A 31-year-old man with Fabry disease was treated with hemodialysis because he had developed end-stage renal failure. However, hemodialysis treatment could not be continued due to uncontrolled systemic pain, and subsequently the patient received a renal transplant from his biological father who was not a carrier of Fabry disease. Good renal graft function and better pain control compared with before surgery were achieved, and the patient returned to society. Control of systemic pain, which is a primary symptom in patients with Fabry disease who develop renal failure can be improved.
Half a century has passed since renal transplantation started in Japan, and its outcomes have improved remarkably. Advances in immunosuppressive drug therapy are largely responsible for this improvement, but the accumulation of experience, improvement of perioperative management, and broadening of the acceptable range of primary diseases (i.e., expanded indications) have likely contributed. According to the Annual Progress Report from the Japanese Renal Transplant Registry published by the Japanese Society for Clinical Renal Transplantation in 2017, the most common primary disease in 1,331 recipients who underwent living donor renal transplantation was chronic glomerulonephritis (346 patients, 26%); 1 of 124 (9.3%) patients with a genetic disorder or a congenital metabolic disorder had Fabry disease [1]. Fabry disease is an X-linked disorder of lipid metabolism and is one of main forms of sphingolipidoses. These are characterized by genetic deficiency of a lysosomal hydrolytic enzyme α-galactosidase A (α-Gal A) resulting in accumulation of globotriaosylceramide (GL-3), an intermediate metabolite of sphingoglycolipids, in organs throughout the body causing diverse symptoms. Patients with Fabry disease present with pain in extremities and hypohidrosis from childhood, and with renal dysfunction and cardiac dysfunction in adulthood. Recently, enzyme replacement therapy (ERT) [2,3] has been recommended as a fundamentally curative treatment.
We report a case of good pain control using ERT after living donor renal transplantation in a patient with Fabry disease who was not fit to undergo maintenance hemodialysis.
A 31-year-old man with blood type A.
Chief complaints: Renal dysfunction and systemic pain.
History of present illness: Frequent systemic pain started when he was 11 years old, and renal dysfunction was identified at that time. The diagnosis of Fabry disease was made when he was 29 years old, but ERT started shortly after diagnosis did not achieve satisfactory pain control. Administration of Carbamazepine, Pregabalin, and opioids, and frequent intravenous injections of Lidocaine hydrochloride were needed to relieve the severe pain. Renal function gradually deteriorated despite conservative treatment. Because the patient developed end-stage renal failure at age 30, hemodialysis was started but then discontinued due to uncontrolled systemic pain. He was referred to our department for renal transplant from his biological father.
Clinical Course: Family history was notable for his maternal grandmother having renal disease; Fabry disease was suspected but not confirmed. The donor was his 63-year-old biological father whose blood type was A, and thus it was a blood type compatible living donor renal transplantation. HLA typing test result was one haplo-identical, lymphocyte cross-matching was CDC and FCXM negative. The surgery was performed under general anesthesia, and the donor’s left kidney was transplanted into the right iliac fossa. Induction immunosuppression was performed using anti-CD25 antibody, with Tacrolimus, Mizoribine and Prednisolone. The patient developed transient acute tubular necrosis, and a duration of about 10 days was needed before satisfactory renal graft function was seen. Respiratory failure was aggravated due to alveolar hemorrhage on postoperative day 9 but alleviated by steroid pulse therapy and antibacterial therapy under ventilator management by postoperative day 14.
For pain control (Figure 1), Carbamazepine, Pregabalin, and Lidocaine were administered daily before surgery (solid line), whereas Carbamazepine daily and Lidocaine as needed were administered after surgery (dotted line). Postoperative pain control was satisfactory, and he was discharged from the hospital with a serum creatine level of 1.0 mg/dL on postoperative day 36. ERT given after surgery was continued after discharge, and this with administration of carbamazepine daily and lidocaine as needed achieved good pain control (Numeric Rating Scale pain; NRS scire 0-6/10). Immunosuppression mycophenolate mofetil (MMF) had severe side effects (i.e., bone-marrow suppression), so MMF was switched to Everolimus (1.5 mg/day). The patient made remarkable progress without further side effects. As of 5 years after surgery have passed after surgery, the patient has had sustained good renal graft function with serum creatinine of 1.2 mg/dL, and no significant complications.
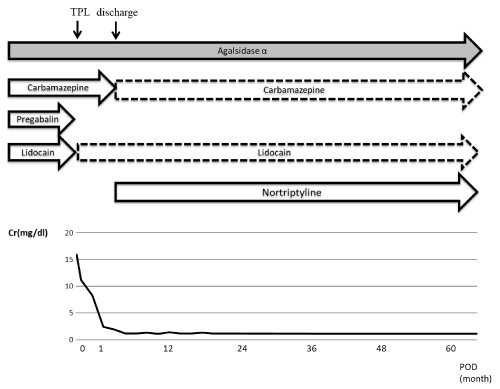
Figure 1. Figure showing the clinical course including pain control (solid arrow: continuous administration, dotted-line arrow: administration as needed).
Fabry disease is an inborn error of lipid metabolism. The major symptoms are pain in the extremities and hypohidrosis due to the global accumulation of GL-3, especially in the nervous system; renal dysfunction develops in middle age, with cardiac and cerebrovascular complications resulting in death in some cases. Fabry disease can be divided into classical Fabry disease and atypical Fabry disease. In classical Fabry disease, a vast amount of GL-3 accumulates in the systemic vascular system, the heart, kidneys, and autonomic nervous system; in atypical Fabry disease, disease onset is mostly after middle age, and is further subdivided into cardiac Fabry disease (symptoms localized to the heart) and renal Fabry disease (renal dysfunction is the primary presentation, while other organ dysfunction is secondary).
Renal failure in Fabry disease is caused by the accumulation of GL-3 in all constituent cells of the kidneys including the podocytes of the glomerulus, Bowman capsule, mesangial cells, Henle’s loop, distal and proximal tubular cells, and arteriolar endothelial cells. Therefore, pathological changes include glomerular sclerosis, interstitial fibrosis, and tubular cell atrophy [4].
Any form of renal replacement therapy can be selected for treatment of end-stage renal failure in Fabry disease. The prognosis is reported to be poorer than that of end-stage renal failure due to glomerulonephritis [5], but it was shown to be improved by renal transplantation [6]. Shah, et al. [7] reported that patient survival was slightly worse in Fabry disease than in other primary diseases although graft survival was similar. Regarding the prognosis of end-stage renal failure in Fabry disease, Sofue, et al. [8] reported that the improvement of patient survival by ERT is limited because ERT prevents GL-3 accumulation in the renal transplant, but not in other organs. Ersözlü, et al. [9] reported that, among 17 patients with Fabry disease who had undergone renal transplantation, there were 7 deaths, 6 of which were due to a cardiovascular cause. In the past several years, excellent clinical results of renal transplantation have been increasingly reported [9-11]; therefore, we suggest that renal transplantation could well be regarded as first-line choice for renal replacement therapy.
Pain in the extremities is one of the symptoms of Fabry disease. According to the Fabry disease guidelines published by Eng, et al. in 2006 [11], its pathophysiologic findings involve ischemic injury and metabolic failure due to GL-3 accumulation in neurovascular endothelial cells and neurons in the peripheral nervous system, resulting in nerve cell dysfunction. Therefore, ERT is an essential treatment. Successful pain control after renal transplantation was reported in several studies, and immunosuppressive therapy was performed safely with the combined use of ERT in those studies [12]. However, the mechanism of improvement of pain control has not been clarified. Future accumulation of cases, through which the mechanism will be revealed, is awaited.
Fabry disease is sometimes difficult to diagnose due to mild symptoms or could even be missed due to poor awareness of the disease. The prevalence rate of Fabry disease in male dialysis patients is about 1% [13]. Thus, this condition cannot be ignored, and should be kept in mind. Patients with as yet unknown hemizygosity for Fabry disease developed Fabry disease after receiving transplants from heterozygous donors without previous diagnosis of Fabry disease; short-term outcomes were favorable while long-term outcomes were poor [14,15]. ERT was not performed in those patients. By contrast, patients were stable after similar transplant from heterozygous donors to hemizygous recipients, when Fabry disease was diagnosed upon transplantation and ERT was started [16], suggesting the utility of ERT to some extent. Nonetheless, long-term outcomes are still unclear, so it is recommended that Fabry disease be diagnosed before transplantation to avoid transplantation from heterozygous donors.
An understanding of Fabry disease facilitates diagnosis of a disease that previously was missed, thereby addressing the attendant problems. This study showed that continuing ERT after renal transplantation may improve symptoms (e.g., better pain control) and renal transplant prognosis (graft survival) in patients with Fabry disease.
- Japanese Society for Clinical Renal Transplantation and The Japan Society for Transplantation (2016) Annual progress report from the japanese renal transplant registry: Number of renal transplantations in 2016 and follow-up survey. Jpn J Transplant 52: 2-3.
- Schiffmann R, Murray GJ, Treco D, Daniel P, Sellos-Moura M, et al. (2000) Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc Natl Acad Sci 97: 365-370.
- Schiffmann R, Kopp JB, Austin HA, Sabnis S, Moore DF, et al. (2001) Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285: 2743-2749.
- Alroy J, Sabnis S, Kopp JB (2002) Renal pathology in Fabry disease. J Am Soc Nephrol 13: S134-138.
- Sessa A, Moroni M, Battini G, Righetti M, Mignani R (2004) Chronic renal failure, dialysis, and renal transplantation in Anderson-Fabry disease. Semin Nephrol 24: 532-536.
- Inderbitin D, Avital I, Larbiader F, Vogt B, Candinas D (2005) Kidney transplantation improves survival and is indicated in Fabry’s disease. Transplant Proc 37: 4211-4214.
- Shah T, Gill J, Molhotra N, Takemoto SK, Bunnapradist S (2009) Kidney transplant outcomes in patients with Fabry disease. Transplantation 87: 280-285.
- Sofue T (2015) Fabry Disease and Renal Transplant. Journal of Japanese Society for Clinical Renal Transplantation 3: 23-30.
- Ersözlü S, Desnick RJ, Huynh-Do U, Canaan-Kühl S, Barbey F, et al. (2018) Long-term outcomes of kidney transplantation in Fabry disease. Transplantation 102: 1924-1933.
- Cybulla M, Kurschat C, West M, Nicholls K, Torras J, et al. (2013) Kidney transplantation and enzyme replacement therapy in patients with Fabry disease. J Nephrol 26: 645-651.
- Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, et al. (2006) Fabry disease: guidelines for the evaluation and management of multi organ system involvement. Genet Med 8: 539-48.
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, et al. (2007) Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med 146: 77-86.
- Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, et al. (2003) Fabry disease: detection of undiagnosed hemodialysis patients and identification of a "renal variant" phenotype. Kidney Int 64: 801-807.
- Kochar O, Wick MR, Kerr SE, Oglesbee D, Cathro HP (2011) Unexpected Fabry disease in a renal allograft kidney: an underrecognized cause of poor allograft function. Ultrastruct Pathol 35: 92-96.
- Paull LS, Lipinski MJ, Wilson WG, Lipinski SE (2012) Female with Fabry Disease unknowingly donates affected kidney to sister: A call for pre-transplant genetic testing. JIMD Rep 4: 1-4.
- Nishioka R, Sofue T, Moritoki M, Nishijima Y, Nishioka S, et al. (2015) A case of living-donor kidney transplantation from a heterozygote mother to a hemizygote son of Fabry disease diagnosed by donated allograft biopsy. The Journal of the Japanese Society of Internal Medicine 104: 775-780.

