The synthesis of indium(III) complex of orotic acid (HOA) was described and the composition and the structure of In(III) complex ware determined by means of analytical and spectral analyses. Detailed vibrational analysis of HOA, sodium salt of HOA (NaOA) and In(III)-OA systems based on both the calculated and experimental spectra confirmed the suggested metal-ligand binding mode. The calculated vibrational wavenumbers including IR and Raman scattering activities for the ligand and its In(III) complex were in good agreement with the experimental data. The vibrational analysis performed for the studied species, orotic acid, sodium salt of orotic acid and its In(III) complex, helped to explain the vibrational behaviour of the ligand vibrational modes, sensitive to interaction with In(III). The compounds HOA, NaOA and InOA were investigated for possible antioxidant activity in a model of non-enzyme-induced lipid peroxidation on isolated rat microsomes. On isolated rat microsomes, administered alone, the compounds didn’t revealed pro-oxidant effects. In conditions of non-enzyme-induced lipid peroxidation, only the complex InOA showed antioxidant activity. HOA and NaOA didn’t reveal antioxidant activity. We suggest that the antioxidant activity of the complex InOA, might be due to the presence of indium(III) in the structure of InOA.
Key words
In(III) complex, orotic acid, FT-Raman, FT-IR, antioxidant activity
Introduction
Coordination compounds of orotic acid and its substituted derivatives continue to attract attention because of the multidentate functionality of the ligand and its role in bioinorganic and pharmaceutical chemistry [1-5]. Orotic acid (2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid, HOA) (Figure 1) is a natural substance classified more than 40 years ago as vitamin B13 and mainly used in the past for the treatment of pernicious anaemia. Its major metabolic role within the human body consists of that it is the first fully formed intermediate in the manufacture of the pyrimidine bases required for the RNA/DNA synthesis. Metal orotates are also widely applied in medicine [6,7].
Figure 1. The structure of the ligand orotic acid
The structure of sodium salt of orotic acid NaOA is presented in (Figure 2). Orotic acid acts as a diacid in aqueous solution [8,9]. The coordinated orotate anions exhibit a ligand surface with double or triple hydrogen-bonding capabilities, depending on the metal coordination mode, and have thus a potential to adopt several modes of interligand hydrogen bonding. Orotic acid has demonstrated versatile coordination modes during the formation of coordination frameworks, that is why it was a challenge for us to obtain new metal coordination complexes with orotic acid, especially in view of their application as anticancer and antioxidant agents. We have recently synthesized lanthanide(III) complexes with a number of biologically active ligands, and we reported their significant antioxidant and cytotoxic activity in different human cell lines [10-16]. These promising results prompted us to search for new metal complexes with orotic acid. Thus, the aim of this work was to synthesize and characterize a complex of indium(III) with orotic acid in view of determination of its antioxidant activity.
Figure 2. The structure of sodium salt of orotic acid
Group IIIa metals especially gallium and indium are of major current interest as components of medicinal inorganic therapeutic and diagnostic agents. It has been shown that not only gallium, but also indium and in some cases aluminum salts prevent the growth of various experimental solid tumors [17,18]. Gallium is the second metal ion, after platinum, to be used in cancer treatment. Its activities are numerous and various. It modifies three-dimensional structure of DNA and inhibit’s its synthesis, modulates protein synthesis, inhibit’s the activity of a number of enzymes, such as ATPases, DNA polymerases, ribonucleotide reductase and tyrosine-specific protein phosphatase. Gallium alters plasma membrane permeability and mitochondrial functions [17,18]. It has been revealed that indium curcumin complex, diacetylcurcumin, and indium diacetylcurcumin have anticancer activity. New Ga(III) and In(III) compounds with a better bioavailability are now under clinical investigations and could improve the anticancer and antioxidant activity first demonstrated with their inorganic salts [18].
In this paper we report analytical and spectroscopic results about the new In(III) complex of orotic acid (HOA). The In(III)-OA binding mode was identified and characterized by elemental analysis, IR and Raman spectroscopies. For estimation of the most preferred reactive sites of HOA for electrophilic attack and metal binding, DFT calculations of the vibrational structure of HOA have been performed. The compounds HOA, NaOA and InOA have been investigated for possible antioxidant activity in a model of non-enzyme-induced lipid peroxidation on isolated rat liver microsomes, model of lipid membrane.
Materials and Methods
Chemistry
Synthesis of the coordination complex: The compounds used for preparing the solutions were Sigma-Aldrich products, p.a. grade: In(NO3)3. The sodium salt of orotic acid was used for the preparation of the metal complex as a ligand. The complex was synthesized by reaction of indium(III) nitrate and the sodium salt of orotic acid in aqueous solution, in amounts equal to metal: ligand molar ratio of 1: 3. The complex was prepared by adding an aqueous solution of indium(III) salt to an aqueous solution of the sodium salt of orotic acid. The reaction mixture was stirred with an electromagnetic stirrer at 25 oC for one hour. At the moment of mixing of the solutions, precipitate was obtained. The precipitate was filtered (pH of the filtrate was 5.0), washed several times with water and dried in a desiccator to constant weight.
The complex was insoluble in water, methanol and ethanol and well soluble in DMSO.
Chemistry device descriptions: The carbon, hydrogen and nitrogen contents of the compound were determined by elemental analysis. The water content was determined by thermogravimetrical analysis.
The solid-state infrared spectra of the ligand and its In (III) complex were recorded in KBr in the 4000-400 cm-1 frequency range by FT-IR 113V Bruker spectrometer.
The Raman spectra of orotic acid and its new In(III) complex were recorded with a Dilor Labram spectrometer (Horiba-Jobin-Yvon, model LabRam) using the 784.8 nm excitation line from a near infrared Diode laser. The Labram integrated system is coupled trough an Olympus LMPlanFL 50x objective to the optical microscope. The spectra were collected in the backscattering geometry with a resolution of 2 cm-1. The detection of Raman signal was carried out with a Peltier-cooled CCD camera. The laser power of 35 mW was used in our measurements.
Computational details: The geometry of orotic acid was optimized using the Gaussian 03 program [19]. Becke’s three-parameter exchange functional (B3) [20] with Perdew and Wang’s gradient-corrected correlation functional (PW91) [21,22] and Becke’s three-parameter hybrid exchange functional (B3) [23,24] using the LYP correlational functional of Lee, Yang and Parr (LYP) [25,26] were employed in the DFT calculations. The 6-311++G** Pople split valence basis sets along with the LANL2DZ basis set implemented in the Gaussian 03 program19 were chosen in the geometry optimization and normal modes calculations.
Using the fully optimized molecular geometry we performed the density functional theory (DFT) calculations on harmonic vibrational modes for the ligand. Harmonic vibrational wavenumbers including IR and Raman intensities were calculated analytically for the fully optimized molecular geometry of the ligand. Only real harmonic vibrational wavenumbers were obtained for all structures, confirming the localization of global minima on the potential energy surfaces.
Pharmacology
In our experiments, KH2PO4, K2HPO4, KCl, (Scharlau Chemie SA, Spain), 2-thiobarbituric acid (4,6-dihydroxypyrimidine-2-thiol; TBA), Fenol reagent, FeSO4, Ascorbinic acid (Sigma Aldrich), trichloroacetic acid (TCA) and Glycerol (Valerus, Bulgaria) were used.
Animals: Male Wistar rats (body weight 200-250 g) were used. The rats were housed in plexiglass cages (3 per cage) in a 12/12 light/dark cycle, under standard laboratory conditions (ambient temperature 20 ± 2 � C and humidity 72 ± 4 %) with free access to water and standard pelleted rat food 53-3, produced according to ISO 9001:2008.
Animals were purchased from the National Breeding Centre, Sofia, Bulgaria. At least 7 days of acclimatization was allowed before the commencement of the study. The health was monitored regularly by a veterinary physician. The vivarium (certificate of registration of farm ? 0072/01.08.2007) was inspected by the Bulgarian Drug Agency in order to check the husbandry conditions (? A-11-1081/03.11.2011). All performed procedures were approved by the Institutional Animal Care Committee and made according Ordinance ? 15/2006 for humaneness behaviour to experimental animals. The principles stated in the European Convention for the Protection of Vertebrate Animals used for Experiments and other Scientific Purposes (ETS 123) (Council of Europe, 1991) were strictly followed throughout the experiment.
Isolation of liver microsomes: Liver is perfused with 1.15 % KCl and homogenized with four volumes of ice-cold 0.1 M potassium phosphate buffer, pH=7,4. The liver homogenate was centrifuged at 9 000 x g for 30 min at 4°C and the resulting post-mitochondrial fraction (S9) was centrifuged again at 105 000 x g for 60 min at 4°C. The microsomal pellets were re-suspended in 0.1 M potassium phosphate buffer, pH=7.4, containing 20 % Glycerol. Aliquots of liver microsomes were stored at -70°C until use [27].
The content of microsomal protein was determined according to the method of Lowry using bovine serum albumin as a standard [28].
FeSO4/Ascorbic acid-induced lipid peroxidation in vitro: As a system, in which metabolic activation may not be required in the production of lipid peroxide, 20 µM FeSO4 and 500 µM Ascorbic acid were added directly into rat liver microsomes and incubated for 20 min at 37 °C [29].
Microsomes’ incubation with HOA, NaOA and InOA: Liver microsomes were incubated with concentration 100 μM of the investigated compounds [30].
Lipid peroxidation in microsomes: After incubation of microsomes (1 mg/ml) with the compounds, we added to the microsomes 1 ml 25 % (w/v) trichloroacetic acid (TCA) and 1 ml 0.67 % 2-Thiobarbituric acid (TBA). The mixture was heated at 100 °C for 20 min. The absorbance was measured at 535 nm, and the amount of MDA was calculated using a molar extinction coefficient of 1.56 x 105 M-1cm-1 [29].
Statistical analysis: Statistical analysis was performed using statistical programme ‘MEDCALC’. Results are expressed as mean ± SEM for 5 experiments. The significance of the data was assessed using the nonparametric Mann-Whitney test. A level of P < 0.05 was considered significant. Three parallel samples were used.
Results and Discussion
Chemistry
The new complex was characterized by elemental analysis. The content of the metal ion was determined after mineralization. The water content in the complex was determined thermogravimetrically. IR and Raman spectra confirmed the nature of the complex.
The data of the elemental analysis of the new indium(III) complex obtained serving as a basis for the determination of its empirical formula are presented below.
Elemental analysis of In(III) complex of orotic acid: (% calculated/found): In(OA)3.4H2O: C: 25.57/25.47; H: 2.41/2.24; N: 11.93/11.79; H2O: 10.23/10.25; In: 23.72/24.06, where HOA = C5N2O4H4 and OA- = C5N2O4H3-.
The mode of bonding of the ligand to In(III) ions was elucidated by recording the IR and Raman spectra of the complex as compared with those of the free ligand and the theoretical predictions. The vibrational fundamentals from the IR and Raman spectra were analysed by comparing these modes with those from the literature [31-35] in combination with the results of our DFT calculations (i.e., harmonic vibrational wavenumbers and their Raman scattering activities) for the ligand [34] and for the In(III) complex.
Vibrational spectroscopy
In (Table 1) the selected calculated and experimental IR and Raman data together with their tentative assignments are given. The vibrational IR and Raman spectra of HOA, sodium salt of orotic acid NaOA and In(III)–OA are presented in (Figures 3) and (Figures 4). Significant differences in the IR and Raman spectra of the complex were observed as compared to the spectra of the ligand and confirmed the suggested metal-ligand binding mode. The vibrational fundamentals from the IR and Raman spectra were analysed by comparing these modes with those from the literature34 in combination with the results of our DFT calculations (i.e., harmonic vibrational wavenumbers and their Raman scattering activities).
Table 1. Selected theoretical and experimental IR and Raman wavenumbers (cm-1) of orotic acid (HOA) and its In(III) complex (InOA) and their tentative assignment
Frequency
scaled |
Experimental Raman
HOA InOA |
Experimental IR
HOA InOA |
Assignments |
253 |
|
306 |
|
|
ν(O1-In) |
269 |
|
|
|
|
ν(O1’-In), ν(O3’-In) |
337 |
|
345 |
|
|
ν(C6-C7) |
380 |
395 |
383 |
|
|
ν(O1’-In), ν(C7’-In), ν(O3’-In), δ(C5’-C4’-O4’), δ(N3’-C2’-O2’), δ(N1’-C2’-O2’), δ(N3’-C4’-O4’) |
409 |
|
|
|
|
δ(C5-C4-O4), γ(O5-H6), δ(C2-N3-C4), δ(N1-C2-O2) |
434 |
|
440 |
|
473 |
ν(O1’-In), ν(O3’-In), ν(C7’-In) |
464 |
460 |
470 |
451 |
|
δ(C6-C7-O1), ν(O1-In), δ(C7-O1-In), δ(N1-C2-O2) |
480 |
|
|
|
|
γ(O5-H6), γ(H1-O5) |
506 |
513 |
520 |
507 |
517 |
γ(C7-O1), γ(N1-C6), γ(C6-C7) |
510 |
|
|
|
|
γ(C6’-C7’), δ(N1’-C6’-C5’) |
529 |
|
|
|
|
δ(C5’-C4’-N3’), δ(N3’-C4’-O4’), δ(C6’-C5’-C4’), δ(C2’-N1’-C6’) δ(N3’-C2’-O2’), δ(N1’-C2’-N3’), δ(C2’-N3’-H3’) |
533 |
546 |
553 |
555 |
538 |
γ(HI-O5), δ(N3-C4-O4), δ(C6-C5-C4), δ(C5-C4-N3), δ(C2-N1-C6) |
579 |
|
|
|
|
δ(C2-N3-C4), γ(C4-N3), δ(N1-C2-N3) |
582 |
|
|
|
|
δ(C2’-N3’-C4’), γ(C4’-N3’) |
596 |
608 |
602 |
603 |
595 |
ν(In-O5) |
615 |
|
|
|
|
γ(N1’-H1’) |
659 |
|
|
|
|
δ(N3-C2-N1), γ(N3-H3), γ(H1-N1) |
691 |
653 |
646 |
|
633 |
γ(N3’-H3’), γ(N1’-H1’) |
696 |
|
|
|
|
γ(N3-H3), δ(N1-C2-N2), γ(N1-H1) |
758 |
|
|
|
|
γ(O2-C2), γ(H5-C5) |
765 |
753 |
744 |
758 |
|
γ(C2’O), γ(C2’N) |
769 |
|
|
|
|
γ(C2-O2), γ(C2N) |
782 |
|
|
|
|
δ(C7O2), ν(O1-In) |
795 |
792 |
786 |
786 |
743 |
δ(C7’O2), ν(C7’-In) |
800 |
|
|
|
|
γ(O1-In), w(CO2), γ(C-H, N-H) |
810 |
|
|
|
|
w(CO2), γ(C-H) |
885 |
887 |
893 |
892 |
|
γ(C5’-H5’), γ(C5’-C4’), γ(C6’-C5’), γ(C4’-N3’) |
891 |
|
|
|
|
γ(C5-H5) |
930 |
933 |
949 |
930 |
911 |
ν(C6’-C7’) |
931 |
|
|
|
|
ν(C6-C7), δ(C2-N3-C4) |
973 |
1011 |
1016 |
1015 |
1027 |
δ(C2-N3-H3), δ(C2-N3-H3), δ(C6-C5-H5), δ(C2-N1-C6) |
992 |
1047 |
1047 |
1042 |
|
δ(C6’-C5’-C4’), δ(N1’-C6’-C5’), ν(C6’-C7’), δ(C2’-N1’-C6’) |
1071 |
|
1114 |
|
|
δ(C4’-C5’-H5’), δ(C6’-C5’-H5’) |
1075 |
1134 |
1145 |
1125 |
1137 |
δ(C6-C5-H5), δ(C4-C5-H5) |
1165 |
|
1205 |
|
|
ν(C4-N3) |
1169 |
1254 |
1241 |
1241 |
|
ν(C4’-N3’) |
1247 |
1282 |
1300 |
1284 |
1258 |
δ(N1’-H1’), δ(C5’-H5’) |
1281 |
1326 |
1319 |
1345 |
1320 |
δ(N1-H1), δ(C5-H5) |
1383 |
1414 |
1380 |
1407 |
1401 |
νs(C7’O2),ν(C6’-C7’) |
1464 |
1522 |
1479 |
1522 |
1460 |
ν(N1’-C6’), ν(C7’-O3’), ν(C6’-C7’) |
1512 |
|
|
|
|
νas(C7’-O2) |
1612 |
1615 |
1622 |
1617 |
1631 |
ν(C6-C5) |
1623 |
|
|
|
|
ν(C6’-C5’) |
1721 |
1657 |
1671 |
1684 |
1698 |
ν(C4-O4) |
1725 |
|
|
|
|
ν(C4’-O4’) |
1727 |
|
1748 |
|
|
ν(C7-O3) |
1736 |
1715 |
1714 |
1712 |
|
ν(C2-O2) |
1766 |
|
|
|
|
ν(C2’-O2’) |
3138 |
|
|
|
|
ν(C5’-H5’) |
3354 |
|
|
3520 |
3437 |
ν(N1-H1), ν(H1-O5) |
3642 |
3144 |
|
3232 |
3199 |
ν(O5-H6) |
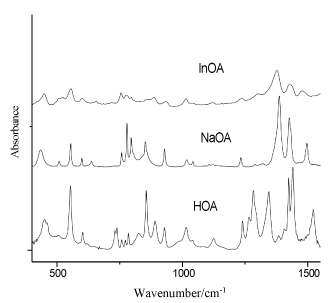
Figure 3. IR spectra of orotic acid (HOA), sodium salt of orotic acid (NaOA) and its In(III) complex (400-2000 cm-1)
In the 3600-2000 cm-1 spectral region from the IR spectrum the O-H and N-H stretches give rise to medium IR bands (Figure 3). The O-H and N-H bands appear overlapped in the same spectral region, and the involvement of these groups in hydrogen bonds affects their wavenumbers and produces a relevant band broadening [32-35]. In the IR spectrum of orotic acid the medium band at 3520 cm-1 was assigned to the N-H stretching modes, while the shoulder at 3232 cm-1 was attributed to the O-H stretching modes (Table 1). The wavenumber region 2700-2500 cm-1 in the IR spectra of orotic acid and its complex is typical of strongly hydrogen bonded intermolecular complexes with overtones and combinations of lower frequency modes of the bonded molecules [36-43].
When the carbonyl is hydrogen bonded but not dimerized, a bond active in both IR and Raman spectra appears at 1730-1705 cm-1. In our IR spectra, one very strong band can be observed in this region (at 1712 cm-1 for orotic acid), which was assigned to the symmetrical stretching mode of C2=O2 and to the N-H stretching mode. In this region were observed one medium band at 1715 cm-1 in the Raman spectrum of the free ligand, and two shoulders in the Raman spectrum of the complex (Figure 3) [36-40]. The very strong bands at 1684 cm-1 were assigned to the symmetrical stretching modes of C4=O4 and to the C6=C5 stretching modes. In the Raman spectra, these vibrations can be observed as very strong bands at 1657 and 1671cm-1 for orotic acid and its In(III) complex, respectively.
The asymmetrical COO- stretching mode was observed as a medium band at 1522 cm-1 (in the IR spectrum of orotic acid) and as a shifted shoulder (in the IR spectra of the complex). The symmetrical COO- stretching mode was observed in the IR spectra at 1407 and 1401 cm-1 for the ligand and its In(III) complex, respectively, while in the Raman spectra this vibration appears as a strong peak at 1414 cm-1 for the free ligand and as a medium band for the complex at 1380 cm-1 (Figure 4). In the 1800-900 cm-1 spectral region of the IR spectra, the bands at 1345 and 1320 cm-1 for the free ligand and its complex (Figure 3) can be due to the stretching modes of N-H. These vibrations are rather different and shifted in the Raman spectra for the free ligand and for the complex.
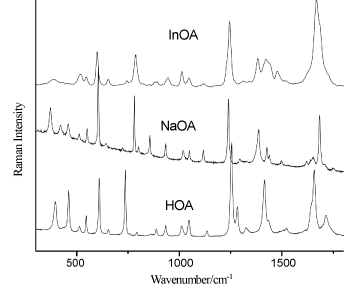
Figure 4. Raman spectra of the solid state of orotic acid (HOA), sodium salt of orotic acid (NaOA) and its In(III) complex. Excitation: 784.8 nm, 35 mW
The C5-H5, N1-H1, and C-O-H bending modes are present in the IR spectra as well as in the Raman spectra. In the IR spectra they are observed at 1284 cm-1 for the free ligand, while in the Raman spectra they are detected at 1282 cm-1. The weak peak at 1241 cm-1 that appear only in the IR spectrum of the free ligand, was assigned to the stretching modes of C2-N3-C4 and C6=C5-C4, while in the Raman spectra these vibrations appear also for the complex. The bands around 1015 cm-1, weak in IR and medium in Raman spectra, can be due to the symmetrical C=O stretching mode, whereas bands around 930 cm-1 almost weak in IR and medium in Raman spectra, were attributed to the symmetrical C(ring)-C(carboxyl) bridge bond stretching mode. The uracilate ring bending vibration and the skeletal deformation bands of the free orotic acid, mainly in the 900-300 cm-1 wavenumber region, show considerable changes on complex formation (Figures 3) (Figure 4) (Table 1). These changes may be attributed to distorsion of the uracilate rings upon coordination.
The new bands at 440-470 cm-1 in the IR and Raman spectra, which appear only for the In(III) complex, can be due to the indium-oxygen interactions [35]. In the low wavenumbers region of the Raman spectrum of orotic acid (Figure 4), the medium strong band at 395 cm-1 is importantly shifted to the shorter wavenumbers in the Raman spectrum of the In(III) complex and became weaker. This one and the new neighbouring band at 345 cm-1 (in the Raman spectrum of the complex) can be due to the indium-oxygen vibration modes [44-48]. The metal affects the carboxylate anion as well as the ring structure. The ionic potential of the metal is the most important parameter responsible for the influence of the metal on the rest of the molecule [49-51]. The carboxylic acids interact with the metals as symmetric [52,53], bidentate carboxylate anions and both oxygen atoms of the carboxylate are symmetrically bonded to the metal [54]. In this sense, we can observe in the Raman spectra of the In(III) complex a very weak peak at 306 cm-1, which can be due to the O-In-O vibration modes (Table 1) [48,55-57].
From our previous results regarding the newly synthesised lanthanide complexes and this work, it is clear that the nature of orotic acid makes its various anionic forms versatile ligands for use with a variety of metals and for a variety of objectives/advantages, including variable coordination modes. Thus, the ligand orotic acid has great potential as a generally useful polyfunctional ligand in metal coordination chemistry and it will prove attractive to a variety of coordination chemists.
On the basis of the detailed vibrational analysis the most probable structure of the obtained In(III) complex was suggested (Figure 5).
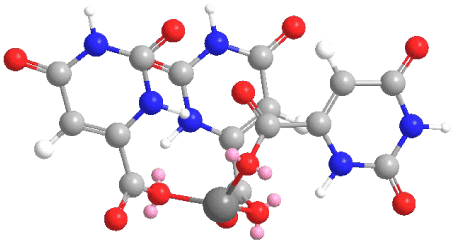
Figure 5. The suggested structure of In(III) complex of orotic acid
Pharmacology
Effects of HOA, NaOA and InOA on isolated rat liver microsomes: One of the most suitable sub-cellular in vitro systems for investigation of drug metabolism is isolated microsomes.
Administered alone, HOA, NaOA and InOA, didn’t reveal statistically significant toxic effects on isolated rat microsomes. The level of malondialdehyde (MDA), marker for lipid peroxidation, was not increased statistically significant from all compounds, compared to the control (non-treated microsomes) (Figure 6).
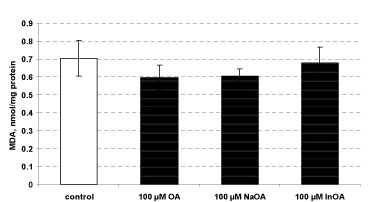
Figure 6. Effects of HOA, NaOA and InOA (at concentration 100 μM), administered alone, on isolated rat microsomes
In conditions of non-enzyme-induced lipid peroxidation, only the complex InOA revealed statistically significant antioxidant activity, compared to toxic agent – Fe2+/AA (iron/ascorbate). HOA and NaOA didn’t show antioxidant activity at this toxicity model (Figure 7).
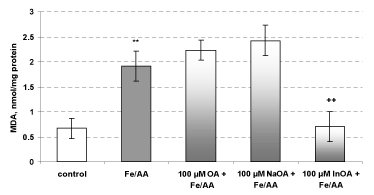
Figure 7. Effects of HOA, NaOA and InOA (at concentration 100 μM), in non-enzyme-induced lipid peroxidation, on isolated rat microsomes
** P < 0.01 vs control (non-treated microsomes)
+ P < 0.01 vs toxic agent (Fe2+/AA)
Microsomes incubation with Fe2+/AA, resulted in statistically significant increase of the amount of MDA with 191 % vs control (non-treated microsomes). In non-enzyme-induced lipid peroxidation model, the pre-teratement only with the complex InOA, at concentration 100 μM, significantly reduced lipid damage by 64 %, as compared to the toxic agent (Fe2+/AA). HOA and NaOA didn’t show antioxidant activity in this toxicity model (Figure 7).
The microsomal fraction, which is prepared by differential centrifugation, contents fragments from the endoplasmatic reticulum and preserve the enzyme activity, mostly cytochrome P450 enzymes. Microsomes are used as a model of lipid membrane in experiments, related to the process of lipid peroxidation [58]. Here, we show that only the complex InOA revealed statistically significant antioxidant effect in non-enzyme-induced lipid peroxidation in isolated microsomes. The effects of InOA might be due to the presence of metal ions.
Conclusion
The complex of indium(III) with orotic acid has been synthesized and characterized by elemental and vibrational (IR, Raman) analyses. The vibrational analysis performed for the studied species, orotic acid and its In(III) complex, helped to explain the vibrational behaviour of the ligand vibrational modes, sensitive to interaction with In(III). The most probable metal-ligand binding mode in the In(III) complex of orotic acid was elucidated. It is suggested that orotic acid binds through the oxygen atoms of the carboxylic groups from the ligands.
The results from the preliminary pharmacological investigations of orotic acid, sodium salt of orotic acid and In(III) complex demonstrate the antioxidant potential of the In(III) complex which is in line with our preceding papers concerning the activity of lanthanide coordination compounds with various biologically active ligands. The complex formation proved to be beneficial for the exerted efficacy of the In(III) complex vs. the corresponding ligand and its sodium salt. Thus, the newly synthesised In(III) complex necessitates further more detailed pharmacological evaluation.
Acknowledgement
The authors gratefully acknowledge the financial support from the Medical University-Sofia Grant Commission (Grant ?78/2018).
References
- Dodin G, Dubois JE (1980) Tautomerism of orotic acid dianion. effect of calcium and magnesium cations on the tautomeric constant and on tautomerization dynamics. J Am Chem Soc 102: 3049-3056.
- Arrizabalaga P, Castan P, Dahan F (1983) Coordination sites of 5-nitro-6-carboxyuracil: UV study and x-ray structure determination of diammine(5-nitroorotato)copper(II) hydrate and hexaamminebis(5-nitroorotato)tricopper(II) pentahydrate. Inorg Chem 22: 2245-2252.
- Arrizabalaga P, Castan P, Laurent JP (1984) Intramolecular influence of a carboxylic function on platinum blue synthesis, A systematic study of complexes originating from acid amides. J Am Chem Soc 106: 4814-4818.
- Lea MA, Luke A, Assad A, Patel M, Reddy PA (1992) Inhibitory action of orotate, 2-thioorotate and isoorotate on nucleotide metabolism and nucleic acid synthesis in hepatoma cells. Int J Biochem 24: 1453-1459. [Crossref]
- Casas JS, Sordo J, Hiller W, Strahle J (1991) Reaction of dimethylhydroxythallium(III) with 2-thioorotic acid, Crystal structure of dimethyl(2-thioorotato)thallium(III) monohydrate. Inorg Chim Acta 181: 43-49.
- Hueso-Ureña F, Moreno-Carretero MN, Salas-Peregrin JM, De Cienfuegos-Lopez GA (1995) Silver(I), palladium(II), platinum(II) and platinum (IV) complexes with isoorotate and 2-thioisoorotate ligands: synthesis, I.R. and N.M.R. spectra, thermal behaviour and antimicrobial activity. Trans Metal Chem 20: 262-269.
2021 Copyright OAT. All rights reserv
- Hueso-Ureña F, Moreno-Carretero MN, Romero-Molina MA, Salas-Peregrin JM, Sanchez-Sanchez MP (1993) Transition metal complexes with monodeprotonated isoorotic and 2-thioisoorotic acids: Crystal structure, spectral and magnetic study, and antimicrobial activity. J Inorg Biochem 51: 613-632.
- Kumberger O, Riede J, Schmidbaur H (1993) Preparation and crystal structure of zinc bis[orotate(1-)] octahydrate. Zeits Naturforsch B 48: 961-964.
- Bach I, Kumberger O, Schmidbaur H (1990) Orotate complexes. Synthesis and crystal structure of lithium orotate(I) monohydrate and magnesium bis[orotate(I)] octahydrate. Chem Berich 123: 2267-2271.
- Kostova I, Trendafilova N, Momekov G (2005) Theoretical and spectroscopic evidence for coordination ability of 3,3’-benzylidenedi-4-hydroxycoumarin. New neodymium (III) complexes and its cytotoxic effect. J Inorg Biochem 99: 477-487.
- Kostova I, Traykova M (2006) Cerium(III) and neodymium(III) complexes as scavengers of X/XO-derived superoxide radical. Med Chem 2: 463-470. [Crossref]
- Kostova I, Peica N, Kiefer W (2006) Theoretical and spectroscopic studies of lanthanum (III) complex of 5-aminoorotic acid. Chem Phys 327: 494-505.
- Kostova I, Momekov G (2008) Synthesis, spectral and pharmacological studies on lanthanide(III) complexes of 3, 5-pyrazoledicarboxylic acid. J Coord Chem 61: 3776-3792.
- Kostova I, Stefanova T (2009) Synthesis, characterization and cytotoxic/cytostatic activity of Sm(III) and Gd(III) complexes. J Coord Chem 62: 3187-3197.
- Kostova I, Valcheva-Traykova M (2015) New samarium(III) complex of 5-aminoorotic acid with antioxidant activity. Appl Organomet Chem 29: 815-824.
- Kostova I, Peica N, Kiefer W (2007) Theoretical and spectroscopic studies of 5-aminoorotic acid and its new lanthanide (III) complexes. J Raman Spectrosc 38: 205-216.
- Collery P, Keppler B, Madoulet C, Desoize B (2002) Gallium in cancer treatment. Crit Rev Oncol Hematol 42: 283-296. [Crossref]
- Mohammadi K, Thompson KH, Patrick BO, Storr T, Martins C (2005) Synthesis and characterization of dual function vanadyl, gallium and indium curcumin complexes for medicinal applications. J Inorg Biochem 99: 2217-2225.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA et al. (2003) Gaussian 03, Revision B, 04, Gaussian Inc, Pittsburgh PA, 12, 2003.
- Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98: 5648-5652.
- Perdew JP (1991) Electronic structure of solids; Eds. P. Ziesche, H. Eschrig; Akademie Verlag, Berlin, 11, 1991.
- Perdew JP, Wang Y (1992) Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys Rev B Condens Matter 46: 12947-12954. [Crossref]
- Becke AD (1992) Density-functional thermochemistry. II. The effect of the Perdew-Wang generalized-gradient correlation correction. J Chem Phys 97: 9173-9177.
- Gelfand LS, Iaconianni FJ, Pytlewski LL, Speca AN, Mikulski CM (1980) Nicotinic and isonicotinic acid n-oxide interactions with 3d metal perchlorates. J Inorg Nucl Chem 42: 377-385.
- Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37: 785-789. [Crossref]
- Dunning TH, Hay PJ (1976) Modern Theoretical Chemistry; Eds. HF Schaefer; Plenum, New York, 3, 805, 1976.
- Deby C, Goutier R (1990) New perspectives on the biochemistry of superoxide anion and the efficiency of superoxide dismutases. Biochem Pharmacol 39: 399-405. [Crossref]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. [Crossref]
- Kim HJ, Chun YJ, Park JD, Kim SI, Roh J K, et al. (1997) Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by red ginseng saponin through cytochrome P450 inhibition. Planta Med 63: 415-418. [Crossref]
- Oh KY, Roberts VH, Schabel MC, Grove KL, Woods M, Frians AE (2015) Gadolinium chelate contrast material in pregnancy: fetal biodistribution in the nonhuman primate. Radiology 276: 110-118. [Crossref]
- Hernanz A, Billes F, Bratu I, Navarro R (2000) Vibrational analysis and spectra of orotic acid. Biopolymers 57: 187-198. [Crossref]
- Icbudak H, Olmez H, Yesilel OZ, Arslan F, Naumov P (2003) Syntheses, characterization and crystal structures of novel amine adducts of metal saccharinates, orotates and salicylates. J Mol Struct 657: 255-270.
- Papaefstathiou GS, Manessi S, Raptopoulou CP, Behrman EJ, Zafiropoulos TF (2004) The first metal complex of 5-hydroxyorotic acid: dimethylammonium bis (N, N-dimethylformamide) bis (5-hydroxyorotato(-2)) gallate (III). Inorg Chem Comm 7: 69-72.
- Kostova I, Peica N, Kiefer W (2007) Theoretical and spectroscopic studies of new lanthanum (III) complex of orotic acid. Vibr Spectrosc 44: 209-219.
- Lencioni S, Pellerito A, Fiore T, Giuliani AM, Pellerito L (1999) Organometallic complexes with biological molecules. X: Dialkyltin (IV) and trialkyltin (IV) orotates: Spectroscopic and in vivo investigations. Appl Organomet Chem 13: 145-158.
- Exner K, Fischer G, Bahr N, Beckmann E, Lugan M (2000) Proximate, syn-periplanar bisdiazene skeletons: Syntheses, structures, homoconjugate reactivity and photochemistry. Eur J Org Chem 2000: 763-785.
- Castaneda JP, Denisov GS, Kucherov SY, Schreiber VM, Shurukhina AV (2003) Infrared and ab initio studies of hydrogen bonding and proton transfer in the complexes formed by pyrazoles. J Mol Struct 660: 25-40.
- González-Sánchez F (1958) Infra-red spectra of the benzene carboxylic acids. Spectrochim Acta 12: 17-33.
- Hadzi D, Sheppard N (1953) The infra-red absorption bands associated with the COOH and COOD groups in dimeric carboxylic acids. I. The region from 1500 to 500 cm-1. Proc Roy Soc Ser A 216: 247-266.
- Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, Edited by Jovanovich HB, Academic Press Inc., San Diego 140, 1991.
- Dolish FR, Fateley WG, Bentley FF (1974) Characteristic Raman Frequencies of Organic Compounds, John Wiley&Sons Inc. 128, 1974.
- Orza JM, Garci´a MV, Alkorta I, Elguero J (2000) Vibrational spectra of 3,5-dimethylpyrazole and deuterated derivatives. Spectrochim Acta A 56: 1469-1498. [Crossref]
- Szabó A, Cešljevic VI, Kovács A (2001) Tautomerism, hydrogen bonding and vibrational properties of 4-acetyl-3(5)-amino-5(3)-methylpyrazole. Chem Phys 270: 67-78.
- Sourisseau C, Fouassier M, Mauricot R, Boucher F, Evain M (1997) Structure and bonding in cerium oxysulfide compounds. II Comparative lattice dynamics calculations on Ce2O2S and Ce2.O2.5S. J Raman Spectrosc 28: 973-978.
- Lu Y, Deng G, Miao F, Li Z (2003) Metal ion interactions with sugars. The crystal structure and FT-IR study of the NdCl3-ribose complex. Carbohydr Res 338: 2913-2919. [Crossref]
- Sohn JR, Chun EW, Pae YI (2003) Spectroscopic studies on ZrO2 modified with MoO3 and activity for acid catalysis. Bull Korean Chem Soc 24: 1785-1792.
- Jayaraman A, Sharma SK, Wang SY, Shieh SR, Ming LC (1996) Pressure-induced phase transitions in KDy(MoO4)2 and KY(MoO4)2: a high-pressure Raman study. J Raman Spectrosc 27: 485-490.
- Fielicke A, Meijer G, Von Helden G (2003) Infrared multiple photon dissociation spectroscopy of transition metal oxide cluster cations. Eur Phys J 24: 69-72.
- Lewandowski W, Dasiewicz B, Koczon P, Skierski J, Dobrosz-Teperek K (2002) Vibrational study of alkaline metal nicotinates, benzoates and salicylates. J Mol Struct 604: 189-193.
- Koczon P, Lewandowski W, Mazurek AP (1999) Vibrational (FT-IR and FT-Raman) and NMR studies on selected metal (Ca, Mn, Zn) complexes with ortho-, meta-, and para-iodobenzoic acids. Vibr Spectrosc 20: 143-149.
- Kakiuchi M, Abe T, Nakayama H (2001) D/H fractionation factor between water vapor and crystal water of copper chloride dihydrate: Statistical mechanical approach based on Raman spectra. Geochem J 35: 285-293.
- Wang K, Li YS (1997) Silver doping of polycarbonate films for surface-enhanced Raman scattering. Vibr Spectrosc 14: 183-188.
- Boerio FJ, Hong PP, Clark PJ, Okamoto Y (1990) Surface-enhanced Raman scattering from model acrylic adhesive systems. Langmuir 6: 721-727.
- Kwon YJ, Dong HS, Sang JA, Myung SK, Kwan K (1994) Vibrational spectroscopic investigation of benzoic acid adsorbed on silver. J Phys Chem 98: 8481-8487.
- Galdecka E, Galdecki Z, Huskowska E, Amirkhanov V, Legendziewicz J (1997) Crystal structure and optical properties of Ln(III) octahedral complexes with hexamethylphosphortriamide;[Ln(HMPA)6](ClO4)3. J Alloys Comp 257: 182-190.
- de Andrés A, Taboada S, Martínez JL, Salinas A, Hernández J, et al. (1993) Optical phonons in R2BaMO5 oxides with M=Co, Ni, Cu, and R=a rare earth. Phys Rev B Condens Matter 47: 14898-14904. [Crossref]
- Cho BO, Lao SX, Chang JP (2003) Origin and effect of impurity incorporation in plasma-enhanced ZrO2 deposition. J Appl Phys 93: 9345-9351.
- Zaidi SI, Agarwal R, Eichler G, Rihter BD, Kenney ME (1993) Pharmacodynamic effects of new silicon phthalocyanines: In vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem Photobiol 58: 204-210.







