68; p=0.020).
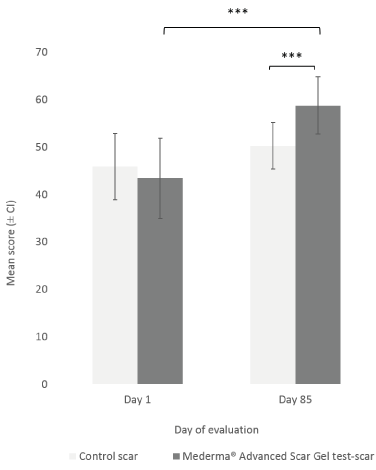
Figure 1. Investigator’s assessment of target scars overall appearance after week 12 (Day 85). Mean scores (± CI, confidence intervals) of overall target scar appearance as rated by the Investigator at Day 1 (baseline) and Day 85. Asterisks (***) indicate statistically significant difference (p value <0.001) between Mederma®Advanced Scar Gel (black graph) and control (grey graph) scars at Day 85 or test-scars between Day 1 and Day 85
The overall appearances of target test-scars and control-scars were also rated by subjects at each evaluation visit. The number of subjects who reported scar’s overall appearance slightly better or much better with Mederma®Advanced Scar Gel compared to the control-scar, increased over time (Figure 2); from 53.0% at Day 15 to 72.0% at Day 29 to 83.0% at Day 57; 83.0% of the subjects found that the overall appearance of the test-scar was slightly better or much better than the control-scar at Day 85 (Figure 2).
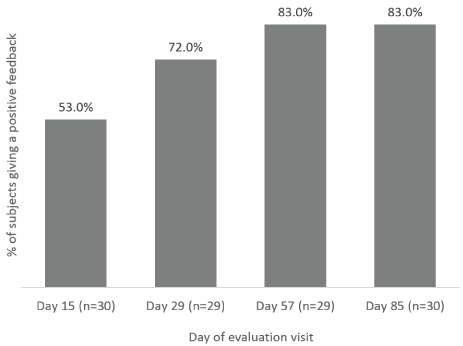
Figure 2. Subjects’ assessment of target scars overall appearance. Percentage of subjects assessing scar overall appearance as “slightly better” or “much better” with Mederma® Advanced Scar Gel compared to the control-scar
Other cosmetic outcomes
At each evaluation visit, the Investigator rated individual parameters of scar appearance; that are softness, redness and texture (roughness) of the target scars. Significant improvements in the test-scar softness were observed at Day 57 (6.30 ± 0.21; p=0.014) and Day 85 (6.67 ± 0.25; p<0.001) with Mederma®Advanced Scar Gel as compared to baseline (Day 1) (5.60 ± 0.48). No significant change in softness was observed for the control-scar. The improvement from baseline in scar softness was significantly greater with Mederma®Advanced Scar Gel compared to control at the end of the 12-week gel application period (6.67 ± 0.25 vs 5.60 ± 0.22; p=0.002). In addition, test-scar redness using Mederma®Advanced Scar Gel significantlydecreased from Day 15 (3.07 ± 0.56) as compared to Day 1 (3.90 ± 0.71). The improvement from baseline in scar redness was significantly greater with Mederma®Advanced Scar Gel than without the gel at Day 85 (-2.00 ± 1.36 vs -0.60 ± 1.35; p<0.001). Finally, statistically significant improvement in target test-scars’ roughness (texture) was observed from Day 29 (3.07 ± 0.41), compared to Day 1 (4.27 ± 0.63). Moreover, at each visit subjects also rated softness, redness, texture and discomfort of the target scars (Figure 3). Significant improvements in test-scar softness from baseline (5.80 ± 0.74) were observed at Day 57 (7.07 ± 0.39; p=0.002) and Day 85 (7.23 ± 0.43; p=0.003). No significant change in softness was observed for the control-scar. The improvement from baseline in scar softness was significantly greater with Mederma®Advanced Scar Gel than without the gel, from Day 57 (7.07 ± 0.39 vs 5.21 ± 0.56; p< 0.001 at Day 57; 7.23 ± 0.43 vs 5.27 ± 0.59; p=0.002 at Day 85) (Figure 3a). Likewise, statistically significant improvements in scar redness were observed at all evaluation visits with Mederma®Advanced Scar Gel (4.40 ± 0.66 at Day 1; 3.57 ± 0.59 at Day 15; 2.73 ± 0.59 at Day 29; 2.45 ± 0.63 at Day 57; 2.23 ± 0.49 at Day 85) (Figure 3b). No significant change in redness was observed for the control-scar. The improvement from baseline in scar redness was significantly greater with Mederma®Advanced Scar Gel than without gel from Day 15 (3.57 ± 0.59 vs 4.63 ± 0.89; p=0.025 at Day 15; 2.73 ± 0.59 vs 4.38 ± 0.80; p<0.001 at Day 29; 2.45 ± 0.63 vs 4.21 ± 0.67; p<0.001 at Day 57; 2.23 ± 0.49 vs 4.77 ± 0.73; p<0.001 at Day 85) (Figure 3b). Similarly, scar roughness (texture) decreased with the IP, as reported by subjects, from Day 29 (4.10 ± 0.78 at Day 1; 3.60 ± 0.62 at Day 15; 3.40 ± 0.53 at Day 29; 3.03 ± 0.47 at Day 57; 2.77 ± 0.49 at Day 85) (Figure 3c). No significant change in scar texture was observed for the control-scar. The improvement from baseline in scar texture was significantly greater with Mederma®Advanced Scar Gel than without gel from Day 57 (3.03 ± 0.47 vs 4.41 ± 0.70; p=0.04 at Day 57; 2.77 ± 0.49 vs 4.37 ± 0.67; p=0.012 at Day 85) (Figure 3c). The discomfort of the scar was significantly reduced with Mederma®Advanced Scar Gel at all post-baseline visits (5.27 ± 1.03 at Day 1; 4.47 ± 0.91 at Day 15; 3.90 ± 0.84 at Day 29; 2.97 ± 0.62 at Day 57; 3.10 ± 0.66 at Day 85) (Figure 3d). No significant change in discomfort was observed for the control-scar. The improvement in the scar discomfort from baseline was significantly greater with Mederma®Advanced Scar Gel than without gel from Day 29 (3.90 ± 0.84 vs 4.76 ± 0.79; p=0.011 at Day 29; 2.97 ± 0.62 vs 4.41 ± 0.77 p<0.001 at Day 57; 3.10 ± 0.66 vs 5.00 ± 0.87; p<0.001 at Day 85) (Figure 3d).
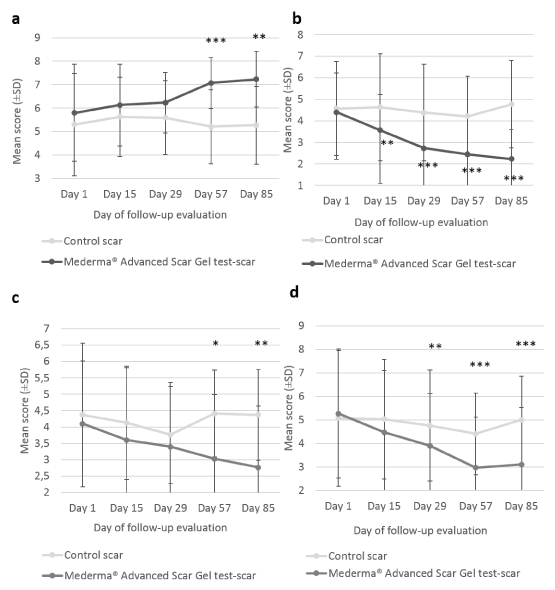
Figure 3. Subjects’ assessment of targets scar softness, redness, discomfort and texture. Mean scores (± CI, confidence intervals) of target scar (a) softness, (b) redness, (c) texture and (d) discomfort, as rated by the subject over time. Asterisk indicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between Mederma® Advanced Scar Gel (black graph) and control (grey graph)
Finally, at each evaluation visit, subjects assessed the itching sensation, reduction of scar visibility, improvement of scar’s thickness and smoothness. During the first month (at Day 15 and Day 29), 9 subjects attributed temporary positive scores to “itching” on the test-scar. From Day 29 to the end of the study, neither scratch marks nor itching were observed or reported. The reduction in scar visibility was significantly greater with the IP than without it from Day 15 (0.30 ± 0.19 vs 0.00 ± 0.00; p=0.005 at Day 15; 0.83 ± 0.21 vs 0.07 ± 0.09; p<0.001 at Day 29; 1.37 ± 0.29 vs 0.00 ± 0.00; p<0.001 at Day 57 and 1.97 ± 0.32 vs 0.03 ± 0.07; p<0.001 at Day 85) (Figure 4a). At the end of the 12-week application period, subjects reported improvement of target scar with Mederma®Advanced Scar Gel while no change was reported for control-scar.Target scar’s thickness improvement scores at all post-baseline visits were significantly greater with Mederma®Advanced Scar Gelthan without it, at all post-baseline visits (0.20 ± 0.15; p=0.012 at Day 15; 0.53 ± 0.20; p<0.001 at Day 29, 1.43 ± 0.29; p<0.001 at Day 57; 1.47 ± 0.34 at Day 85 p<0.001) (Figure 4b). The subjects did not notice any improvement from baseline in control-scar thickness. Likewise, test-scar smoothness improvement was significantly greater than control-scar at all post-baseline visits (0.63 ± 0.22; p<0.001 at Day 15; 0.93 ± 0.21; p<0.001 at Day 29; 1.27 ± 0.28; p<0.001 at Day 57; 1.60 ± 0.35; p<0.001 at Day 85) (Figure 4c) and no change in smoothness was reported for control-scar.
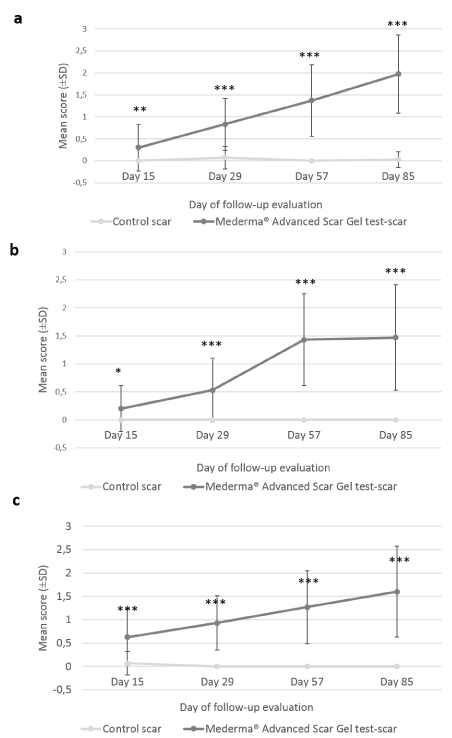
Figure 4. Subjects’ assessment of target scars visibility, thickness and smoothness. Mean scores (± CI, confidence intervals) of target scar (a) visibility, (b) thickness and (c) smoothness as rated by the subject over time. Asteriskindicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between Mederma® Advanced Scar Gel (black graph) and control (grey graph)
Figure 5. Subjects’ positive cosmetic acceptability of Mederma® Advanced Scar Gel. Percentage of subjects who totally and somewhat agreed with the proposed statements at Day 85 (end of study). Only positive answers given by more than 60.0% of subjects are presented
First Positive Visible Effects of Mederma®Advanced Scar Gel
The first subjects to observe and report the first visible effects of Mederma®Advanced Scar Gel on their target scar did so after the first few applications and quite early during the 12-week follow-up period (Table 2). At Day 2, the first subject reported the first visible effects on roughness reduction and on softness. The first effect observed by a participant on scar size reduction, redness reduction and overall appearance improvement was reported at Day 4. The first subject reported the first visible effect on thickness was noticed by the first subject on Day 6 and on discomfort improvement at Day 8 (Table 2).
Table 2. First day of visible effect(s) reported by the first subjects
|
Mederma® Advanced Scar Gel scar (N=30) |
First day of first visible effect on: |
|
Scar softness |
Day 2 |
Roughness reduction |
Day 2 |
Scar size reduction |
Day 4 |
Scar redness reduction |
Day 4 |
Overall appearance improvement |
Day 4 |
Scar thickness |
Day 6 |
Discomfort improvement |
Day 8 |
Cosmetic acceptability
At the end of the study, participating subjects were asked 18 questions concerning their perception of Mederma®Advanced Scar Gel, to which they could totally or somewhat agree, neither agree nor disagree, somewhat disagree or disagree. Overall, on 13 out of 18 questions, 60.0% - 100.0% of subjects gave positive answers, i.e. totally or somewhat agreed with the proposed statements (Figure 5)and 80.0% of subjects disagreed with the statement that the product stained fabric, like clothes and towels. Precisely, 63.3% - 76.7% of subjects found that Mederma®Advanced Scar Gel improved the appearance of their scar and 60.0% - 83.3% of subjects positively appreciated the sensory properties (fragrance, colour and texture) of this cosmetic product. After 12 weeks of daily application on skin, all subjects (100.0%) found the product easy to apply and more than 66.7% appreciated its pleasant aspect, easiness to penetrate the skin without leaving oily effect and discreetness after application (Figure 5). In addition, 66.7% - 96.7% would willingly and easily fit the product into their daily skincare routine and recommend to anyone with bothersome acne scars. Finally, even though only a small zone of the skin was tested with Mederma®Advanced Scar Gel, more than half of the subjects were more confident and felt less self-conscious about their acne scars after having used Mederma®Advanced Scar Gel.
Safety and cutaneous tolerability
Safety and cutaneous tolerability of Mederma®Advanced Scar Gel were excellent throughout the study, for almost all participating subjects; only 2 (6.7%) out of 30 included subjects experienced 3 adverse events (AEs) of mild to moderate intensity during the study. Of these AEs, only 1 (a mild erythema) was considered as likely related to the IP and was ongoing at the end of the study; the subject reported mild signs of irritation at Day 85. A second subject reported moderate signs of irritation at Day 57.
Discussion
The blinded Investigator and subjects both assessed that overall appearance of target test-scars was significantly improved after a 12-week application period; an improvement statistically significantly greater compared to control-scar from Day 29. Overall, 83.0% of the subjects found overall scar appearance with the product was slightly better or much better than control-scar from Day 57. When considering individual appearance parameters, the Investigator reported that scar redness significantly decreased over time, statistically significantly more on the test-scar than the control-scar. Subjects also reported statistically greater improvements in test-scar redness, texture, softness and discomfort. Of course, during the follow-up period, a subjects’ perception may evolve and acne scars may naturally change over time; this variability may be reflected by slight evolution of control-scar appearance evaluations.
Our results were in line with previously published articles that have shown that onion extract-based formulations significantly improved appearance of several types of scars [10-12], among which a study showing the cosmetic benefits of once-daily application of Mederma®Advanced Scar Gel [12]. In our study, randomisation of the scar on which the product was applied and comparison to a control-scar provided a rigorous tool to evaluate cause-effect relationship between Mederma®Advanced Scar Gel application and cosmetic outcomes. Of course, objectivity of subjects self-rating assessments of appearance improvements may be challenged as subjects were not blinded to the test-scar. However, their assessments were very similar to those of the Investigator, who was blinded to the test-scar, supporting cosmetic outcomes in favour of Mederma®Advanced Scar Gel. Moreover, in cosmetic studies such as this one, subject self-rating of aesthetic responses provides useful “consumer-based” information on acceptability of cosmetic care products. Knowing how atrophic acne scars can have a substantial negative impact on subjects’ self-esteem and social interactions [14], cosmetic care products that improve scars’ overall appearance, contribute in providing high subject satisfaction. In our study, this was clearly reflected in the well-appreciated cosmetic acceptability of Mederma®Advanced Scar Gel and excellent local tolerability. The IP was applied on only a small area of the skin (only one target acne scar one side of the face) and it is evident that a certain percentage of subjects may not feel better or more confident about their appearance after 12 weeks of gel application, since they may have more than one acne scar to “take care of”. Having said that, more than 66.0% of subjects would purchase and use the product on a daily basis and more than 73.0% would recommend it to persons with bothersome acne scars. In addition, the convenient once-a-day application regimen was approved by 96.7% of subjects. This globally reflects a very positive acceptance of Mederma® Advanced Scar Gel by users.
In conclusion, under these study conditions, Mederma®Advanced Scar Gel provided a benefit in the cosmetic appearance of acne scars; first cosmetic effects (roughness reduction and softness) were visible 2 days after application, 6 out of 7 cosmetic outcomes (scar roughness, size and redness reduction, softness, overall appearance improvement, scar thickness) were visible from the first week of once-daily application. Efficacy in cosmetic appearance of acne scars was optimal after 2 months of use.
Acknowledgments
HRA Pharma funded this study. We are thankful to CPCAD and investigational site staff for their collaboration and work.
Conflicts of interest
JZB (Medical Operations Lead), PA (Scientific Affairs Assistant & Clinical Operations Assistant), TJ (Global Category Lead Wound Care) and CAA (Global Head of Medical Affairs) are employees at HRA Pharma. CQR (CPCAD Managing Director) is an employee at CPCAD (Centre Pharmacologie Clinique Appliqué à la Dermatologie), the organization that has conducted the study. MK (Medical writer) is an employee at ICTA, the organization to which the article writing was subcontracted.
References
- Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, et al. (2008) The prevalence of acne in adults 20 years and older. J Am Acad Dermatol58:56-9.[Crossref]
- Connolly D, Vu HL, Mariwalla K, Saedi N (2017) Acne Scarring-Pathogenesis, Evaluation, and Treatment Options. J Clin Aesthet Dermatol10:12-23.[Crossref]
- Layton AM, Henderson CA, Cunliffe WJ (1994) A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol19:303-8.[Crossref]
- Lee WJ, Jung HJ, Lim HJ, Jang YH, Lee SJ, et al. (2013) Serial sections of atrophic acne scars help in the interpretation of microscopic findings and the selection of good therapeutic modalities. J Eur Acad Dermatol Venereol27:643-6.[Crossref]
- Jacob CI, Dover JS, Kaminer MS (2001) Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol45:109-17.[Crossref]
- Rivera AE (2008) Acne scarring: a review and current treatment modalities. J Am Acad Dermatol59:659-76.[Crossref]
- Koo J (1995) The psychosocial impact of acne: patients' perceptions. J Am Acad Dermatol32:S26-30.[Crossref]
- Lanoue J, Goldenberg G (2015) Acne scarring: a review of cosmetic therapies. Cutis95:276-81.[Crossref]
- Augusti KT (1996) Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J Exp Biol34:634-40.[Crossref]
- Clarke LF, Baker B, Trahan C, Myers L, SE M (1999) A prospective double-blinded study of Mederma skin Care vs. Placebo for post-traumatic scar reduction. Cosmetic Dermatology.
- Zurada JM, Kriegel D, Davis IC (2006) Topical treatments for hypertrophic scars. J Am Acad Dermatol55:1024-31.[Crossref]
- Draelos ZD, Baumann L, Fleischer AB, Plaum S, Avakian EV, et al. (2012) A new proprietary onion extract gel improves the appearance of new scars: a randomized, controlled, blinded-investigator study. J Clin Aesthet Dermatol5:18-24.[Crossref]
- Fitzpatrick TB, Pathak M, Parrish JA (1974) Protection of human skin against the effects of the sunburn ultraviolet (290-320 nm). In: Fitzpatrick TB, al. (Eds) Sunlight and Man, normal and abnormal photobiological responses pp. 751.
- Tan J, Beissert S, Cook-Bolden F, Chavda R, Harper J,et al. (2001) Evaluation of psychological well-being and social impact of atrophic acne scarring: A multinational, mixed-methods study. JAAD Int6:43-50.[Crossref]




