A novel series of rhodanine based amide derivatives have been prepared through multi-component reaction of rhodanine-N-acetic acid with aromatic aldehydes and alkyl isocyanides in the presence of aniline. The products were isolated in 85-95% yields by easy workup procedure. The synthesized compounds have also been evaluated for their antibacterial effects against four pathogenic bacteria, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis. On the basis of the obtained results, some compounds showed moderate growth inhibitory effect against all tested bacteria.
rhodanine, alkyl isocyanides, aniline, multi-component reaction, antibacterial activity
A major dispute of modern chemistry is the plan of new chemical reaction successions that supply novel compounds in high yields. Multicomponent reactions (MCRs) [1] have become precious tools for the synthesis of highly functionalized organic molecules and pharmacologically important heterocyclic compounds because of their convergence, simplicity and synthetic efficiency, atom economy, and other suitable characteristics from the point of view of green chemistry [2]. MCRs are quicker and cheaper than conventional reactions, since they are performed by mixing compounds together in one pot, without separating any intermediate. Isocyanide-based MCRs (IMCRs) are especially important, because they are more diverse and flexible than other MCRs [3,4]. Amides are an incredibly vital group of organic compounds with a variety of functions. Some derivatives of amides reveal biological properties such as vermifuge [5], antihistamine [6], fungicide and antibacterial [7,8]. The conventional approach for the synthesis of amides is the reaction of carboxylic acids with amines at high temperature. Because of carboxylic acid’s low activity, several procedures for their activation have been reported in the literature [9]. Negative aspects of these procedures consist of modest yields, by-products, costly coupling reagents and difficulty in removal of surplus reagents. Consequently, the growth of a new and uncomplicated synthetic procedure for the preparation of amides has become an interesting challenge.
On the other hand, rhodanine (2-thioxothiazolidin-4-one) as a privileged scaffold is found in a variety of biologically active compounds especially with antiviral [10], antibacterial [11], antifungal [12], antitubercular [13], anticancer [14], anticonvulsant [15], and hypnotic activities [16]. Therefore, following our interest in isocyanide-based reactions and our studies towards the development of new directions for the synthesis of novel organic compounds with biological activities [17-19], we herein report the multi-component reaction of aromatic aldehydes 1 with aniline 2, rhodanine-N-acetic acid 3 and isocyanides 4 in THF as a suitable procedure affording rhodanine-based amides 5 in good yields (Figure 1). The synthesized amides were also screened for their antibacterial activity by the disc diffusion method.
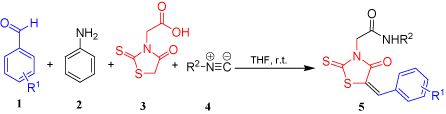
Figure 1. Multi-component reaction of rhodanine-N-acetic acid with aromatic aldehydes and alkyl isocyanides in the presence of aniline
General information
All chemicals and reagents were purchased from Merck (Darmstadt, Germany) or Aldrich (Missouri, United States), and used without purification. Melting points were measured on an Electrothermal 9100 (Staffordshire, United Kingdom) apparatus. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 AVANCE (Massachusetts, United States) spectrometer at 400.13 and 100.61 MHz, respectively. Chemical shifts are given in parts per million (δ) relative to internal tetramethylsilane standard and coupling constants (J) are reported in hertz (Hz). IR spectra were recorded on a Bruker Tensor 27 (Massachusetts, United States) spectrometer. Mass spectra were determined on a Finnigan-Matt 8430 (Waltham, United States) mass spectrometer operating at an ionization potential of 70 eV. Elemental analyses were carried on a Perkin-Elmer 2400II CHNS/O Elemental Analyzer (Massachusetts, United States).
General procedure for the synthesis of compounds (5a-5j)
A mixture of aromatic aldehydes 1 (1 mmol), aniline 2 (0.1 mL, 1 mmol), rhodanine-N-acetic acid 3 (0.2 g, 1 mmol) and alkyl isocyanides 4 (1 mmol) in 5.0 mL THF was stirred at 25 °C for the definite time period. After completion of the reaction, checked by TLC, the solvent was removed under reduced pressure to provide desired product. The structures of products 5a-j were determined on the basis of their elemental analysis, 1H and 13C NMR, IR and mass spectra.
General procedure for evaluation of antibacterial activity
The in vitro biocidal screening, antibacterial activities of the synthesized 2-(5-arylidene-4-oxo-2-thioxothiazolidin-3-yl)-N-alkylacetamide derivatives 5a-j was assayed using Kirby–Bauer disc diffusion method where a filter disc was impregnated with the compounds and placed on the surface of inoculated agar plates [20]. The synthesized compounds were dissolved into DMSO to achieve 20 mg mL-1 solution then filter sterilised using a 0.22 m Ministart (Sartorius).
The antibacterial activity of the compounds was investigated against four bacterial species. Test organisms included Escherichi coli PTCC 1330, Pseudomonas aeruginosa PTCC 1074, Staphylococcus aureus ATCC 35923 and Bacillus subtilis PTCC 102 [21]. Late exponential phase of the bacteria was prepared by inoculating 1% (v/v) of the cultures into the fresh Muller-Hinton broth (Merck) and incubating on an orbital shaker at 37°C and 100 rpm overnight. Before using the cultures, they were standardized with a final cell density of approximately 108 cfu mL-1. Muller-Hinton agar (Merck) were prepared and inoculated from the standardized cultures of the test organisms then spread as uniformly as possible throughout the entire media. Sterile paper discs (6 mm diameter, Padtan, Iran) were impregnated with 20 µL of the compound solution then allowed to dry. The impregnated disc was introduced on the upper layer of the seeded agar plate and incubated at 37°C for 24 hours. The antibacterial activities of the synthesized compounds were compared with known antibiotic gentamicin (10 µg/disc) and chloramphenicol (30 µg/disc) as positive control and DMSO (20 µL/disc) as negative control. Antibacterial activity was evaluated by measuring the diameter of inhibition zone (mm) on the surface of plates and the results were reported as Mean ± SD after three repeats.
The desired product 5a, confirmed by NMR and Mass spectra, was obtained in 90% yield (Figure 1) [22]. To illustrate the role of aniline, the reaction was checked in the absence of aniline. Interestingly, this reaction miscarried under reflux conditions, even after 24 h, and starting materials were recovered. Additionally, the three-component reaction of benzilidenerhodanine-N-acetic acid (1.0 mmol), benzaldehyde (1.0 mmol) and tert-butyl isocyanide (1.0 mmol) without aniline showed no progress in boiling THF even after long reaction time. Obviously, these results prove that aniline is a vital component for this reaction. Afterward the effect of solvent on this reaction was screened. The model reaction was carried out in different solvents including CH2Cl2, 1,4-dioxane, EtOH and H2O under similar conditions at ambient temperature (Table 1). As revealed in table 1, the maximum yield was observed in THF (Table 1). Therefore, the best result is achieved by the use of substrates in equimolar ratio in THF at room temperature.
Table 1. Optimization of reaction conditions for the synthesis of amide 5aa
Entry |
Solvent |
Temp/oC |
Time/h |
Yield/%b |
1 |
THF |
25 |
13 |
90 |
2 |
1,4-Dioxane |
25 |
46 |
25 |
3 |
CH2Cl2 |
25 |
40 |
20 |
4 |
EtOH |
25 |
48 |
5 |
5 |
H2O |
25 |
48 |
5 |
6 |
THF |
70 |
13 |
91C |
aReaction conditions: benzaldehyde (1 mmol), aniline (1 mmol), rhodanine-N-acetic acid (1 mmol), tert-butylisocyanide (1 mmol); bYields refer to isolated products; Cbenzaldehyde (2 mmol)
To explore the substrate scope of this novel procedure, the condensation reactions of various aromatic and heteroaromatic aldehydes 1a-1i with rhodanine-N-acetic acid 3, and alkyl-isocyanides (tert-butyl isocyanide 4a and cyclohexylisocyanide 4b) in the presence of aniline 2 were studied in THF at room temperature. Under optimum reaction conditions, a novel series of rhodanine based amides were synthesized, the results are listed in table 2. In all cases, aromatic aldehyde substituted with either electron-donating or electron-withdrawing groups underwent the reaction smoothly and gave desired products in excellent yields (85%-95%). The reaction products were isolated by simple workup procedure and did not need any further purification steps. As can be seen from table 2, the presence of electron-withdrawing substituents on the aromatic aldehyde accelerated the reaction rate, whereas electron-releasing substituent reduced the rate of reaction. However, the nature of the substituents did not affect the yield of the products.
Table 2. Synthesis of rhodanine-based amides 5a
|
R1 |
R2 |
Product |
M.p. (°C) |
Time (h) |
Yield (%) b |
1 |
H |
tert-Butyl |
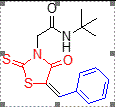
5a |
225-227 |
13 |
90 |
2 |
4-NO2 |
tert-Butyl |
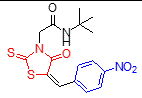
|
250-252 |
12 |
95 |
3 |
4-CN |
Cyclohexyl |
5b
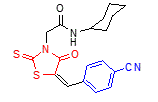
5c |
240-242 |
12 |
95 |
4 |
2-Cl |
tert-Butyl |
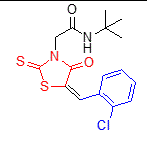
5d |
215-218 |
12.5 |
90 |
5 |
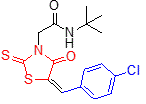
4-Cl |
tert-Butyl |
5e |
249-251 |
12.5 |
95 |
6 |
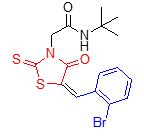
2-Br |
tert-Butyl |
5f |
230-232 |
12 |
90 |
7 |
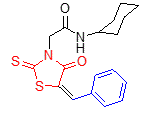
H |
Cyclohexyl |
5g
|
247-250 |
13 |
90 |
8 |
2-MeO |
tert-Butyl |
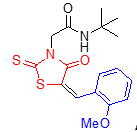 5h 5h
|
262-264 |
14 |
85 |
9 |
4-MeO |
Cyclohexyl |
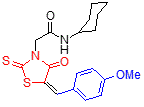 5i 5i
|
250-252 |
14 |
85 |
10 |
2-furyl |
tert-Butyl |
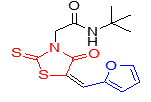
5j |
205-208 |
12 |
90 |
aReaction and conditions: aldehydes (1 mmol), aniline (1 mmol), rhodanine-N-acetic acid (1 mmol), isocyanides (1mmol), THF (5 mL), room temperature, stirring; b Yields refer to isolated products.
The structure of rhodanine-based amides 5 was deduced from 1H and 13C NMR, IR and mass spectra as well as elemental analyses. The physical properties, MS data and elemental analysis results for these compounds are listed in table 2, and the spectral data are shown in table 2. For example, the mass spectrum of 5a displayed the molecular ion (M+) peak at m/z 334, which this consistent with the structure of compound. The 1H NMR spectrum of 5a showed two sharp signals at 1.22 and 4.39 ppm for the tert-butyl and methylene groups, a multiplet at 7.52-7.59 ppm and a doublet at 7.66 ppm corresponding to five aromatic protons, and two singlets at 7.81 and 8.16 ppm for vinyl proton and NH, respectively. The 13C NMR spectrum of 5a demonstrated 12 signals in agreement with the anticipated structure.
Although a mechanism for the formation of product 5 has not yet been established experimentally, a possible explanation is anticipated in figure 2. First, the Knoevenagel condensation between aldehyde 1 and rhodanine-N-acetic acid 3 in the presence of aniline 2 generates benzilidenerhodanine-N-acetic acid 7. According to the well-established chemistry of the reaction of isocyanides with acids [23], it is sensible to suppose that protonation of the isocyanide 4 by the carboxylic acid 3. Then nucleophilic attack of carboxylate 8 to nitrilium 9 produces O-acyl-imine 10 which on quenching with aniline rearranges to intermediate 12. Finally, removal of N-phenyl from this intermediate gives rhodanine-based amide 5 [24].
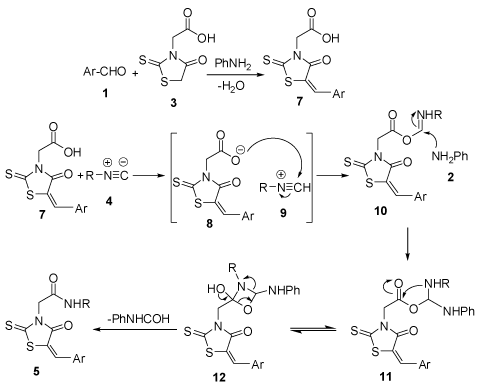
Figure 2. Proposed mechanism for the synthesis of rhodanine-based amides 5
The antibacterial activity of synthesized compounds 5a-5j was evaluated against gram positive (S. aureus and B. subtilis) and gram negative bacteria (E. coli and P. aeruginosa) by the disc diffusion method; the results are illustrated in table 3. In addition, the finding towards inhibition of microorganisms was compared with that of positive controls, gentamicin and chloramphenicol, and DMSO as a negative control. As presented in the table 3, compounds 5a, 5e, 5f and 5j exhibited moderate growth inhibitory effect against all tested bacteria, whereas compound 5i showed no activity. Compounds 5b, 5c and 5h displayed good activity against Gram positive bacteria while these compounds remained inactive against two Gram-negative bacteria. Moreover, compound 5d showed good activity against all bacteria except B. subtilis. In general, the antibacterial activities of these compounds tended to be more potent against Gram-positive species than Gram-negative bacteria, which can be related to the variation in their cell wall structure.
Table 3. Antibacterial activity of the compounds 5a-5j using Kirby-Bauer technique (zone of growth inhibition, mm)a
Compound |
E. coli/mm |
P. aeruginosa/mm |
S. aureus/mm |
B. subtili/mm |
5a |
8.5 ± 0.7 |
8.5 ± 0.7 |
12.5 ± 0.7 |
10.5 ± 0.7 |
5b |
NEb |
NE |
16.5 ± 0.7 |
10.5 ± 0.7 |
5c |
NE |
NE |
12.5 ± 0.7 |
10.5 ± 0.7 |
5d |
10.5 ± 0.7 |
10.0 ± 1.4 |
15.5 ± 0.7 |
NE |
5e |
11.5 ± 0.7 |
11.0 ± 0.0 |
10.5 ± 0.7 |
12.5 ± 0.7 |
5f |
10.0 ± 1.4 |
9.0 ± 1.4 |
14.5 ± 0.7 |
15.0 ± 1.4 |
5g |
10.0 ± 1.4 |
9.5 ± 0.7 |
12.5 ± 0.7 |
NE |
5h |
NE |
NE |
15.5 ± 0.7 |
8.0 ± 0.0 |
5i |
NE |
NE |
NE |
NE |
5j |
9.5 ± 0.7 |
9.5 ± 0.7 |
12.5 ± 0.7 |
14.5 ± 0.7 |
Gentamicin
(10 μg/disc) |
19.6 ± 1.1 |
15.6 ± 0.5 |
20.3 ± 1.5 |
26.0 ± 1.7 |
Chloramphenicol (30 μg/disc) |
20.7 ± 1.5 |
NE |
21.7±0.6 |
22.3±1.2 |
aConcentration of compounds 5a-5j: 20 mg mL-1; bNo effect
In summary, we have reported an efficient approach for the synthesis of rhodanine-based amides via multi-component reaction of aromatic aldehydes, rhodanine-N-acetic acid and alkyl isocyanides in the presence of aniline in THF at ambient temperature. The advantages of the present procedure include good functional group tolerance, high yields of products, simple operation, and absence of any tedious workup or purification. On the basis of the obtained results from antibacterial test, compounds 5a, 5e, 5f and 5j showed moderate growth inhibitory effect against all tested bacteria. Among compounds, 5b exhibited the most antibacterial activity against S. aureus.
We gratefully acknowledge financial support from the Research Council of Mazandaran University.
Electronic Supplementary Material associated with this article can be found in the online version of this paper.
- Jorge LS, Chueire AG, Rossit AR (2010) Osteomyelitis: a current challenge. Braz J Infect Dis 14: 310-315. [Crossref]
- Walter G, Kemmerer M, Kappler C, Hoffmann R (2012) Treatment Algorithms for Chronic Osteomyelitis. Dtsch Arztebl Int 109: 257–64. [Crossref]
- Ikpeme IA, Ngim NE, Ikpeme AA (2010) Diagnosis and treatment of pyogenic bone infections. Afr Health Sci 10: 82-88. [Crossref]
- Jorge LS, Chueire AG, Fucuta PS, Machado MN, Oliveira MGL, et al. (2017) Predisposing factors for recurrence of chronic posttraumatic osteomyelitis: a retrospective observational cohort study from a tertiary referral center in Brazil. Patient Saf Surg 11: 17. [Crossref]
- Agaja SB, Ayorinde RO (2008) Chronic osteomyelitis in Ilorin, Nigeria. S Afr J Surg 46: 116-118. [Crossref]
- Li Q, Cui H, Dong J, He Y, Zhou D, et al. (2015) Squamous cell carcinoma resulting from chronic osteomyelitis: a retrospective study of 8 cases. Int J Clin Exp Pathol 8: 10178–10184. [Crossref]
- Biruk WL, Wubshet K (2007) Chronic Osteomyelitis at Tikur Anbessa Hospital, Addis Ababa University, Ethiopia. East Cent Afr J Surg 12.
- Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ 3rd, et al. (2015) Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am 97: 837-845. [Crossref]
- Onche II, Obiano SK (2004) Chronic osteomyelitis of long bones: reasons for delay in presentation. Niger J Med 13: 355-358. [Crossref]
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, et al. (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm 70: 195–283. [Crossref]
- Dartnell J, Ramachandran M, Katchburian M (2012) Haematogenous acute and subacute paediatric osteomyelitis A systematic review of the literature. J Bone Jt Surg Br 94: 584-595. [Crossref]
- Beckles VL, Jones HW, Harrison WJ (2010) Chronic haematogenous osteomyelitis in children: a retrospective review of 167 patients in Malawi. J Bone Joint Surg Br 92: 1138-1143. [Crossref]
- Stanley CM, Rutherford GW, Morshed S, Coughlin RR, Beyeza T (2010) Estimating the healthcare burden of osteomyelitis in Uganda. Trans R Soc Trop Med Hyg 104: 139-142. [Crossref]
- Ouedraogo S, Zida M, Walla A, Tall M (2017) Epidemiological, bacteriological and therapeutic aspects of chronic osteomyelitis in a sub-Saharan environment. Med Sante Trop 27: 292–295. [Crossref]
- Ibingira CB (2003) Chronic osteomyelitis in a Ugandan rural setting. East Afr Med J 80: 242-246. [Crossref]
- Ali AM, Maya E, Lakhoo K (2014) Challenges in managing paediatric osteomyelitis in the developing world: Analysis of cases presenting to a tertiary referral centre in Tanzania. Afr J Paediatr Surg 11:308–11. [Crossref]
- Bickler SW, Rode H (2002) Surgical services for children in developing countries. Bull World Health Organ 80: 829-835. [Crossref]
- Bahebeck J, Atangana R, Techa A, Monny-Lobe M, Sosso M, et al. (2004) Relative rates and features of musculoskeletal complications in adult sicklers. Acta Orthop Belg 70: 107-111. [Crossref]
- Panteli M, Giannoudis PV (2017) Chronic osteomyelitis: what the surgeon needs to know. EFORT Open Rev 1: 128-135. [Crossref]
- Hatzenbuehler J, Pulling TJ (2011) Diagnosis and management of osteomyelitis. Am Fam Physician 84: 1027-33. [Crossref]
- Pommelet V, Vincent QB, Ardant MF, Adeye A, Tanase A, et al. (2014) Findings in patients from Benin with osteomyelitis and polymerase chain reaction– confirmed mycobacterium ulcerans infection. Clin Infect Dis 59: 1256–1264. [Crossref]
- Nwadiaro HC, Ugwu BT, Legbo JN (2000) Chronic osteomyelitis in patients with sickle cell disease. East Afr Med J 77: 23-26. [Crossref]
- Thanni LO (2006) Bacterial osteomyelitis in major sickling haemoglobinopathies: geographic difference in pathogen prevalence. Afr Health Sci 6: 236–239. [Crossref]
- Mthethwa PG, Marais LC (2017) The microbiology of chronic osteomyelitis in a developing world setting. SA Orthop J 16: 39-45.
- Beckles VL, Jones HW, Harrison WJ (2011) Chronic haematogenous osteomyelitis in children: A retrospective review of 167 patients in Malawi. J Bone Jt Surg Br 92: 1138-43. [Crossref]
- Nwagbara IC, Opara KO (2017) Chronic Osteomyelitis of the Long Bones. Orient J Med 29: 3-4.
- Gashegu J, Byamungu T, Ngarambe C, Bayisenga J, Kiswezi A (2018) Treatment of chronic osteomyelitis with locally made calcium sulfate bone cement pellets impregnated with antibiotics at University Teaching Hospital of Butare (CHUB), Rwanda. East Cent Afr J Surg 23: 1.
- Marais LC, Ferreira N, Aldous C, Le Roux TL (2016) The outcome of treatment of chronic osteomyelitis according to an integrated approach. Strategies Trauma Limb Reconstr 11: 135–142. [Crossref]
- Orimolade EA, Salawu L, Oginni LM (2007) Clinical and laboratory features of Nigerian patients with osteomyelitis. Singap Med J 48:917–921. [Crossref]
- Marais LC, Ferreira N, Le Roux TL (2014) The management of chronic osteomyelitis: Part I – Diagnostic work-up and surgical principles. SA Orthop J 13: 2.
- Baldan M, Gosselin RA, Osman Z, Barrand KG (2014) Chronic osteomyelitis management in austere environments: the International Committee of the Red Cross experience. Trop Med Int Health 19: 832-837. [Crossref]
- Museru LM, Mcharo CN (2001) Chronic osteomyelitis: a continuing orthopaedic challenge in developing countries. Int Orthop 25: 127-131. [Crossref]
- Alonge TO, Ogunlade SO, Omololu AB, Fashina AN, Oluwatosin A (2002) Management of chronic osteomyelitis in a developing country using ceftriaxone-PMMA beads: an initial study. Int J Clin Pract 56: 181–183. [Crossref]









 5h
5h 5i
5i
