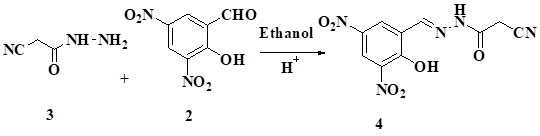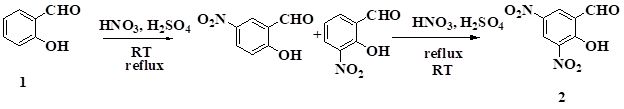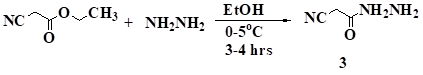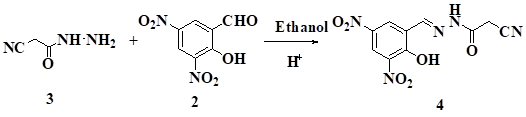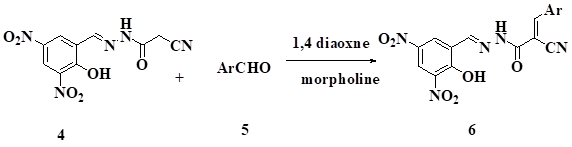The (E)-N'-(2-hydroxy-3, 5-dinitrobenzylidene)-2-cyanoacetohydrazide was obtained by reacting2-hydroxy-3,5 -dinit robenzaldehydewith 2-cyanoacetohydrazide. The new compounds were characterized by IR, NMR and Mass spectroscopy.
Cyanoacetohydrazide, 2-Hydroxy-3,5dinitrobenzylidene
Hydrazide and hydrazones are important precursors, used for the synthesis of N-heterocycles [1,2]. The antibiotic resistant organisms are considered as important pipeline for the discovery of new antimicrobial agents [3]. Hydrazide and hydrazones showed Pharmacological profiles such as antimicrobial [4,5] anti-tubercular [6,7] anticonvulsant [8,9] anti-inflammatory [10,11] antidepressant [12] antitumor [13] and analgesic activities [14]. Hydrazones also act as orally effective drugs for the treatment of iron overload disease or genetic thalassemia [15] and Anti-hepatitis C virus activity (HCV) activity [16]. We focused our work on synthesis of novel multi-functionalized heterocycles having potential bioactivity. We have concentrated our efforts towards the synthesis of (E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-2-cyanoacetohydrazide.
Experimental section
Synthesis of 2-hydroxy-3,5-dinitrobenzaldehyde, 2: The mixture of Salicylaldehyde (0.2 mol,20.96 ml) and 10 ml Conc. HCl was cooled in ice-salt mixture (0oC). Then the reaction mass was added drop wise to ice cooled nitrating mixture (Conc.H2SO4: Conc.HNO3; 2:1) at 0 oC for 20 minutes. It was stirred for 2-3 hrs at room temperature. The product obtained as the mixture of 3-and 5-nitrosalicylaldehyde was filtered, washed with water and dried. This product is used for further nitration.
The mixture of 3-and 5-nitrosalicylaldehyde obtained by the nitration of salicylaldehyde was stirred with ice cold nitrating mixture of conc.H2SO4 and HNO3 in 2:1 proportion.(33g ,0.156 mol) After 30 minutes the reaction mass was poured on ice. The yellow solid of 2-hydroxy-3,5-dinitrobenzaldehyde2 was obtained in 85% yield, M.P.70-74 oC. Its structural assignment of this compound was performed by IR,1H NMR and elemental analysis.
M.P.:70-74oC;IR V cm-1:1490 (NO2),2750(CH), 1725(CO), 3200 (OH); 1H NMR 500 MHz (CDCl3): 11.95 (s, 1H, OH), 10.44 (S, 1H, CH), 8.95 (S, 1H, CH), 9.23(S, 1H, CH);Anal.calcd. forC7H4N2O6( Mol. Wt.: 212.12): Calcd C, 39.64; H, 1.90; N, 13.21 Found: C, 39.60; H, 1.92; N, 13.24
Synthesis of (E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-2-cyanoacetohydrazide,4
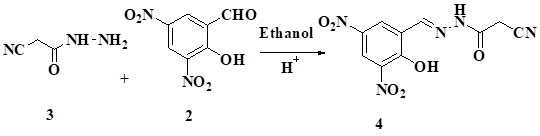
2-Hydroxy-3,5-dinitrobenzaldehyde 2 (0.212 g ,1mmol) in ethanol was added 1 drop of acetic acid and stirred for 30 min. To this reaction mixture 2-cyanoacetohydrazide(0.1 g, 1mmol ), 3 was added and stirred at room temperature. The yellow product obtained was recrystalised using ethanol. Yellow powder, M.P. 230oC; Yield: 0.129 g , 88% ; IR (Platinum ATR) cm−1: 2260 (CN ), 3271 (OH) and 3197 (NH) proton.1H NMR (DMSO-d6): δ, 10.2 (s, 1H, OH). 3.90 (s, 2H, CH2), 9.5 (s, 1H, NH), 9.8 (s,1H,CH), 10.8-11 (s, 2H,ArH).
C10H7N5O6 Mol. Wt.: 293.192.CalcdC, 40.97; H, 2.41; N, 23.89; Found C,, 40.98; H, 2.39; N, 23.90;
Synthesis of N'-(2-hydroxy-3,5-dinitrobenzylidene)- 2-cyano-3- substituted phenyl acrylohydrazide derivatives, 6: Equimolar mixture of 2-hydroxy-3,5-dinitrobenzylidene) -2-cyanoacetohydrazide 4 (0.1g,3 mmol) in 1,4-diaoxane containing (0.06ml, 3m mol) morpholine was stirred for 30 min. To this reaction mixture the (0.06 ml, 3mmol) benzaldehyde was added and refluxed at 140oC for 1 hrs(TLC Checked, Hexane: Ethyl acetate,8:2 v/v).The reaction mass was poured in to crush ice light brown color product was filtered and recrystallized from ethanol afforded compound 6a.
Similar procedure was used for the synthesis of compounds (6b-f).
(2E,14E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-2-cyano-3-phenylacrylohydrazide, 6a
Light brown solid, Yield: 60%, 0.62 mg, M.P. 60-65°C; IR (Platinum ATR) cm−1:stretching frequencies at 2333, 2920, 1658 cm−1 for CN, NH, C=O cm−1 respectively.1H NMR (500 MHz DMSO-d6): δ, 10.2 (s, 1H, OH). 8.56-8.51(s,J=7 Hz ,2H,CH),8.01 (s,1H, NH), 8.09(s,1H,CH),8.61(s,1H,CH),7.28-7.80 (m,5H,ArH) ppm.C17H11N5O6Mol. Wt.: 381.299,Calcd: C, 53.55; H, 2.91; N, 18.37, Found :C, 53.45; H, 2.96; N, 18.42
(2E,14E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-2-cyano-3-(4-methoxyphenyl)acrylohydrazide, 6b
Brown solid, Yield :66%, 0.72 mg, M.P.90-95°C; IR (Platinum ATR) cm−1:2337,2920,2854 cm-1for CN, NH, OH respectivly 1H NMR (500 MHz, CDCl3) δ 9.89 (s, 1H,OH ), 8.02 (s, 1H,CH), 7.99 (bs,1H,NH), 3.90 (s,3H ,CH3), 2.89 (s, 1H,CH), 6.93 (d, 2H J = 4.4 Hz,CH), 7.00 (d, 2H J = 4.4 Hz,CH), 8.62-9.20 (s, 2H, J= 4.4 Hz CH) ppm.C18H13N5O7Mol. Wt.: 411.33, Calcd:C, 52.56; H, 3.19; N, 17.03 ,Found C, 52.50; H, 3.25; N, 17.33
(2E,14E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-3-(4-chlorophenyl)-2-cyanoacrylohydrazide, 6c
Brown solid, Yield :70%, 0.80 mg, M.P.128-130°C; IR (Platinum ATR) cm−1:2337, 3093, 2967 cm-1for CN ,OH,NH. 1H NMR (500 MHz, CDCl3) δ 9.99 (s, 1H,OH), 8.61 (s, 1H,NH), 8.02-8.03 (s, J = 8.5 Hz, 2H), 7.52-7.26(dd, J=4.5, 4H, CH), 7.96 (s, 1H,CH), 7.98 (s, 1H,CH). C17H10ClN5O6Mol. Wt.: 415.744, Calcd :C, 49.11; H, 2.42; Cl, 8.53; N, 16.85. Found : C, 49.08; H, 2.45; Cl, 8.50; N, 16.88.
(2E,14E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-3-(4-bromophenyl)-2-cyanoacrylohydrazide, 6d
Brown solid, Yield :61%, 0.64 mg, M.P. 112-115°C;IR (Platinum ATR) cm−1:2337, 3093, 2967,650 cm-1for CN ,OH,NH, Br. 1H NMR (500 MHz, CDCl3) δ 9.99 (s, 1H,OH), 8.61 (s, 1H,NH), 8.71-8.90 (s, J = 8.5 Hz, 2H), 8.75 – 7.80 (dd, J=4.5, 4H, CH), 8.26 (s, 1H,CH),8.36 (s, 1H,CH)..
C17H10BrN5O6Mol. Wt.: 460.195, Calcd : C, 44.37; H, 2.19; Br, 17.36; N, 15.22. Found : C, 44.27; H, 2.29; Br, 17.16; N, 15.42.
(2E,14E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-3-(3-chlorophenyl)-2-cyanoacrylohydrazide, 6e
Brown solid, Yield :60%, 0.60 mg, M.P.102-105°C; IR (Platinum ATR) cm−1:
2337.72 for CN,3086 for OH and 2920 cm-1 forNH 800 cm-1for chlorine .1H NMR (500 MHz, CDCl3) δ1H NMR (500 MHz, CDCl3) δ 9.98 (s,1H ,OH), 7.80– 8.20 (m, 4H, ArH), 7.25 (t, J = 7.9 Hz, 1H),8.95-9.61 (s, J = 7.9 Hz ,2H), 8.01 (s,1H,NH),7.91 (s,1H,CH),C17H10ClN5O6 ,Mol. Wt.: 415.74Calcd: C, 49.11; H, 2.42; Cl, 8.53; N, 16.85. Found: C, 49.10; H, 2.32; Cl, 8.63; N, 16.84.
(2E, 14E)-N'-(2-hydroxy-3,5-dinitrobenzylidene)-2-cyano-3-(3-nitrophenyl)
Acrylohydrazide, 6f
Brown solid, Yield :62%, 0.69 mg, M.P. 118-120°C; IR (Platinum ATR) cm−1: 2337.72 for CN,3 086 for OH and 2920 cm-1 forNH .1H NMR (500 MHz, CDCl3) δ1H NMR (500 MHz, CDCl3) δ 10.13 (s,1H ,OH), 7.98 – 8.23 (m,4H,CH), 7.77 (t, J = 7.9 Hz, 1H),8.93-9.51 (s, J = 7.9 Hz ,2H), 7.99 (s,1H,NH), 8.74(s,1H,CH) C17H10N6O8Mol. Wt.: 426.3 , Calcd:C, 47.90; H, 2.36; N, 19.71. Found: C, 47.98; H, 2.32; N, 19.67
The physical constant ie melting point of all new compounds was reported with the help of Gallencamp melting point equipment (Model no MFB-595) using open capillary tubes. All the recorded melting points are uncorrected. Bruker FTIR-TENSOR-II was used to record IR spectra of the compounds. 1HNMR spectra of the compounds were recorded on Bruker advance II NMR instrument at 500 MHz frequency. The CDCl3 or DMSO was used to record the NMR using TMS as internal standard. Chemical shifts are given in δ ppm and splitting of NMR samples are given as singlet(s), broad singlet (bs), doublet (d), triplet (t), multiplets (m).The reactions were monitored on thin layer chromatography (TLC 0.2 mm silica gel 60 F254 Merck plates) plates using UV light 254 and 366 nm. All commercial grade chemicals were purchased from S.D. Fine chemicals, Sigma Aldrich, Merck, Lobachemie and used without further purification while solvents were purified by standard literature procedures.
Compound 2 was synthesized by nitration of salicylaldehydeto get ortho and para products. Further nitration of ortho and para products gave 2-hydroxy-3,5-dinitrobenzaldehyde 4 The structure of 2 was established on the basis of IR, 1H NMR data and comparison with the literature M.P.
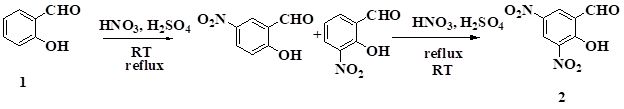
Compound 3 was synthesized by reaction of ethyl cyanoacetate and hydrazine hydrate in ethanol at 0-5oC temperature. The spectral and analytical data of these compounds was found identical with that of reported data.
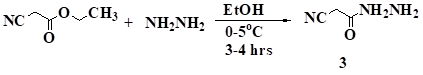
The reaction of 2-cyanoacetohydrazide 3 and 2-hydroxy-3,5-dinitrobenzaldehyde 2 in presence of ethanol and catalytic amount of acetic acid gave 2-hydroxy-3,5-dinitrobenzylidene)-2-cyanoaceto hydrazide 4. The compound 4 was characterized by IR, 1H NMR data. For e.g. IR spectrum showed stretching frequencies at 2260 cm-1 for CN, 3271 cm-1 for OH and 3197 cm-1for NH proton 1HNMR, 10.2 δ (s, 1H, OH), 3.90 δ(s,2H,CH2), 9.5 δ (s,1H, NH), 9.8 δ (s,1H,CH), 10.8-11 δ(s, 1H,CH)
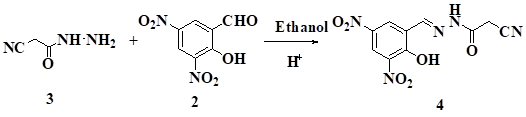
Benzalidine derivative 6 was synthesized by reaction of 2-hydroxy-3,5-dinitrobenzylidene)–2–cyanoacetohydrazide4with different aromatic aldehydes 5 a-f in presences of 1,4 diaoxaneand catalytic amount of morpholine in 60-70 % yield.
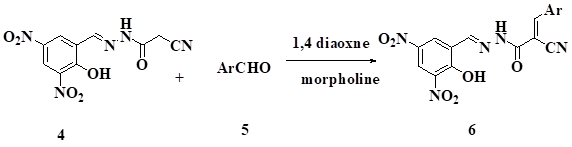
Antimicrobial assay
The antimicrobial (Table 1) assay of compound 6 was carried out using agar well plate method. The antibacterial and antifungal assays were performed in Muller-Hinton agar and crazek doxagar. The standard strains used for the antimicrobial assay was procured from Micro bialCulture Collection Centre, Pune, India. Antimicrobial evaluation was performed using the bacteria reseeded in Muller-Hinton broth for 24 hr at 37oC and fungi reseeded in crazek doxagar for 48 hr at 25oC. The antibacterial activity of tested samples were studied intriplicate against Gram positive bacteria Staphylococcus aureus(ATCC 29737) and Gram negative bacteria Escherichia coli (ATCC 25922). The same samples were tested for antifungal activity in triplicate against Candidaalbican s(MTCC 277) and Aspergillus Niger (MCIM 545) (Table 2).
Table 1. The compound 6 was well characterized byFTIR and 1H NMR
Compd. |
Ar |
% Yield |
5a |
Benzaldehyde |
60 |
5b |
Anisaldehyde |
66 |
5c |
3- Nitrobenzaldehyde |
62 |
5d |
4-Chlorobenzaldehyde |
70 |
5e |
4-Bromobenzaldehyde |
61 |
5f |
3- Chlorobenzaldehyde |
60 |
Table 2. Antimicrobial screening of compounds (7a-h): Inhibition Zone Diameter (mm)
Compound |
Ar |
E. coli |
S. aureus |
A.niger |
C. albicans |
6a |
Benzaldehyde |
13 ± 0.8 |
14 ± 1.2 |
13 ± 0.6 |
14 ± 0.8 |
6b |
Anisaldehyde |
15 ± 1.1 |
16 ± 0.7 |
17 ± 1.1 |
16 ± 0.8 |
6c |
4-Chlorobenzaldehyde |
16 ± 0.8 |
16 ± 0.8 |
18 ± 0.5 |
18 ± 0.9 |
6d |
4-Bromobenzaldehyde |
17 ± 0.8 |
18 ± 0.3 |
17 ± 0.7 |
18 ± 0.4 |
6e |
3- Chlorobenzaldehyde |
15 ± 1.1 |
16 ± 0.6 |
17 ± 0.3 |
16 ± 0.7 |
6f |
3-Nitrobenzaldehyde |
18 ± 0.8 |
17 ± 0.4 |
19 ± 0.3 |
18 ± 0.5 |
| |
DMSO |
11 ± 0.7 |
12 ± 0.9 |
12 ± 0.6 |
13 ± 0.3 |
| |
Gentamicin |
22 ± 0.4 |
23 ± 0.7 |
- |
- |
|
Fluconazole |
- |
- |
23 ± 0.8 |
24± 0.5 |
Gentamicin (10 μg/ mL) and Fluconazole (20 μg/ mL) Inhibition Zone = 9-14 mm: slight activity, 15-19 mm: moderate activity, 20 -24 mm : high activity, >25 mm: excellent activity
The solution of these compounds were prepared in DMSO at desired concentrations of 40, 20, 10 μg/ mL loaded as negative control. The Gentamicin (10 μg/ mL) and Fluconazole (20 μg/ mL) were used as standards for evaluating the antibacterial and antifungal activity. The zone of inhibition (mm) was determined as per National Committee for Chemical Laboratory Standards (NCCLS, M7-A5, and January 2000).The antimicrobial activity of 3- nitro benzaldehyde was more than 4-chlorobenzaldehyde and 4- bromobenzaldehyde.
The compound 6f exhibited excellent antibacterial activities against Gram positive and Gram negative bacteria viz. Staphylococcus aureus, Escherichia coli with MIC 10 μg/ mLas compared with Gentamicin (10 μg/ mL). Similarly, compound6fshowed excellent antifungal activities against Aspergillus Niger and Candida albicans with MIC 10 μg/ mLas compared with Fluconazole (20 μg/ mL). The compound 6b,6c,6dand 6e showed moderate antibacterial activity against Escherichia coli (ATCC25922) with MIC 20 μg/ mL when compared with standard antibacterial drug Gentamicin (10 μg/ mL). The compounds 6b, 6c, 6dand6eshowed excellent antifungal activities against Aspergillus Niger (MCIM 545). Similarly, compounds 6b, 6c,6d show equivalent antifungal activities against Candida albicans (MTCC 277) with MIC 20 μg/ mLas compared with standard antifungal drug Fluconazole (20 μg/ mL) (Table 3).
Table 3. Antimicrobial screening of compounds (7a-h): MIC in μg / mL values
Compound |
Ar |
E. coli |
S. aureus |
A.niger |
C. albicans |
6a |
Benzaldehyde |
80 |
40 |
80 |
40 |
6b |
Anisaldehyde |
40 |
40 |
20 |
20 |
6c |
4-chlorobenzaldehyde |
20 |
20 |
20 |
20 |
6d |
4-brombenzaldehyde |
20 |
20 |
20 |
20 |
6e |
3- chlorobenzaldehyde |
40 |
40 |
20 |
40 |
6f |
3- nitrobenzaldehyde |
10 |
10 |
10 |
10 |
| |
Gentamicin |
10 |
10 |
- |
- |
| |
Fluconazole |
- |
- |
20 |
20 |
Gentamicin (10 μg/ mL) and Fluconazole (20 μg/ mL) Inhibition Zone = 9-14 mm: slight activity, 15-19 mm:
moderate activity, 20 -24 mm : high activity, >25 mm: excellent activity
- Rallas S, Gulerman N, Erdeniz H, Farmaco (2002) 57: 171-174.
- Gursoy A, Terzioglu N, Otuk G (1997) Eur J Med CheM 32: 753-757.
- Walsh C (2000) Nature 406: 775-778.
- Rollas S, Kalyoncuoglu N, Sur-Altiner D, Ye-genoglu Y (1993) Pharmazie 48: 308-309.
- Papakonstantinou- Garoufalias S, Pouli N, Marakos P, Chytyro- Glouladas A, Farmaco (2002) 57: 973-977.
- Kidwai M, Kumar R, Srivastava A, Gupta HP (1998) Bioorganic Chemistry 26: 289-294.
- Kachhadia VV, Patel MR, Joshi HS (2005) Journal of the Serbian Chemical Society 70: 153-161.
- Kadaba PK (2003) Current Medicinal Chemistry 10: 2081-2108.
- kucukguzel I, Kucukguzel SG, Rollas S, Sanis GO, Ozdemir O, (2004) 59: 893- 901.
- Mullican MD, Wilson MW, Connor DT, Kostlan CR, Schrier DJ (1993) Journal of Medicinal Chemistry 36: 1090-1099.
- Palaska E, Şahin G, Kelicen P, Durlu NT, Altinok G, (2002) 57: 101-107.
- Varvaresou A, Siatra-Papastaikoudi T, Tsotinis A, TsantiliKakoulidou A, Vamvakides A (1989) Farmaco 53: 320-326.
- Holla BS, Veerendra B, Shivanada MK, Poo- jary B (2003) European Journal of Medicinal Chemistry 38: 759-767.
- Lange JH, Stuivenberg HH, Coolen HK, Adolfs TJ, McCreary AC (2005) Journal of Medicinal Chemistry 48: 1823-1838.
- Szuber N, Buss JL, Lin SS, Felfly H, Trudel M (2008) Exp. Hematol 36: 773-785.
- Soad A, EL-Hawash M, Abeer E. Abdel, (2006) Arch Pharm Chem. Life Sci 339: 14-23.