Patients with medically intractable epilepsy are candidates for surgical treatment. The main goals of treatment consist in the ablation of epileptogenic areas, surgical sectioning of the corpus callosum in order to avoid interhemispheric transmission of epileptic electrical activity and stimulation of brain areas capable of inducing inhibition of epileptic activity. Another alternative is the injury of structures such as the dentate nucleus of the cerebellum, which has shown beneficial effects in patients. However, most of these surgical treatments involve healthy brain areas and implicate excessive costs. In this review we discuss previous works that describe surgical techniques and their main post-surgical complications.
cerebellum, epilepsy surgery, dentatotomy, kainic acid
Epilepsy is a disorder affecting more than 1% of the general population [1]. Patients with this neurological condition present different types of injuries associated with epileptic activity, the most common include bruises, cuts, burns and falls. Some patients have more serious injuries such as fractures, concussions, head injuries with intracerebral haemorrhage or breathing problems. These are usually seen in people who have generalized seizures with falls, prolonged seizures, or repeated seizures [2]. In addition, patients may lose days of work due to seizures; others may face the adversity of costs and economic effects of antiepileptic drugs (AEDs) and comorbidity [3]. Epilepsy contributes to 0.5% of disability-adjusted life-years (DALYs) due to all diseases and injuries [4].
Despite the introduction of many AEDs over the past three decades, 30–40% of people with epilepsy who have access to such medications still have seizures not completely controlled by these AEDs [4]. Compared to individuals with controlled epilepsy, individuals with drug-resistant epilepsy have a significant impairment in their quality of life. Drug-resistant epilepsy is defined by the International League Against Epilepsy (ILAE) as the failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom [5].
Patients with epilepsy may suffer increased morbidity and a higher rate of mortality [6], social isolation, dependent behaviour, as well as neuropsychological and neurocognitive issues [7,8]. Unfortunately, a pharmacological treatment to relieve patients is not always the answer.
Thus, it is estimated that one-quarter of people with refractory seizures can be potential candidates for surgical therapy. However, reports indicate that only 60–90% of patients receiving surgical treatments can expect to become free of disabling seizures [9]. Normally the severity and frequency of seizures decrease, but they are not always seizure free. This being said, it is necessary to search for new alternatives for an effective treatment. The ideal treatment would be one that reduces the risk of memory, language and visual impairments, among other complications, that arise after conventional surgical procedures.
Considerable progress has been made in understanding the pathophysiological mechanisms underlying epileptic seizures in those patients who are resistant to AEDs and whose seizures have not yet been controlled. For these, the offered alternative is to undergo a surgical procedure; these surgical treatment modalities have varying degrees of clinical and experimental support. Even though removing a portion of the brain almost always carries other consequences, many of the subjects remain fully functional. This measure is highly invasive, although in many cases, long-lasting beneficial results have been achieved in patients.
The first surgical procedures in epileptic patients were performed during the 19th century; when the insight of epilepsy as a cortical disorder of the brain emerged [10]. Today thanks to scientific and technological advances such as magnetic resonance imaging, or metabolic studies like positron emission tomography, single photon emission computed tomography, electroencephalogram and video-electroencephalogram monitoring, as well as electrocorticography, trans-operative, deep electrode registration and meshes to identify epileptogenic areas, there is now a bigger contribution to the accurate pre-operative evaluation and diagnosis of the epileptic zone to be possibly removed. Surgeries are planned to remove possible epileptogenic zones while preserving functional areas. Surgery outcome depends largely on the ability to locate the seizure focus along with the extension of the structural lesion. Due to a greater resection of the epileptic zone, this procedure offers a greater control of drug-resistant epilepsy than pharmacological treatment [11].
Surgery should not be thought of as a last resort. In order for a patient to be considered a potential candidate for surgery they must present drug-resistant epilepsy for at least 3 years and enough evidence that seizure onset is focal. Candidates should be reduced to those who will most likely benefit from surgery enough to make a difference in their quality of life and exclude patients who could result harmed in the short and long term (Table 1). Surgeries are not recommended in patients whose epileptic focus is located on an eloquent zone [12]. Preoperatively, these surgery candidates should undergo the intracarotid amobarbital procedure (also called the Wada test) which is fundamental to define the hemispheric dominance of language and evaluate cognitive functions essential to prevent any postoperative deficit [13]. In recent years, this procedure has been largely replaced by functional MRI given its greater safety profile [75].
Table 1. Criteria for the selection of candidates for epilepsy surgery
1. Confirmed diagnosis of epilepsy |
2. Patients who meet established criteria for epilepsy refractory to drug treatment |
3. Duration of seizures for at least 3 years still under pharmacological treatment |
4. Probability of a disabilization of the epileptogenic focus through its resection |
5. Resectable focus (except in candidates for callosotomy, vagus nerve stimulation and deep brain stimulation) |
6. Probability of seizures control with an improvement in the quality of life |
A prospective series study reported temporal lobe lobectomy as a surgical procedure done to treat drug-resistant epilepsy. Nevertheless, it showed that 2.9% of participants had some major complication (resolution > 3 months) and that a 7.8% had a minor complication (resolution < 3 months) after surgery. Extensive lobe resections increase the risk of complications, especially the appearance of visual disturbances. The most frequent post-surgical complications in adults are presented in order of frequency in table 2, with visual field involvement (quadrantopia [more frequent] or hemianopia) being the most frequent neurological complication. The age groups that most frequently present major complications are subjects 10 to 20 years of age along with subjects 40 to 50 years of age. Subjects 60 to 70 years of age tend to present minor complications compared to other age groups.
Table 2. Complications that occur in patients more frequently after epilepsy surgery
Surgical complications |
1. Infection |
2. Hematoma |
3. Pulmonary thromboembolism |
4. Leakage of artificial cerebrospinal fluid |
5. Hydrocephalus |
6. Cerebral edema |
Neurological complications |
1. Impacts of the visual field (hemianopia and quadrantanopia) |
2. Hemi / monoplegia |
3. Sensitive deficit |
4. Alterations of the cranial nerves |
5. Aphasias |
The three main surgeries performed in adults for the treatment of epilepsy that generate the greatest number of complications are temporal lobe resection, frontal lobe resection and parietal lobe resection. Conversely, lesions caused by stereotactic surgeries in specific brain areas present the lowest risk of developing complications. These procedures are less invasive and have a higher safety profile [14,15]. Studies conducted with emphasis on the complications of epilepsy surgery have shown that the short- and long-term results of neurosurgical procedures with large invasion of brain tissue for drug-resistant epilepsy may affect the quality of life of patients regardless of whether seizure control was obtained or not, due to neurological complications that limit functionality. Therefore, neo-aortic procedures that intend to provoke injury or stimulation of nuclei, or signalling pathways of cortical entry or exit, such as the cortico-cerebellar pathways, to be discussed later, can be a safe procedure with favourable results for seizure control with minimal risks of short and long-term complications.
Lobectomies
The most common surgeries consist in removing a small portion of the brain to resect the epileptic focus. Bailey (1951) was the first to try temporal lobectomy to treat psychomotor seizures and the first to use electrography for intraoperative localization [16]. At the same time, Penfield (1950) initiated temporal lobe resections for the treatment of epilepsy, defining lobectomy as a type of surgery that can be done when a person presents seizures that always begin in the same cerebellar lobe [17]. Temporal lobe epilepsy surgeries are between 70-80% of all surgical interventions performed in patients suffering from refractory epilepsy [18]. The most common procedure is standardized anterior temporal lobectomy (ATL), which involves removing 4–6 cm of the anterior temporal lobe, including the amygdala and hippocampus. Patients with drug resistant epilepsy who can be treated with lobectomy are only those in whom the area of the epileptogenic focus (a term introduced by Gibbs and Lennox in 1938) [19], is detected in the temporal lobe, 80% of TLE’s have onset in the hippocampus [20]. Seizure freedom has been reported in 70-80% of patients who undergo ATL [76], however, not all procedures can be done without damaging important functions; memory and language may be affected if this procedure is performed on the dominant hemisphere. One multi-institutional study reported surgical procedures registered on a 30-day outcome data after temporal lobectomy for medically intractable epilepsy, demonstrating a mortality rate of 1.4%, a major complication rate of 6.5%, and a readmission rate of 11% [21] Table 2.
Amygdalohippocampectomy
Selective amygdalohippocampectomy (SAH) is the most specific indication for mesolimbic temporal epilepsy. Studies report up to 65% of completely seizure-free patients in whom this surgical procedure is done [20]. A recent systematic review and meta-analyses comparing ATL and SAH demonstrated patients were more likely to achieve a better outcome after ATL, with a summary risk difference of 8% [77].
Corpus callosotomy
Corpus callosotomy was introduced as a palliative treatment for severe epilepsy, especially in children suffering from drop attacks, both tonic and atonic, e.g. Lennox–Gastaut syndrome [23,78]. Callosotomy involves cutting the nerve fibres connecting one side of the brain with the other, preventing the extension of abnormal activity to the middle part of the brain; an effort to limit the spread of epileptic activity between both hemispheres. This procedure theoretically reduces the severity and frequency of seizures. Although, long-term results suggest only 35% of callosotomy patients remain seizure-free after 5 years of surgery, the frequency of these seizures remains reduced (<50%) in most patients [24]. This surgery is considered palliative, meaning it does not offer a cure for epilepsy, but a reduction in seizure severity and a significative improvement in patients´ cognitive capacities. [25].
Hemispherectomy
When seizures are really devastating, neurosurgeons prefer to perform hemispherectomies which will undoubtedly paralyze the patient’s hemibody. This type of surgery removes and disconnects one half of the brain. At the beginning of the 20th century, hemispherectomy was introduced as a neurosurgical procedure by Dandy (1928) [26]. However, it was not until the 1930s that major advances were made in epilepsy surgery. After hemispherectomy, most patients will experience some degree of paralysis and a variety of deficits. When the procedure is done, patients may refer a loss of peripheral vision. In adult subjects, reduced neuroplasticity is no longer significant enough to recover this function. Retrospectively in one study 12 adult patients (within 18-56 years) were analysed, all of them with intractable epilepsy due to a unihemispheric pathology. Patients underwent a functional hemispherectomy, 83% of the patients were seizure-free, and 17% had recurrent seizures at last follow-up. Postoperative functional assessment revealed deterioration of motor function in 58% patients, whereas a 41% remained unchanged; language was unchanged in 66% patients [27].
In many cases, patients with medically intractable epilepsy may not be candidates for epilepsy surgery. For example, when the epileptic focus lies within an eloquent zone or when there are multiple epileptic zones. In these cases, there are other surgical alternatives such as brain stimulation [28].
Minimal invasion epilepsy neurosurgery
Not all surgical procedures offered to patients are done by removing brain tissue [29]. Other surgical procedures are also offered. Among these, the chronic implantation of electrodes for vagus nerve stimulation (VNS). This type of surgical procedure was approved by the Food and Drug Administration (FDA) in 1997 for medically intractable epilepsy and in 2005 for drug resistant depression [30]. This therapy helps with focal and multifocal epilepsy. Nevertheless, VNS therapy complications appear early, with some complications related to the procedure like intraoperative bradycardia, asystolia during the lead impedance test, peritracheal hematomas and infections (3-8%). When VNS is done and the vagus nerve is injured, there are alterations such as hoarseness, dyspnea and dysphagia owed to paralysis of the vocal cords and other complications due to the device implanted, including late infections or problems in wound healing. Late complications might include delayed arrhythmias, laryngopharyngeal dysfunction (hoarseness, dyspnea, and coughing), obstructive sleep apnea, stimulation of the phrenic nerve amygdala pain mimicking glossopharyngeal neuralgia [31]. The exact mechanisms on how VNS modulates seizures and mood are still not understood, and there are no indicators as to which patients are most likely to benefit [30]. VNS increases synaptic activity in the thalamus and its cortical projections to decrease the synchronization of synaptic activity in hypothalamus, amygdala, hippocampus and other parts of the limbic system. Another proposed mechanism by which VNS inhibits epileptic seizures is through the decrease in synaptic activity or by an intermittent increase in the release of noradrenaline and serotonin [32,33].
Thalamic nuclei stimulation has also been used for epilepsy control. Prior research conducted by Velasco et. al. reported that bilateral electrical stimulation of the centromedian thalamic nucleus, typically used for the treatment of kinetic disorders, reduced the overall number of tonic-clonic seizures and abscense seizures by nearly 80% in children with Lennox-Gastaut syndrome [34]. These results may be partly due to desynchronization and hyperpolarization of reticulo-thalamic neurons involved in the initiation and propagation of tonic-clonic seizures [35], its clinical efficiency remained consistent during the observation period (21 months). Alternatively, another report discusses a different approach involving VNS and deep brain stimulation in the anterior thalamic nuclei, observing just a minority of patients become totally seizure-free after both stimulations.
Furthermore, cerebellum cortex stimulation has been proposed for patients presenting epilepsy. A decrease of the excitatory activity in thalamic and cortical projections is believed to work [36-40], remaining crucial to advance experimental investigations to understand how brain stimulation works and how it can be implemented on patients with drug-resistant epilepsy. The cerebellum’s ability to inhibit clinical and experimentally induced seizures has been demonstrated by several authors who have examined the effects of cerebellar electrical stimulation on animal models and carried out in humans since the 1970s [36-45]. Generating lesions in deep cerebellar nuclei [46-48] has been an experimental alternative for the control of generalized seizures induced by kindling. Cerebellum nuclei have also been stimulated to counteract epileptic seizures. Stimulation of the dentate nucleus has been reported to generate improvements in patients with different types of intractable epilepsy [36]. The knowledge we have about the cerebellum’s participation in epilepsy is thanks to animal models, in which we have also been able to prove that the stimulation of the cerebellum has beneficial results for epileptic seizures. Seizures induced by electrical stimulation in animal models reproduce the epileptogenic features in the intact brain with low mortality and high reproducibility. Several studies support the effectiveness of this stimulation on difficult epilepsy control. For example, cerebellar cortex stimulation has been reported to counteract epileptic seizures produced by a penicillin cat model [49,50]. Hutton (1972); Cooke and Snider (1955) [43,51], have reported that stimulation on the cerebellum counteracts seizures induced by stimulation of the cerebral cortex.
Moreover, as described by Mutani and Farielo in 1969 [51], even when only the anterior lobe of the cerebellum is stimulated, the seizures produced by cobalt can be inhibited. With this model of cobalt in cats, stimulation of the vermis was reported to produce a prolongation of seizures [52]. Conversely, Maiti and Snider [53], found that vermis stimulation suppresses epileptic activity produced by hippocampus stimulation. Hablitz [44], found that cerebellar stimulation has no effect on the model of penicillin-induced seizures. In cerebellum stimulation reports aiming to eliminate the seizure, we invariably found differences in methodology and models, so the results showed can have contradictory conclusions.
Paradoxically, the model of epileptogenesis that has garnered the most knowledge for the study of epilepsy is the model of electric kindling, described by Graham Goddard in 1969 [54]. Kindling means the repeated application of subthreshold electrical stimuli in specific brain areas, such as the cerebral amygdala. This progressive stimulation produces behavioural and electrographic changes culminating in a complex partial seizure secondarily generalized. Kindling has served to study secondary generalized seizures which have the highest incidence in the epilepsy suffering population, as well as many of the known mechanisms of epileptic seizures. Thanks to this model it has been possible to prove the cerebellum’s participation in the epileptic phenomenon [45,48,55-57].
Likewise, experimentally injuring some cerebellar areas has led to beneficial effects in epileptic seizures, as proven in different animal models by Dow (1962) [41], pioneers on the role of the cerebellum in epilepsy performed studies in rats that showed that the lesion of the cerebellum increases the duration of generalized seizures produced after the application of cobalt in the cerebral cortex.
The cerebellum communicates with the central nervous system through three pairs of peduncles: inferior cerebellar peduncle, medium and superior cerebellar peduncle (SCP), forming the main cerebellar efference through the synaptic action of Purkinje cells [58]. The lesion or the stimulation of the SCP reduces the duration of epileptic activity recorded during the kindling model generalization phase reported by Paz (1991) and Rubio (2004) [45,56]. The SCP is formed by axons coming from the Dentate Nucleus (DN) and Interpositus Nucleus (IN) cells.
Stimulation of the DN has shown inhibition of epileptic seizures caused by a penicillin model [43]. Our investigation group has suggested injuring the cellular DN or IN selectively with kainic acid, considering its ability to injure neuronal bodies without damaging input or output fibers [59,60], in order to decrease the duration of generalized seizures caused by electrical amygdaloid kindling in rats [48]. Histological findings of this study confirm destruction of neuronal bodies of DN and IN without damaging fibers (Figure 1); as it has been described in previous studies where rabbits have been used to determine the participation of these cerebellar nuclei in conditioning [61-63]. In addition, some other studies have described the role played by these cerebellar nuclei in epileptic seizures. A number of authors have suggested that an electrolytic lesion in the DN has a suppressive effect of epileptic activity, while the IN is not involved in this process [46,47]. Unfortunately, the electrolytic lesions are not lesions circumscribing a cell group; instead, they destroy entry and exit fibers.
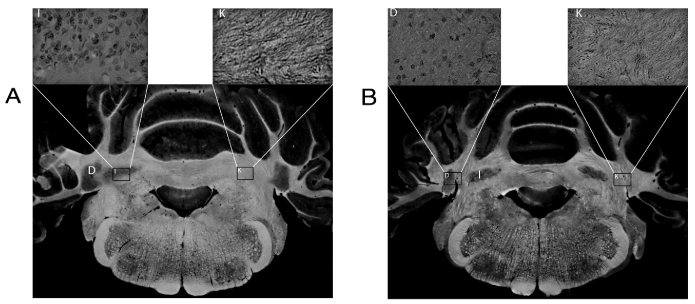
Figure 1. Coronal section of the cerebellum projected onto photographic paper processed using the rapid procedure technique. (a) The interpositus nucleus (I). (b) Dentate nucleus (D). The rectangle shows a microphotographic image of a sagittal section stained with hematoxylin-eosin, indicating the presence of fibers on the lesioned side and cell bodies on the non-lesioned side. Kainic acid (K)
The axons of DN and IN fibers are mostly glutamatergic and aspartatergic [64]. These establish synaptic contacts mainly with brain structures related to epileptic activity, such as: The Red Nucleus (RN), described as a motor nucleus due to its relation to motor coordination. Previous work by our research group demonstrated a delay in behavioural stage 5 and 6 presentation in cats when the RN nucleus is injured through the lesion of the middle cerebellar peduncle [56]. After the dentatotomy, in experimental research, monkeys have correctly performed assigned motor tasks within a few days post-surgery, presenting a total recovery of motor functions; this type of injuries might only cause reversible consequences [65-67]. Even more, this lesion has already been used successfully in human patients [68].
The axons of the DN are found predominantly in the ventral lateral nucleus and the rostral pole of the posterior nuclear group of the thalamus. The thalamus has been considered as a key component of the initiation and propagation circuit of crises, but its exact role in epileptogenesis is unknown [69]. It is known that the thalamic cortical circuits are involved in generalized crises in absence crises models. It has been shown that repeated injections of N-methyl-D-aspartate (NMDA) in the midline of the thalamus in animals induces generalized seizures associated with temporal limbic posterior discharge in the amygdala or hippocampus, suggesting that NMDA receptors in the thalamus are involved in the modulation of temporal limbic excitability [70]. The ventrolateral thalamic nucleus establishes excitatory synaptic contacts with the cerebral cortex and constitutes the path of convergence of DN axons [71]. The thalamus and the motor cortex are known to be at the center of the development and spread of epileptic seizures [72,73] Studies in animals using the amygdaloid electrical kindling model show that when the ventrolateral thalamic nucleus is injured, the duration of epileptic activity is diminished [56].
Prior investigations of stimulation and cerebellar lesion for epilepsy inhibition have been proposed based on anatomical and physiological concepts of cerebellum exerts on the thalamus and cerebral cortex through the synaptic action of Purkinje cells mediated by cerebellum nuclei through SCP involved in the inhibitory effect of epileptic seizures [45,55]. The DN sends cholinergic and glutamatergic fibers to the RN, which is composed of glutamatergic and GABAergic cells. To test the participation of neurotransmitters mentioned above in seizures, a comparison is made between glutamate and gamma-aminobutyric acid (GABA) levels at the RN in a control condition, a kindled stage, and a kindled stage followed by injuries in DN. A significant reduction in glutamate and GABA in the was found in the kindled stage. Additionally, a reversed severity of seizures, restored GABA levels and an increase of GAD65, a GABA-synthesizing enzyme probably owed to an exacerbated demand of GABA, followed the injury carried out on the DN. GABA maintains the inhibitory tone that counterbalances neuronal excitation. As remarked, a decrease in GAD65 expression was found after the DN lesions, indicating that the GABA-synthesizing enzyme was no longer required once excitatory glutamate was eliminated. DN lesions and their consequent biochemical changes are capable of decreasing the generalized seizures induced by kindling stimulation. Thus, we suggest this type of dentate injury as a strategic therapy in medically intractable epilepsy treatment (Figure 2).
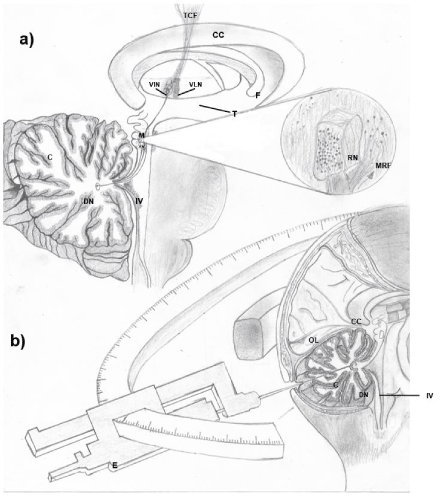
Figure 2. a) The dentorubrotalamocortical pathway of the cerebellum (C) is observed. The main efferences of the dentate nucleus (DN) the red nucleus (RN) and the mesencephalic reticular formation (MRF) which contains fibers with cholinergic and glutaminergic activity. The RN sends nerve fibers to the motor nuclei of the thalamus (T); lateral ventral nuclei (VLN) and inter-medial ventral nuclei (VIN), these neurons send their axons to the motor cortex in the frontal lobe by means of the thalamocortical fibers (TCF). b) a figure of a new proposal for the control of epileptic seizures is shown with the performance of minimally invasive neurosurgery with stereotaxic (E) by means of the damage of DN cells with kainic acid in patients with epilepsy refractory to treatment. Corpus callosum (CC), fourth ventricle (IV), occipital lobe (OL).
Patients with drug-resistant epilepsy may benefit from epilepsy surgery. Per contra, sequels such as alterations in cognitive procedures, motor skills or social stigma cannot be prevented. Therefore, we propose a selective lesion of the Dentate Nucleus as a possible alternative for patients who have uncontrollable seizures and because of functional reasons cannot be offered a conventional surgical procedure.
The authors confirm this article content has no conflict of interest. The authors whose names are included certify they do not have any financial interest, nor did they receive any financing for the realization of this manuscript.
We thank César Mendoza and Emmanuel Ruiz for schematic representation.
- Kwan P, Brodie MJ (2006) Refractory epilepsy: mechanisms and solutions. Expert Rev Neurother 6: 397-406. [Crossref]
- Téllez-Zenteno JF, Nguyen R, Hernández-Ronquillo L (2010) Injuries, accidents and mortality in epilepsy: a review of its prevalence risk factors and prevention. Rev Invest Clin 10: 466-479. [Spanish]. [Crossref]
- Begley CE, Beghi E (2002) The economic cost of epilepsy: a review of the literature. Epilepsia 43: 3-9. [Crossref]
- Benbadis SR, Tatum WO 4th (2001) Advances in the Treatment of Epilepsy. Am Fam Physician 64: 91-98. [Crossref]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, et al (2010) Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51: 1069-1077. [Crossref]
- Spencer S, Huh L (2008) Outcomes of epilepsy surgery in adults and children. Lancet Neurol 7: 525-537. [Crossref]
- Engel J Jr. (2008) Surgical treatment for epilepsy: too little, too late? JAMA 300: 2548-2550. [Crossref]
- Englot DJ, Blumenfeld H (2009) Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res 177: 147-170. [Crossref]
- Engel J Jr, Wiebe S, French J, Sperling M, Williamson P, et al. (2003). Practice parameter: Temporal lobe and localized neocortical resections for epilepsy report of the quality standards subcommittee of the american academy of neurology, in association with the american epilepsy society and the american association of neurological surgeons. Neurology 60: 538-547. [Crossref]
- Sommer W (1880) Erkrankung des Ammonshorns als aetiologisches Moment der Epilepsie. Archiv für psychiatrie und nervenkrankheiten 10: 631-675.
- Awad IA, Rosenfeld J, Ahl J, Hahn JF, Lüders H (1991) Intractable epilepsy and structural lesions of the brain: mapping, resection strategies, and seizure outcome. Epilepsia 32: 179-86. [Crossref]
- Chandra PS, Tripathi M (2010) Epilepsy surgery: recommendations for India. Ann Indian Acad Neurol 13: 87. [Crossref]
- Wagner K, Hader C, Metternich B, Buschmann F, Schwarzwald R, et al. (2012) Who needs a Wada test? Present clinical indications for amobarbital procedures. J Neurol Neurosurg Psychiatry 83: 503-509. [Crossref]
- Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, et al (2013) Complications of epilepsy surgery: A systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia 54: 840-847. [Crossref]
- Bjellvi J, Flink R, Rydenhag B, Malmgren K (2015) Complications of epilepsy surgery in Sweden 1996-2010: a prospective, population-based study. J Neurosurg 122: 519-525. [Crossref]
- Bailey P, Gibbs FA (1951) The surgical treatment of psychomotor epilepsy. J Am Med Assoc 145: 365-370. [Crossref]
- Penfield W, Jasper HH (1947) Heighest level seizures. Research Publications-Association for Research in Nervous and Mental Disease 26: 252-71.
- Zentner J, Hufnagel A, Wolf HK, Ostertun B, Behrens E, et al (1995) Surgical treatment of temporal lobe epilepsy: clinical, radiological, and histopathological findings in 178 patients. J Neurol Neurosurg Psychiatry 58: 666-673. [Crossref]
- Gibbs FHD, Lennox W (1935) The electroencephalogram in epilepsy and in conditions of impaired consciousness. Archives of Neurology and Psychiatry 34: 1133–48.
- Wieser HG, Burcet J, Russi A (2000) Indications of the surgical treatment of epilepsy. Rev Neurol 30: 1190-1196. [Crossref]
- Kerezoudis P, McCutcheon B, Murphy ME, Rajjoub KR, Ubl D, et al. (2018) Thirty-day postoperative morbidity and mortality after temporal lobectomy for medically refractory epilepsy. J Neurosurg 128: 1-7. [Crossref]
- So N, Olivier A, Andermann F, Gloor P, Quesney LF (1989) Results of surgical treatment in patients with bitemporal epileptiform abnormalities. Ann Neurol 25: 432-439. [Crossref]
- Wagenen Van WP, Herren RY (1940) Surgical division of commissural pathways in the corpus callosum: relation to spread of an epileptic attack. Archives of Neurology & Psychiatry 44: 740-59.
- Passamonti C, Zamponi N, Foschi N, Trignani R, Luzi M, et al (2014) Long-term seizure and behavioural outcomes after corpus callosotomy. Epilepsy Behav 41: 23-29. [Crossref]
- Fandiño-Franky J, Torres M, Narino D, Fandiño J (2000) Corpus callosotomy in Colombia and some reflections on care and research among the poor in developing countries. Epilepsia 41. [Crossref]
- Dandy WE (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia: Preliminary report. JAMA 90: 823-825.
- Chen H, Modur PN, Barot N, Van Ness PC, Agostini MA (2016) Predictors of postoperative seizure recurrence: a longitudinal study of temporal and extratemporal resections. Epilepsy Res Treat 2016. [Crossref]
- Chkhenkeli SA (2003) Direct Deep-Brain Stimulation: First Steps Towards the Feedback Control of Seizures. In: Milton J, Jung P (eds) Epilepsy as a Dynamic Disease. Biological and Medical Physics Series. Springer, Berlin, Heidelberg.
- Schmeiser B, Zentner J, Steinhoff BJ, Schulze-Bonhage A, Kogias E, et al. (2017) Functional hemispherectomy is safe and effective in adult patients with epilepsy. Epilepsy Behav 77: 19-25. [Crossref]
- Gschwind M, Seeck M (2016) Modern management of seizures and epilepsy. Swiss Med Wkly 146: 1-18. [Crossref]
- Giordano F, Zicca A, Barba C, Guerrini R, Genitori L (2017) Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia 58: 85-90. [Crossref]
- Henry TH (2003) Vagus nerve stimulation for epilepsy: anatomical, experimental and mechanistic investigations. Schachter SC, Schmidt D. Vagus nerve stimulation Second Edition. Ed. Martin Dunita. United Kingdom, pp. 1-31.
- Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, et al. (1998) Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia 39: 983-990. [Crossref]
- Velasco AL, Velasco F, Jiménez F, Velasco M, Castro G, et al (2006) Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox–Gastaut syndrome. Epilepsia 47: 1203-1212. [Crossref]
- Kim SH, Lim SC, Yang DW, Cho JH, Son BC, et al (2017) Thalamo–cortical network underlying deep brain stimulation of centromedian thalamic nuclei in intractable epilepsy: a multimodal imaging analysis. Neuropsychiatr Dis Treat 13: 2607–2619. [Crossref]
- Šramka M, Chkhenkeli SA (1990) Clinical experience in intraoperational determination of brain inhibitory structures and application of implanted neurostimulators in epilepsy. Stereotact Funct Neurosurg 54: 56-59. [Crossref]
- Davis R, Emmonds SE (1992) Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg 58: 200-208. [Crossref]
- Cooper IS, Amin I, Gilman S (1973) The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc 98: 192-196. [Crossref]
- Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP (1976) Chronic cerebellar stimulation in epilepsy. Clinical and anatomical studies. Arch Neurol 33: 559-570. [Crossref]
- Levy LF, Auchterlonie WC (1979) Chronic cerebellar stimulation in the treatment of epilepsy. Epilepsia 20: 235-245. [Crossref]
- Dow RS, Fernández-Guardiola A, Manni E (1962) The influence of the cerebellum on experimental epilepsy. Electroencephalogr Clin Neurophysiol 14: 383-398. [Crossref]
- Cesa-BianchI MG, Mancia M, Mutani R (1967) Experimental epilepsy induced by cobalt powder in lower brainstem and thalamic structures. Electroencephalogr Clin Neurophysiol 22: 525-536. [Crossref]
- Hutton JT, Frost JD, Foster J (1972) The influence of the cerebellum in cat penicillin epilepsy. Epilepsia 13: 401-408. [Crossref]
- Hablitz JJ (1976) Intramuscular penicillin epilepsy in the cat: effects of chronic cerebellar stimulation. Exp Neurol 50: 505-14. [Crossref]
- Rubio C, Custodio V, Juárez F, Paz C (2004) Stimulation of the superior cerebellar peduncle during the development of amygdaloid kindling in rats. Brain Res 1010: 151-155. [Crossref]
- Tsuru N, Kawasaki H, Genda S, Hara K, Hashiguchi H, et al (1992) Effect of unilateral dentate nucleus lesions on amygdaloid kindling in rats. Epilepsia 33: 213-221. [Crossref]
- Min JK, Valentine PA, Teskey GC (1998) Effect of complete and partial bilateral lesions of the deep cerebellar nuclei on amygdaloid kindling in rats. Epilepsia 39: 692-99. [Crossref]
- Rubio C, Custodio V, González E, Retana-Márquez S, López M, et al. (2011) Effects of kainic acid lesions of the cerebellar interpositus and dentate nuclei on amygdaloid kindling in rats. Brain Res Bull 85: 64-67. [Crossref]
- Gloor P (1978) Generalized epilepsy with bilateral synchronous spike and wave discharge. New findings concerning its physiological mechanisms. Electroencephalogr Clin Neurophysiol Suppl 34: 245-249. [Crossref]
- Bantli H, Bloedel JR, Anderson G, McRoberts R, Sandberg E (1978) Effects of stimulating the cerebellar surface on the activity in penicillin foci. J Neurosur 48: 69-84. [Crossref]
- Cooke PM, Snider RS (1955) Some Cerebellar Influences on Electrically Induced Cerebral Seizures. Epilepsia 4: 19-28. [Crossref]
- Reimer GR, Grimm RJ, Dow RS (1967) Effects of cerebellar stimulation on cobalt-induced epilepsy in the cat. Electroencephalogr Clin Neurophysiol 23: 456-462. [Crossref]
- Maiti A, Snider RS (1975) Cerebellar control of basal forebrain seizures: amygdala and hippocampus. Epilepsia 16: 521-533. [Crossref]
- Goddard GV, McIntyre DC, Leech CK (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25: 295-330. [Crossref]
- Paz C, Reygadas E, Fernández-Guardiola A (1985) Amygdala kindling in totally cerebellectomized cats. Exp Neurol 88: 418-424. [Crossref]
- Paz C, Gutiérrez-Baeza F, Bazán-Perkins B (1991) Transection of the superior cerebellar peduncle interferes with the onset and duration of generalized seizures induced by amygdaloid kindling. Brain Res 558: 90-92. [Crossref]
- Hernández-Cerón M, Martínez-Lazcano JC, Rubio C, Custodio V, González-Guevara E, et al. (2017) Participation of the dentate-rubral pathway in the kindling model of epilepsy. J Neurosci Res 95: 1495-1502. [Crossref]
- Wyckhuys T, Geerts PJ, Raedt R, Vonck K, Wadman W, et al. (2009) Deep brain stimulation for epilepsy: knowledge gained from experimental animal models. Acta Neurol Belg 109: 63-80. [Crossref]
- McGeer PL (1978) Kainic acid as a tool in neurobiology. McGeer Raven Press. NewYork. 123-38.
- Coyle JT, Molliver ME, Kuhar MJ (1978) In situ injection of kainic acid: a new method for selectively lesioning neuronal cell bodies while sparing axons of passage. J Comp Neurol 180: 301-323. [Crossref]
- Katz DB, Steinmetz JE (1997) Single-unit evidence for eye-blink conditioning in cerebellar cortex is altered, but not eliminated, by interpositus nucleus lesions. Learn Mem 4: 88-104. [Crossref]
- Lavond DG, Hembree TL, Thompson RF (1985) Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Res 326: 179-182. [Crossref]
- Anderson ME, DeVito JL (1987) An analysis of potentially converging inputs to the rostral ventral thalamic nuclei of the cat. Exp Brain Res 68: 260-276. [Crossref]
- Monaghan PL, Beitz AJ, Larson AA, Altschuler RA, Madl JE, et al. (1986) Immunocytochemical localization of glutamate-, glutaminase-and aspartate aminotransferase-like immunoreactivity in the rat deep cerebellar nuclei. Brain Res 363: 364-370. [Crossref]
- Amrani K, Dykes RW, Lamarre Y (1996) Bilateral contributions to motor recovery in the monkey following lesions of the deep cerebellar nuclei. Brain Res 740: 275-284. [Crossref]
- Bioulac B, Burbaud P, Varoqueaux D (1995) Activity of area 5 neurons in monkeys during arm movements: effects of dentate nucleus lesion and motor cortex ablation. Neurosci Lett 192: 189-192. [Crossref]
- Beaubaton D, Trouche E (1982) Participation of the cerebellar dentate nucleus in the control of a goal-directed movement in monkeys. Effects of reversible or permanent dentate lesion on the duration and accuracy of a pointing response. Exp Brain Res 46: 127-138. [Crossref]
- Fraioli B, Guidetti B (1975) Effects of stereotactic lesions of the dentate nucleus of the cerebellum in man. Appl Neurophysiol 38: 81-90. [Crossref]
- Bertram EH (2013) Neuronal circuits in epilepsy: do they matter? Exp Neurol 244: 67-74. [Crossref]
- Hirayasu Y, Wada JA (1992) N-Methyl-d-Aspartate Injection in to the Massa Intermedia Facilitates Development of Limbic Kindling in Rats. Epilepsia 33: 965-970. [Crossref]
- Aumann TD, Rawson JA, Finkelslstein DI, Horne MK (1994) Projections from lateral and interpositus cerebellar nuclei to the thalamus of the rat: a light and electron microscopic study using single and double anterograde labeling. J Comp Neurol 349: 165-218.
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH (2002) Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci 22: 1480–1495. [Crossref]
- Rouiller E, Liang F, Babalian A et al. (1994) Cerebellothalamic and pallidothalamic projection to the primary and supplementary motor cortical areas: A multiple tracing study in macaque monkeys. J Comp Neurol 345: 185-213.
- GBD 2016 Epilepsy Collaborators (2019) Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 357-375. [Crossref]
- Szaflarski JP, Gloss D, Binder JR, Gaillard WD, Golby AJ, et al. (2017) Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 88: 395-402. [Crossref]
- Englot DJ, Chang EF (2014) Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev 37: 389-404. [Crossref]
- Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, et al. (2013) Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology 80: 1669-1676. [Crossref]
- Malmgren K, Rydenhag B, Hallböök T (2015) Reappraisal of corpus callosotomy. Curr Opin Neurol 28: 175-181. [Crossref]


