A green and efficient method was developed for the synthesis of some α,β-unsaturated compounds (3a-i) under microwave irradiation reaction. The purpose of this study was to obtain a rapid, efficient, and environmentally friendly methodology from different aromatic aldehydes and cyanoacetamide in presence of sodium acetate as a catalyst through a solvent free condition. All the synthesized compounds were confirmed by the analyses of IR and NMR data. Also, the compounds were screened against four Gram-positive bacteria (Bacillus subtilis, Bacillus cereus, Bacillus megaterium, Staphylococcus aureus), five Gram-negative bacteria (Escherichia coli, Salmonella typhi, Salmonella paratyphi, Shigella dysenteriae, Vibrio cholerae) and three fungi (Macrophomina phaseolina, Aspergillus acheraccus, Penicillium). The antimicrobial screening showed that the majority of the compounds were active against S. typhi, V. cholera and B. subtilis as well asagainst M. phaseolina when compared with standard drugs.
Microwave irradiation, Green synthesis, Aromatic aldehydes, Cyanoacetamide, Antibacterial activity, Antifungal activity
Microwave assisted irradiation (MWI) provides an alternative to the conventional methods, for heating or introducing energy into the system. This method utilizes the ability of mobile electric charges present in liquid or conducting ions in solid to transform electromagnetic energy into heat. The reactions followed by the microwave-assisted irradiation are fast, clean, economic and eco-environmentally benign [1]. This method has been proposed as the ‘technology of tomorrow’ as various advantages were found.
Microwave heating began to gain wide acceptance following the research works in 1986 by a group of Gedye and Giguere/Majetich. Also, the use of microwave heating in chemical modification can be traced little back in 1950s [2,3]. In the early times of microwave method, the experiments were typically carried out in sealed teflon or glass vessels in a domestic household microwave oven without measuring any temperature orpressure [1]. Actually, the kitchen microwave ovens are not designed for laboratory usage where the acids and solvents can corrode the interiors quickly and therefore these are not the safety systems. Due to these non-laboratory microwaves, the results were often violent explosions due to the rapid heating of organic solvents under closed vessel conditions which is little difficult to control. Inthe 1990s several groups started the experiments with solvent free microwave method that eliminated the risk of explosions. In the techniques of laboratory-based microwave, the reagents are pre-adsorbed onto either a more or less microwave transparent inorganic support (silica, alumina or clay) or a strongly absorbing something (graphite) [4] that additionally may have been doped with a reagent or catalyst.
The microwave assisted technique which is well-known for cooking foods, has been successfully making inroads in various chemical laboratories such as organic synthesis [5], nanomaterial synthesis [6-9], nanotechnology [10] and solid-state chemistry. Under MWI conditions, the organic reactions can be accelerated, and the ensuing products selectivity can beobtained through choosing the appropriate MW parameters. Therefore, offer several advantages over conventional heating, such as immediate and rapid heating, high temperature homogeneity, and selective heating [11,12].
2-Cyanoacetamide is commercially available. Cyanoacetamides are very much useful and the versatile reagents for the synthesis of organic compounds as well as the heteroaromatics [13-17] possessing the biological activity and other special properties [18] with the availability of having both the electrophilic and nucleophilic centers. Two of the nucleophilic centers in cyanoacetamidesare restricted on the NH and the C-2 positions as the reactivity order of C-2 > NH. And two electrophilic positions are attentively with the C-1 and C-3 situations as the reactivity order of C-3 > C-1 (Figure 1) [19-21]. It is easy to undergo in the condensation and substitution reactions for the existence of two electron-withdrawing groups in the extraordinary action of cyanoacetamides as CH acidic and the active methylene compounds [22-24].
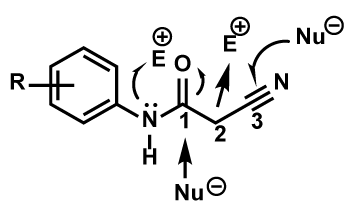
Figure 1. Reactivity of cyanoacetamide derivatives
During the years, microwave assisted techniques have been extensively used for carrying out chemical reactions and have become a very useful energy source for the rapid and efficient synthesis of a variety of organic compounds [25-28] For rising many applications of microwave techniques in organic synthesis, it has made the subject of attention to many researchers and the interest has spread from the academic laboratories to industry and engineering sectors.
A number of review articles have also appeared that cover the underlying theory of microwave dielectric heating, the relevant dielectric parameters, and microwave-assisted organic reactions [29].
In continuation to our earlier studies (Scheme 1a, 1b) directed at the development of practical and efficient chemicalprocesses [30,31] a sustainable green Knoevenagel condensation protocol was reported here using MWI in presence of ammonium acetate under solvent free conditions for the preparation of some cyanoacetamide derivatives (Scheme 1c).
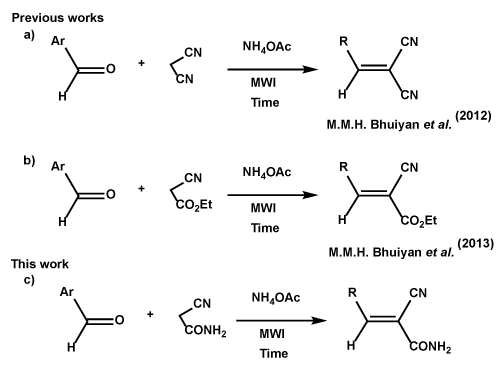
Scheme 1. Microwave-assisted Knoevenagel condensation synthesis (previous and present works)
Physical Measurements
Melting points were recorded with electro-thermal melting point apparatus and were uncorrected. Thin layer chromatography (TLC) was performed on Kieselgel GF254 and the visualization was accomplished by iodine vapor and UV Flame. The infrared (IR) spectra were recorded by FTIR spectrophotometer (Model-8900, Shimadzu, Japan) using KBr matrix in the range 4000-200 cm-1. 1H-NMR (400 MHz and 500 MHz) were recorded on JEOL GS×400, GEOL JNM-AL 400 (400 MHz) and spectrometer in CDCl3 and CD3OD as solvent. Chemical shifts were reported in δ unit (ppm) with reference to TMS as an internal standard and J values were given in Hz. All the reactions were carried out in a commercially available LG microwave oven (MB – 3947C) having a maximum power output of 800 W operating at 2450 MHz.
General procedure for the synthesis of compounds 3a–i
To a mixture of aromatic aldehyde and cyanoacetamide, catalytic amount of ammonium acetate was added, and the reaction mixture was irradiated under microwave condition at 160-320 watt for 30-60 sec. After the completion of reaction (checked by TLC, ethyl acetate: n-hexane = 1:5, v/v), the obtained solid mass was recrystallized with a mixture of ethyl acetate (Table 1) and n-hexane to get the pure solid sample 3a–i.
Table 1. Compounds 3a-i from aldehyde derivatives and cyanoacetamide
Sl. No. |
Substrate
1 |
Reagent
2 |
MWI/
Time
(watt/
sec) |
Product
3 |
Yield
(%) of
3 |
Melting
Point
(°C) |
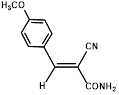
1a |
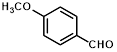
|
2 |
160/30 |
|
99 (3a)
|
193-195 |
1b |
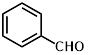
|
2 |
160/40 |
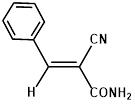
|
99 (3b) |
98-100 |
1c |
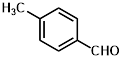
|
2 |
160/30 |
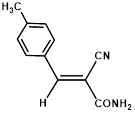
|
99 (3c) |
136-138 |
1d |
|
2 |
160/50 |
|
99 (3d) |
140-142 |
1e |
|
2 |
160/40 |
|
99 (3e) |
176-178 |
1f |
|
2 |
320/60 |
|
93 (3f) |
190-192 |
1g |
|
2 |
160/30 |
|
92 (3g) |
120-122 |
1h |
|
2 |
160/30 |
|
87 (3h) |
120-122 |
1i |
|
2 |
160/40 |
|
81 (3i) |
78-80 |
Physical, analytical, and spectroscopic characterization data of the compounds 3a–i are given hereafter.
Synthesis of 2-(4-methoxyphenylmethylene)-cyanoacetamide (3a)
Yield: 0.82 gm, 4.0741 mmol (99%), Melting Point: 193-195 °C
IR (KBr) νmax (cm-1): 3448.72 (-NH2, sym, str), 3367.71 (-NH2, asym, str), 3066.82 (=C-H), 3178.69 (s, C=C , Ar), 2935.66 (CH3-O, sym, str), 2846.93 (CH3-O, asym, str), 2210.42 (s, C≡N), 1697.36 (s, C-O, amide), 1585.49 (s, C=C), 1180.44 (s, C-O, ether), 1107.14, 1026.13.
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.47 (s, 1H, C=CH), 8.19 (d, 2H, H-2, H-6, J=8.8 Hz), 7.24 (d, 2H, H-3, H-5, J=8.8 Hz), 4.13 (s, 3H, OCH3,), -NH2 (3.31).
Synthesis of 2-(phenylmethylene)-cyanoacetamide (3b)
Yield: 0.97 gm, 5.6391 mmol (99%), Melting Point: 98-100°C
IR (KBr) νmax (cm-1): 3313.71 (-NH2), 3163 (s, C=C, Ar), 2920.23 (=C-H), 2360.87 (s, C≡N), 1697 (s, C-O str, amide), 1573.91 (s, C=C), 1597.06, 1288.45, 1207.44, 1107.14, 1064.71, 1029.99.
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.19 (s, 1H, C=CH), 7.95 (d, 2H, H-2, H-6), 7.54 (m, 3H, H-3, H-4, H-5), 4.84 (s, 2H, -NH2).
Synthesis of 2-(4-methylphenylmethylene)-cyanoacetamide (3c)
Yield: 0.78 gm, 4.1880 mmol (99%), Melting Point: 136-138°C
IR (KBr) νmax (cm-1): 3387(-NH2, sym, str), 3317.56 (-NH2, asym, str), 2920.23 (=C-H), 2360.87 (s, C≡N), 1698 (s, C-O, amide), 1593.20 (s, C=C), 1508.33 (s, C=C, Ar), 1373.32, 1184.29, 1215.15, 1107.14.
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.15 (s, 1H, C=CH), 7.87 (d, 2H, J=8.0 Hz, H-2, H-6), 7.34 (d, 2H, J=8.0 Hz, H-3, H-5), 4.84 (s, 2H, -NH2), 2.41 (s, 3H, CH3).
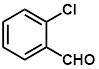
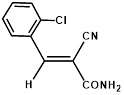
Synthesis of 2-(2-chlorophenylmethylene)-cyanoacetamide (3d)
Yield: 0.93 gm, 4.5241 mmol (99%), Melting Point: 140-142°C
IR (KBr) νmax (cm-1): 3471.87 (-NH2, sym, str), 3414 (-NH2, asym, str), 3155.54 (=C-H, Ar), 2341.58 (s, C≡N), 1604.77(s, C=C), 1037.70 (s, C-Cl).
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.55 (s, 1H, -CH), 8.12 (d, 1H, J=8.4 Hz, H-6), 7.55 (m, 3H, H-3, H-4, H-5), 4.86 (s, 2H, -NH2).
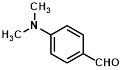
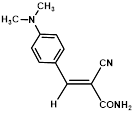
Synthesis of 2-(4-N,N-dimethylaminophenylmethylene)-cyanoacetamide (3e)
Yield: 0.91gm, 4.2431 mmol (99 %), Melting Point: 176-178 °C
IR (KBr) νmax (cm-1): 3406.29 (-NH2, sym, str), 3356.14 (-NH2, asym, str), 3159.40 (=C-H, Ar), 2862 (N-CH3), 2341.58 (s, C≡N), 1685.79(s, C-O, str, amide), 1608.63 (s, C=C), 1562.34, 1438.90, 1365.60, 1238.30, 1188.15, 1064.71.
1H-NMR (400 MHz, CDCl3): δH (ppm): 8.88 (d, 2H, J=8.8 Hz, H-2, H-6), 8.00 (s, 1H, C=CH), 6.78 (d, 2H, J=8.8 Hz, H-3, H-5), 4.84 (s, 2H, -NH2), 3.09 (s, 6H, 2CH3).
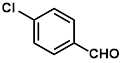
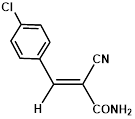
Synthesis of 2-(4-chlorophenylmethylene)-cyanoacetamide (3f)
Yield: 0.56 gm, 2.7310 mmol (93%), Melting Point: 190-192 °C
IR (KBr) νmax (cm-1): 3452.58 (-NH2, sym, str), 3367.71 (-NH2, assym, str), 3155.54 (=C-H,) 2341.58 (s, C≡N), 1701.22 (s, C-O, amide), 1585.49 (s, C=C), 1485.19 (=C-H, Ar), 1458.18, 1381.03, 1207.44, 1091.71, 1006.84.
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.17 (s, 1H, CH), 7.95 (d, 2H, J=8.8 Hz, H-2, H-6), 7.54 (d, 2H, J=8.8 Hz, H-3, H-5), 4.84 (s, 2H, -NH2).
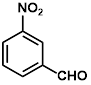
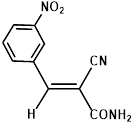
Synthesis of 2-(3-nitrophenylmethylene)-cyanoacetamide (3g)
Yield: 0.63 gm, 2.8890 mmol (92%), Melting Point: 120-122 °C
IR (KBr) νmax (cm-1): 3414 (-NH2, sym, str), 3116.97 (-NH2, assym, str), 2360.87 (s, C≡N), 1700 (s, C-O, amide), 1604.77 (s, C=C), 1527.62 (s, C=C, Ar), 1477.47, 1377.17, 1350.17.
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.83 (s, 1H, H-2), 8.40 (d, 1H, J=8.0 Hz, H-4), 8.39 (s, 1H,C=CH), 8.30 (d, 1H, J=7.2 Hz, H-6), 7.79 (t, 1H, J=8.00 Hz, H-5), 4.84 (s, 2H, -NH2).
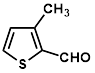
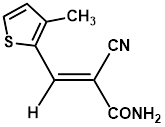
Synthesis of 2-(3-methylthiophene-2-yl-methylene)-cyanoacetamide (3h)
Yield: 0.51 gm, 2.6573 mmol (87%), Melting Point: 120-122°C
IR (KBr) νmax (cm-1): 3360 (-NH2, sym, str), 3336.85 (-NH2, assym, str), 2341.58 (s, C≡N), 2206.57 (thiol), 1697 (s, C-O, amide), 1585 (s, C=C), 1508.33, 1361.74, 1315.45, 1253.73.
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.43 (s, 1H, C=CH), 7.82 (d, 1H, J=5.2 Hz, H-5), 7.08 (d, 1H, J=5.2 Hz, H-4), 4.84 (s, 2H, -NH2), 2.45 (s, 3H, -CH3).
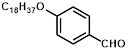
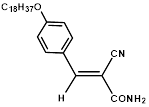
Synthesis of 2-(4-octadecyloxyphenylmethylene)-cyanoacetamide (3i)
Yield: 0.54 gm, 1.2691 mmol (81%), Melting Point: 78-80°C
IR (KBr) νmax (cm-1): 2920.23 (=C-H), 2850.79 (=C-H), 2360.87 (s, C≡N), 1693.50 (C-O, str, amide), 1577.77 (s, C=C), 1261.45 (s, C=C-H, Ar).
1H-NMR (400 MHz, CD3OD): δH (ppm): 8.24 (s, 1H, C=CH), 7.93 (d, 2H, J=8.2 Hz, H-2, H-6), 7.81 (d, 2H, J=8.2 Hz, H-3, H-5), 4.02 (t, 2H, -OCH2, J=6.4 Hz), 3.93 (s, 2H, -NH2), 1.82 (m, 10H), 1.24 (m, 22H), 0.87 (s, 3H, CH3).
Anti-microbial Activity Assessment
In the present study, nine synthesized unsaturated compounds were used against nine human pathogenic bacteria and three phytopathogenic fungi. Among the bacteria strains four was Gram-positive, viz. Bacillus subtilis, Bacillus cereus, Bacillus megaterium, Staphylococcus aureus and five were Gram-negative, viz. Escherichia coli, Salmonella typhi, Salmonella paratyphi, Shigella dysenteriae, Vibrio cholerae. Antifungal activities of the compounds were also studied against three phytopathogenic fungi, viz. Macrophomina phaseolina, Aspergillus acheraccus, Penicillium.
The disc diffusion method [32] and poisoned-food technique [33] were used for antibacterial and antifungal activities, respectively. The tested compounds were dissolved in chloroform and methanol to get a solution of 1 mg ml–1. The inhibition zones were measured in millimeters at the end of an incubation period of 48 hours at (35±2) °C. chloroform and methanol alone showed no inhibition. Nutrient agar (NA) and potato dextrose agar (PDA) were used as basal media to test the bacteria and fungi, respectively. Commercial antibacterial Ampicillin and antifungal Nystatin were also tested under similar conditions for comparison. The test culture of the bacteria and fungi were collected from the Department of Pharmacology, Chittagong Veterinary and Animal Science University, Chittagong, Bangladesh.
The antimicrobial activity results of the synthesized compounds (3a-i) are given below (Table 2-3):
Antibacterial activities
The antibacterial screening data presented in the Table 2 suggest that compound 3i showed highest activities against Gram-positive Bacillus subtilis (15mm) and compound 3e showed highest activities against Gram-negative Vibrio cholerae (15 mm). Most of the tested chemicals showed good inhibition and some were unable to show any inhibition against the tested microorganisms.
Table 2. Zone of inhibition observed against Gram-positive and Gram-negative bacteria by the test chemicals Where, “*” means marked inhibition, “**” means standard inhibition of antibiotic, “---” means no inhibition and “dw” means dry weight
Comp.
No. |
Diameter of Zone of inhibition in mm (200µg (dw)/ disc) |
B. subtilis |
B. cereus |
B. megaterium |
S. aureus |
E. coli |
S. typhi |
S.
paratyphi |
S. dysenteriae |
V. cholerae |
3a |
--- |
6.5 |
--- |
--- |
09 |
--- |
--- |
--- |
06 |
3b |
--- |
--- |
--- |
--- |
--- |
*10 |
--- |
06 |
--- |
3c |
--- |
--- |
--- |
--- |
--- |
*11 |
--- |
--- |
--- |
3d |
07 |
--- |
06 |
8.5 |
--- |
--- |
--- |
06 |
08 |
3e |
--- |
--- |
--- |
--- |
*11 |
--- |
--- |
--- |
*15 |
3f |
--- |
--- |
--- |
--- |
--- |
*10 |
--- |
--- |
--- |
3g |
*11 |
--- |
--- |
--- |
06 |
08 |
--- |
--- |
--- |
3h |
--- |
--- |
--- |
*11 |
*14 |
07 |
--- |
--- |
08 |
3i |
*15 |
*12 |
*12 |
*14 |
*12 |
*14 |
*12.5 |
*11 |
*13 |
**Ampicillin |
*19 |
*18 |
*16 |
*22 |
*10 |
*20 |
*18 |
*22 |
*32 |
Antifungal Activities
Macrophomina phaseolina, Asparagillus acheraccus and Penicilium were selected for mycelial growth test. The result of the percentage inhibitions of mycelial growth of the selected chemicals are presented in Table 3. The efficiency of the chemicals on mycelial growth of the selected fungi are mentioned below:
The antifungal screening data presented in the Table 3 suggest that compound 3i showed maximum inhibition against Macrophomina phaseolina, the compounds 3a and 3i were found to be very effective against the tested fungus while other chemicals showed no inhibition against Aspargillus acheraccus and the compounds 3d and 3i were found to be very effective against the tested fungus while other chemicals showed no inhibition against Penicillium.
Table 3. Percent Inhibition of fungal mycelial growth by the test chemicals. Where, “*” means marked inhibition, “**” means standard inhibition of antibiotic, “---” means no inhibition and “dw” means dry weight
Compound
No. |
% Inhibition of fungal mycelial growth (100µg (dw) /ml PDA) |
Macrophomina phaseolina |
Aspargillus acheraccus |
Penicillium |
3a |
5.01 |
*18.1 |
--- |
3b |
11.11 |
--- |
--- |
3c |
--- |
--- |
--- |
3d |
11.11 |
--- |
13.79 |
3e |
--- |
--- |
--- |
3f |
--- |
--- |
--- |
3g |
33.3 |
--- |
--- |
3h |
12.69 |
--- |
--- |
3i |
*72.22 |
*13.6 |
57.5 |
**Nystatin |
*71.78 |
*12 |
--- |
There are many methods already mentioned in earlier for the synthesis of α,β-unsaturated compounds have been developed and employed successfully on the light of green chemistry aspects. In the present work, we synthesized nine compounds from different aromatic aldehydes and active methylene compound cyanoacetamide in the presence of catalytic amount of NH4OAc through Knoevenagel condensation process using microwave irradiation, as indicated in Scheme 2 and Table 1. The corresponding reactions proceeded smoothly with excellent yields (81-99%). The important feature of this procedure is the survival of a variety of functional groups such as nitro, chloro and amino under the reaction conditions. The structures of the products were established from their IR, and NMR. For example, the IR spectrum of compound 3c displayed a band absorption 2210.42 cm-1 for C≡N group. The absorption bands at 1585.49 cm-1 due to C=C bond of aromatic ring and 1180.44 was due to C-O ether. The 1H NMR spectrum of 3c exhibited two doublet signals resonated at δ 8.19 ppm and at δ 7.24 ppm with both the J values of 8.80 Hz were designated to four aromatic protons of H-2’, H-6’, H-3’ and H-5’. A three-proton singlet displayed at δ 4.13 ppm attributed to –OCH3 group and a two-proton singlet at δ 3.31 ppm. The microanalytical data of the compound 3c is also in good agreement with the assigned structure. Similarly, the peaks in NMR spectra of rest of the compounds were accordance with the assigned structures.
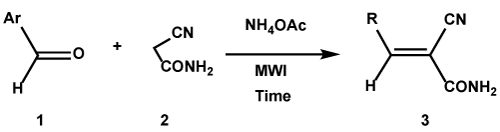
Scheme 2. The reaction of cyanoacetamide 2 with aldehyde derivatives
Most of the compounds showed good to moderate antimicrobial activities and a few of them were unable to show inhibition for some pathogens. For the antifungal activity, all the compounds showed excellent results against all fungi and are comparable to that of the standard antibiotic, ampicillin and Nystatin.
Microwave technique is a convenient way towards the goal of green and sustainable chemistry and is strongly recommended to use in synthetic organic chemistry laboratories. Based upon the fact, the present investigation was planned, and considerable interest has been shown in the synthesis of α,β-unsaturated compounds by Knoevenagel condensation process. The advantages of this method are high yields, relatively short reaction times, low cost, simple experimental and as isolation procedures, and finally, it is in agreement with the green chemistry protocols. A series of title compounds were synthesized from various substituted aromatic aldehydes and cyanoacetamide with sodium acetate as the catalyst to yield 3a-i. The synthesized compounds were confirmed by TLC, IR and NMR spectra. The antimicrobial activity tests were also performed of the synthesized compounds against some bacteria and fungi. Under mild, conventional conditions, these types of reactions can take as long as 15–24 h whereas these reactions reported here are complete within 0.5–1 min under microwave heating is truly remarkable.
The authors are grateful to the Department of Chemistry, University of Chittagong, Chittagong-4331, Bangladesh.
Authors declare that there is no conflict of interest regarding the publication of this paper.
MMHB, MMM and MAA conceptualized and designed the study, drafted the manuscript, and reviewed and revised the manuscript. MMHB, MMM and UHB designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. MRA revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
- Krstenansky JL, Cotterill I (2000) Recent advances in microwave-assisted organic syntheses. Curr Opin Drug Discovery Dev. 4: 454-461. [Crossref]
- Gedye R, Smith F, Westaway K (1986) The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett 27: 279-282.
- Giguere RJ, Bray TL, Duncan SM (1986) Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett 27: 4945-4948.
- Laporterie A, Marquie J, Dubac J (2002) Microwave-assisted reactions on graphite in Microwaves in Organic Synthesis, Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; pp. 219-252.
- Polshettiwar V, Nadagouda MN, Varma RS (2009) Microwave assisted chemistry: A rapid and sustainable route to synthesis of organics and nanomaterials. Aus. J. Chem 62: 16-26.
- Nadagouda MN, Speth TF, Varma RS (2011) Microwave-assisted green synthesis of silver nanostructures, Acc. Chem. Res 44: 469-478.
- Kou J, Varma RS (2012) Beet juice-induced green fabrication of plasmonic AgCl/Ag nanoparticles. ChemSusChem 5: 2435-2441. [Crossref]
- Baruwati B, Varma RS (2009) High value products from waste: Grape pomace extract-a three-in-one package for the synthesis of metal nanoparticles. ChemSusChem 2: 1041-1044. [Crossref]
- Baruwati B, Polshettiwar V, Varma RS (2009) Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem 11: 926-930.
- Virkutyte J, Varma RS (2011) Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem Sci 2: 837-846.
- Polshettiwar V, Varma RS (2008) Microwave-assisted organic synthesis and transformations using benign reaction media. Acc Chem Res 41: 629-639. [Crossref]
- Baig RBN, Varma RS (2012) Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem Soc Rev 41: 1559-1584.
- Konovalova VV, Shklyae YV, Tolstikov AG (2006) Synthesis and biological activity of cyclohexylamine derivatives. Pharm Chem J 40: 298-302.
- Hacksell U, Arvidsson LE, Svensson U (1981) 3-Phenylpiperidines. Central dopamine-autoreceptor stimulating activity. J Med Chem 24: 1475-1482. [Crossref]
- Vittorio V, Clarke GD, Roberto C (1991) (2S)-1-(Arylacetyl)-2-(aminomethyl) piperidine derivatives: Novel, highly selective kappa opioid analgesics. J Med Chem 34: 397-403. [Crossref]
- Domino EF, Luby ED (2012) Phencyclidine/schizophrenia: one view toward the past, the other to the future. Schizophr Bull 38: 914-919. [Crossref]
- Maddox VH, Godefroi EF, Parcell RF (1965) The synthesis of phencyclidine and other 1-arylcyclohexylamines. J Med Chem 8: 230-235. [Crossref]
- Litvinov VP (1999) Cyanoacetic acid and their thio- and selenocarbonyl analogues as promising reagents for fine organic synthesis. Russ Chem Rev 68: 737-763.
- Geissler AE, Huppatz JL, Phillips JN (1980) The herbicidal activity of 2-alkyl-2-cyano acetanilides. Pestic Sci 11: 432-438.
- Qureshi AM, Qadi M, Rauf A (2011) Antimicrobial efficacy of metal-barbiturate conjugates against pathogenic strains of escherichia coli and staphylococcus aureus. Lett Drug Des Discov 8: 980-987.
- Fadda AA, Bondock S, Rabie SR (2008) Cyanoacetamide derivatives as synthons in heterocyclic synthesis, Turk J Chem 32: 259-286.
- Ismail MM, Ammar YA, El-Zahaby HS, Eisa SI, El-Sayed Barakat S (2007) Synthesis of novel 1-pyrazolylpyridin-2-ones as potential anti-inflammatory and analgesic agents. Arch Pharm (Weinheim) 340: 476-482. [Crossref]
- Fouad SA (2014) Synthesis, characterization and anti-breast cancer activity of some new pyrazole, thiazole, chromene and pyridine derivatives, Int J Adv Res 2: 442-453.
- Ammar YA, Abbas SY, Ghorab MM (2016) Transmonocyanoacetylation of phenylenediamines: A simple and efficient synthesis of novel N-(aminophenyl)-2-cyanoacetamides and their derivatives. Tetrahedron Lett 57: 275-277.
- Kappe CO, Pieber B, Dallinger D (2013) Microwave effects in organic synthesis: myth or reality? Angew Chem Int Ed Engl 52: 1088-1094. [Crossref]
- Rathi AK, Gawandea MB, Zboril R (2015) Microwave assisted synthesis-catalytic applications in aqueous media, Coord Chem Rev 291: 68-94.
- Gawande MB, Shelke SN, Zboril R (2014) Microwave assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc Chem Res 47: 1338-1348.
- Varma RS, Baig NR (2012) Organic synthesis using microwaves and supported reagents. in “Microwaves in Organic Synthesis, 3rd Edition, A. de la Hoz and Loupy (Eds.), Wiley-VCH, Weinheim, Chapter 10, pp 427-486.
- Lin SS (2005) Microwave-assisted enzyme-catalyzed reactions in various solvent systems. J Am Soc Mass spectrum 16: 581-588. [Crossref]
- Bhuiyan MMH, Hossain MI, Alam MA (2012) Microwave assisted knoevenagel condensation: Synthesis and antimicrobial activities of some arylidene-malononitriles. Journal of Chemistry 2: 67-73.
- Bhuiyan MMH, Rahman KMM, Alam MA et al. (2013) Microwave Assisted Knoevenagel Condensation: Synthesis and Antimicrobial Activities of Some a-cyanoacrylates, Pak. J. sci. ind. Res 56: 131-137.
- Bauer AW, Kirby WMM, Sherris JC (1996) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Path 45: 493-496. [Crossref]
- Grover RK, Moore JD (1962) Toximetric studies of fungicides against the brown rot organisms. Sclerotinia fructicola and S. laxa. Phytopathology 52: 876-880.





















