Abstract
Background: Accumulating evidence suggests that Regulatory T cells (Tregs) have a crucial role in immune tolerance and long-term graft survival. However, the influence of immunosuppressive drugs on the level of Tregs has not been fully understood. Therefore in this study we prospectively compare the effect of two different calcineurin inhibitor (CNI)-based immunosuppressive protocols on Tregs frequencies and Treg-related genes expression in renal transplant recipients.
Methods: The study included 24 renal transplant recipients who received induction therapy (Antithymocyte globulin) and were on triple immunosuppressive therapy; one group on Tacrolimus (TAC), mycophenolate moftile (MMF) and prednisolone(P) and other group on TAC, Sirolimus (SRL) and P. The frequency of circulating Treg cells were analyzed by flow cytometry before and 4 months after transplantation. Inaddition, the mRNA expression of FOXP3, T-bet, GATA3 and RORγt were estimated by quantitative RT-PCR before and 4 months after transplantation.
Results: All recipients had significantly increased CD4+CD25+FOXP3+Treg cell levels after transplantation compare to baseline. Patients receiving MMF protocol had significantly higher CD4+CD25+FOXP3+Treg cells compared to patients on SRL. There was no significant difference between two group MMF and SRL in frequency of CD3+CD8+CD28- Tregs. FOXP3 mRNA levels were increased 4 months after transplantation and the expression was significantly higher in group MMF recipients. On the other hand T-bet and RORγt expression was significantly lower in group SRL in comparison to MMF group. We did not observe significant difference in GATA3 mRNA level between the two groups.
Conclusions: Our results suggest that regimen containing MMF despite increasing CD4+CD25+FOXP3+Tregs significantly, but cannot decrease RORγt and T-bet expression as well as the regimen containing SRL.
Keywords
regulatory T cells, renal transplantation, calcineurin inhibitor, mycophenolate moftile, sirolimus, immune tolerance
Introduction
Recent improvement in immunosuppressive medications and therapy, make kidney transplantation as a standard treatment for end-stage renal disease (ESRD) [1]. However, these drugs cannot prevent the chronic rejection of the transplantation, and also prolonged use of these drugs increase the risk of various types of malignancies and infections [2]. So avoiding long-term immunosuppression with the goal of achieving immunological tolerance can be considered as a final solution for long-term survival of allograft [3]. There is scattered evidence that allograft tolerance is often accompanied with a specialized population of regulatory T lymphocytes (Treg) [4]. Currently, most therapeutic regimens based on Calcineurin inhibitors (CNI) like Tacrolimus (TAC), significantly decrease the rate of acute rejection [5]. But long-term treatment with CNI is associated with side effects such as nephrotoxicity and vascular disease [6]. Therefore, mTOR inhibitors
such as Sirolimus (SRL) and antimetabolite like Mycophenolate Mofetil (MMF) are used in combination with CNI to decrease the CNI dose and hence its related nephrotoxicity Sid effects [7]. The results of different studies in recent years have shown that Sirolimus decrease the rate of allograft rejection and also selectively expand Treg populations [8-11]. In addition various transplant models has revealed that MMF has a positive effect on the process of tolerance induction [12-14]. Considering the Regulatory T cells subsets (Tregs) and their crucial role in immune tolerance and long-term graft survival, we evaluated two subset of Treg ,FoxP3+ CD4+ CD25+ Tregs [15,16] and the CD8+ CD28- Tregs that can induce tolerance [17-19]. Since the central role of Tregs in the induction of tolerance and the irreversible side effect of immunosuppressive agents on allograft survival, in this study we aimed to investigate the frequency of (CD4+ CD25+ FOXP3+ and CD8+ CD28-) Tregs and also the expression of Foxp3, T-bet , GATA-3 and RORγT genes in peripheral blood of kidney transplant recipients under immunosuppression either MMF/Tacrolimus or SRL/Tacrolimus before transplantation and 4 months after transplantation.
Materials and methods
Patients and study design
In this study we analyzed 24 blood samples from recipient of first kidney transplant (15 males and 9 females) between May 2016 and august 2017 at the transplantation unit of Labbafinejad medical center (Tehran, Iran). The local ethics committee approved all aspects of the study protocol and also written informed consent was obtained from all patients before inclusion in this study. Peripheral blood was collected just before the assumption of immunosuppressive therapy and at 16 weeks after transplantation and immunosuppressive utilization. At the time of transplant, operation patients were randomly grouped according to one of the two immunosuppressive regimen: (1) MMF group: Mycophenolate mofetil (MMF), Tacrolimus (TAC) and prednisolone (n=14), and (2) SRL group: Sirolimus (SRL), TAC and prednisolone (n=10).
All recipients received induction therapy with Anti-thymocyte globulin (ATG) (3 mg/kg) for 4 days and receive prednisolone 250 mg for 2 days and then 1 mg/kg (max 60 mg) for 3 days, the medication has been reduced to 15 mg in 14 days and the 10 mg dose is continued for up to 30 days to ultimately reach 5 mg per day.
MMF group: In this group the initial dose of TAC was 0.1 mg/kg per day orally, and target through levels were 8-10 ng/ml during the first 3 months and 5-8 ng/ml afterward. The dose of MMF (360 mg was administered in 3 divided dose for 7 days) and then will increase to 720 mg/day.
SRL group: The initial dose of TAC in this group is lower 0.08 mg/kg per day, and target through levels were 6-7 ng/ml during the first 6 months and 4-5 ng/ml afterward. The dose of Sirolimus was 2 mg for 96 hours of surgery and then 1 mg/day to reach a plasma level of 3-5 ng/ml in the first 6 months and then increase the dose to reach a plasma level of 6-8 ng/ml.
PBMC isolation: The blood specimens were colleted in EDTA tubes and peripheral blood mononuclear cells (PBMC) were isolated from whole blood by density gradiant centrifugation on ficoll-plaque (ino-train,germany). PBMC was frozen in a cryoprotective media containing 10% dimethyl sulfoxide (DMSO) and 90% fetal bovine serum (FBS) and stored at −196?C.
Flow cytometric analysis: For analysis of Tregs, after thawing PBMC, this cell were washed twice with phosphate-buffered saline containing 0.3% fetal bovine serum. To determine surface markers, the cells were first stained with the following fluorochrome-conjugated monoclonal antibodies (all from eBioscience, San Diego, CA): Anti-CD3-PE-Cyanine 5.5, Anti-CD8a-FITC, Anti-CD28-PE, Anti-CD4-FITC, Anti-CD25-PE. After primary incubation (30 min at 4?C) cells were washed, and for staining of intracellular FoxP3 (anti-FOXP3-PE-Cyanine5.5, eBioscince), the cell fixed and permeabilized using Foxp3/Transcription Factor Staining Buffer Set supplied by eBiosciences. As a control for correcting fluorescence compensation and confirmation of antibody specificity, the appropriate mouse immunoglobulin isotype include: IgG1 K Isotype control (FITC and PE) and IgG2a K Isotype control-PE-Cyanine (eBioscience) were used. Data were obtained on FACSCalibur flow cytometer system and analyzed by CellQuest software (BD Biosciences).
Quantitative mRNA analysis by Real-time PCR
Total RNA was extracted from PBMC (5*106) using the TRI Reagent RNA Isolation Reagent (Sigma-Aldrich T9424) according to the manufacturer,s instructions. RNA quantitiy and purity were measured by NanoDrop ND-1000 Spectrophotometer. DNA templete was removed by the addition of RNase-free DNase I (CinnaClon, Iran) before reverse transcription. In brief, RNA samples were reverse transcribed to complementary DNA using High Capacity cDNA Reverse Transcription kit (Cat no. 4368814, Applied Biosystems, Foster City, CA), and stored at -70?C untile use. Real-time qPCR were performed to quantify of transcription expression level on a Step OnePlus real-time PCR (Applied Biosystems). The TaqMan Gene Expression Assay (primer probe) used for transcription factors T-bet (Hs00894392_m1), GATA-3 (Hs00231122_m1), RORγt (Hs01076112_m1) and FOXP3(Hs0108534_m1) were also from Applied Biosystems. Gene expression was normalized to 18s rRNA (Hs99999901_s1, Applied Biosystems) as the endogenous control. In this study, pretransplant samples were taken as control sample. Transcript levels calculated using the relative quantification method 2-ΔΔct.
Statistical analysis
Data were analyzed using SPSS 16.0 software. The comparison of value before transplantation and four months after transplantation were performed using Paired t-test and between groups was made by unpaired t-test. Values are presented as the Mean ( ± SD) and P value less than 0.05 was recognized as significant.
Results
Basic characteristics of recipients
Demographic data and baseline characteristics are presented in Table1, whereas Table 2 shows patients' clinical and biochemical data. There was no significant difference in age and gender between groups. All patients were first kidney transplants. 12 patients (50%) were living un-related donor transplants and 12 (50%) were cadaveric donor transplants. improvement of renal function was observed as early as 4 months after transplantation in both groups. Relative to baseline value, serum creatinine decreased with a mean of 1.71 ± 0.89 (SD) in MMF group and 1.25 ± 0.42(SD) in SRL group at 4 months after transplantation. The glomerular filtration rate was
Table 1. Demographic and baseline characteristic of renal transplant recipients. Data are expressed as the number of subjects or mean ± SD. MMF, mycophenolate moftile. SRL, sirolimus
Parameter |
groups |
MMF |
SRL |
N |
14 |
10 |
Gender (M:F) |
9:5 |
6:4 |
Age (year) |
|
|
Original renal disease, n (%) |
34 ± 11.26 |
32 ± 7.05 |
Proteinuria |
2 (14.28%) |
2 (20%) |
Hypertension |
4 (28.57%) |
2 (20%) |
Renal cyst |
0 |
1 (10%) |
Lupus |
1 (7.14%) |
0 |
Diabetes |
1 (7.14%) |
2 (20%) |
Nephrotic syndrome |
2 (14.28%) |
0 |
IgM nephropathy |
0 |
1 (10%) |
Reflux |
0 |
1 (10%) |
Congenital |
1(7.14%) |
0 |
Unknown |
3 (21.42%) |
1 (10%) |
Donor type, n (%) |
|
|
Living unrelated |
7 (50%) |
5 (50%) |
Cadaveric |
7 (50%) |
5 (50%) |
Measured and show a significant increase at 4 months after transplantation. There was no significant difference in GFR rate between two groups (Table 2). BUN levels also significantly decreased in both groups after transplantation and we didn’t see significant difference between two groups.
Table 2. Clinical and biochemical data (mean ± SD) of renal transplant pateints. GFR, Glomerular filtration rate. BUN, Blood urea nitrogen. UA, Uric acid. AST, Aspartate aminotransferase. ALT, Alanine aminotransferase. ALK, Alkaline phosphatase. Ca, Calcium. Na, Sodium. P, phosphorus. K, Potassium. *Before versus after transplantation was significant (p < 0.05).
Parameter |
groups |
MMF |
SRL |
Baseline |
4m |
Baseline |
4m |
Creatinine (mg/dL) |
20 ± 5.37 |
1.71 ± 0.89* |
9.77 ± 4.10 |
1.25 ± 0.42* |
GFR (mL/min) |
6.84 ± 2.85 |
53.47 ± 21.46* |
7.19 ± 2.26 |
68.79 ± 17.19* |
BUN |
109 ± 28.25 |
51.09 ± 55.21* |
104 ± 24.82 |
39 ± 11.67* |
UA |
6.81 ± 1.30 |
6.88 ± 2.07 |
6.60 ± 1.33 |
5.62 ± 2.28 |
AST |
10.50 ± 4.90 |
21.30 ± 9.23* |
9.87 ± 4.48 |
22.87 ± 4.88* |
ALT |
12.20 ± 5.73 |
37.7 ± 22.15* |
11.87 ± 5.74 |
30.25 ± 15.67* |
ALP |
276 ± 102.57 |
259 ± 59.47 |
269 ± 130.46 |
245 ± 147.44 |
Ca |
9.36 ± 0.58 |
9.47 ± 0.62 |
8.67 ± 0.55 |
9.30 ± 0.44 |
Na |
142 ± 2.82 |
139 ± 515 |
138 ± 3.33 |
138 ± 2.47 |
P |
5.85 ± 1.91 |
3.98 ± 0.46 |
6.05 ± 1.70 |
4.06 ± 1.01 |
K |
4.58 ± 0.95 |
6.91 ± 7.72 |
5.18 ± 0.77 |
4.07 ± 0.57 |
Frequencies of total CD4+ T cells in recipients
As shown in Figure1, the frequencies of CD4+ T cells in MMF group had decreased significantly 4 months after transplantation (29.10% ± 11.70%) compared with before transplantation (39.92% ± 10.49%, P=0.02) (Figure 1A). Similarly, in SRL group, the frequencies of CD4+ T cells were significantly decreased 4 months after transplantation (26.61% ± 10.11) compare with before transplantation (38.46% ± 12.57, p=0.04) (Figure 1B). However, there was no significant difference in the frequencies of CD4+ T cells between MMF and SRLgroup (P>0.05) (Figure 1C).
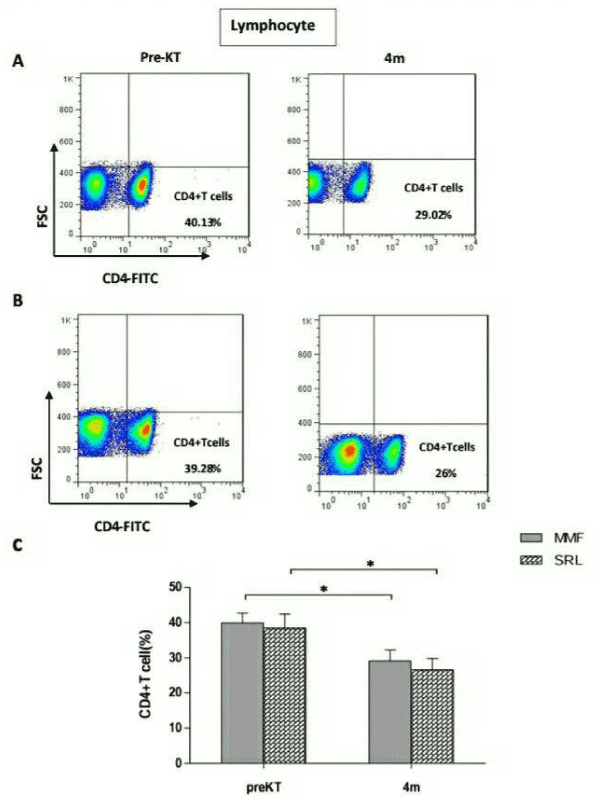
Figure 1. Frequencies of CD4+ T cells in 24 renal transplant recipients taking Mycophenolate mofetil (MMF) or sirolimus (SRL) at 4 months(4m) after transplantation compared to before transplantation (PreKT). The frequencies of CD4+T cells was measured by flowcytometry. A: representative FACS picture from recipients in MMF group before and after transplantation. B: representative FACS picture from recipients in SRL group before and after transplantation. C: collective analysis of result from groups of different drugs. Bar shows median. *P<0.05 for 4m vs. PreKT.
Frequencies of CD4+CD25+FOXP3+Treg in recipients
We next examined CD4+CD25+ T Subsets that represent FOXP3 as a specific transcription factor in regulatory T cell. The frequencies of CD4+CD25+FOXP3+ Tregs was (3.16 ± 1.25) before transplantation, and increased significantly to (4.14 ± 0.93, P=0.02) at 4 months after transplantation, in the MMF group (Figure 2A). In the SRL group, as in the MMF group, the frequencies of CD4+CD25+FOXP3+Tregs was significantly higher 4 months after transplantation (3.36 ± 0.81) compared with before transplantation (3.01 ± 0.75, P=0.04) (Figure 2B). However, patients receiving MMF presented higher level of CD4+CD25+FOXP3+ Tregs (4.14 ± 0.93) compared with patients on SRL (3.36 ± 0.81, P=0.04) (Figure 2C).
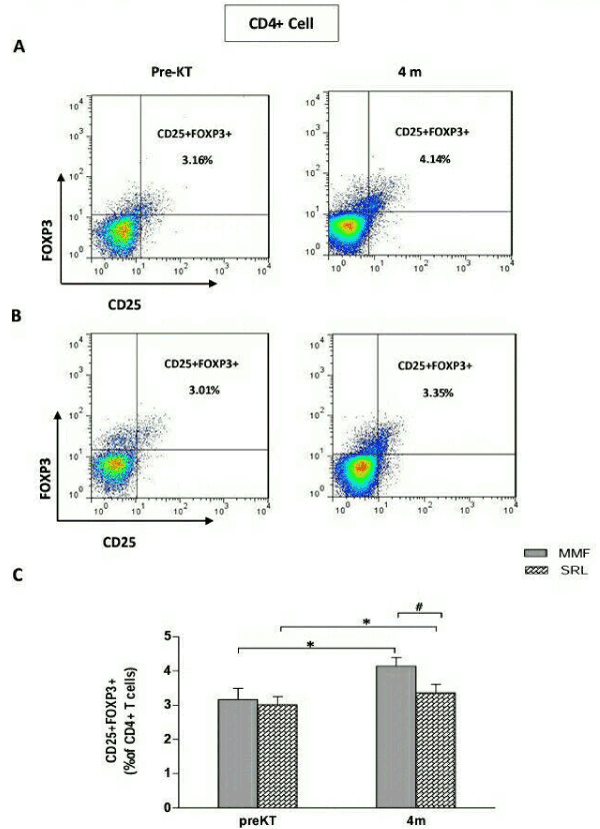
Figure 2. Frequencies of CD4+CD25+FOXP3+ Treg cells in 24 renal transplant recipients taking Mycophenolate mofetil (MMF) or sirolimus(SRL) at 4 months(4m) after transplantation compared to before transplantation(PreKT). The frequencies of CD4+CD25+FOXP3+ Treg cells was measured by flowcytometry. A: representative FACS picture from recipients in MMF group before and after transplantation. B: representative FACS picture from recipients inSRL group before and after transplantation. C: collective analysis of result from groups of different drugs. Bar shows median. *P<0.05 for 4m vs. PreKT. #P<0.05 for MMF group vs. SRL group.
Frequencies of CD3+CD8+CD28-Treg in recipients
CD8+CD28- T cells are other type of regulatory cells, which have recently been given more attention and whose role and frequencies are controversial in the solid organ transplantation. Thus, we analyzed the frequencies of CD3+CD8+CD28-Tregs in peripheral blood of all recipients. Before transplantation, the frequencies of CD3+CD8+CD28- Tregs was (7.37 ± 3.59) and this didn’t change significantly at 4 months (8.37 ± 5.75, P=0.4) after transplantation in the MMF group (Figure 3A). In the SRL group, also showed no significant change at 4 months (7.85 ± 3.94) after transplantation compared to before transplantation (7.21 ± 5.04, P=0.7) (Figure 3B). There was no significant difference in CD3+CD8+CD28-Tregs among group of different drugs (P=0.6) (Figure 3C).
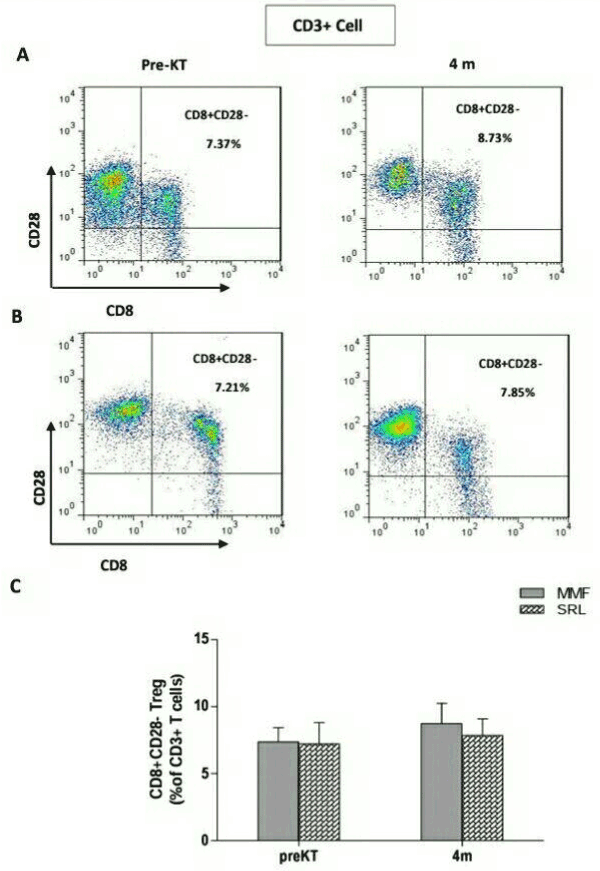
Figure 3. Frequencies of CD3+CD8+CD28- Treg cells in 24 renal transplant recipients taking Mycophenolate mofetil (MMF) or Sirolimus (SRL) at 4 months(4m) after transplantation compared to before transplantation (PreKT). The frequencies of CD3+CD8+CD28- Treg cells was measured by flowcytometry. A: representative FACS picture from recipients in MMF group before and after transplantation. B: representative FACS picture from recipients in SRL group before and after transplantationC: collective analysis of result from groups of different drugs. Bar shows median
Expression of specific transcription factors for CD4+ T cell subsets in recipients
2021 Copyright OAT. All rights reserv
To further estimate the effect of immunosuppressive drug regimen on T cells, we quantified Treg and T helper –specific transcription factor FOXP3, T-bet, GATA3 and RORγt in kidney transplant recipients. FOXP3 mRNA levels were increased 4 months after transplantation compared to before transplantation in all recipients. However, the expression of FOXP3 mRNA was significantly higher in MMF group compared to SRL group (P=0.01). In contrast, the mRNA level of T-bet and GATA3 were reduced 4 months after transplantation compared with before transplantation. As presented in Figure 4, SRL group showed a significantly lower level of T-bet mRNA expression than MMF group (P=0.04). And there was no significant difference in GATA3 mRNA expression between two groups (P=0.1). The expression of RORγt not only decrese but also increase a bit after transplantation in MMF group. In contrast, in SRL group the expression of RORγt decreased 4 months after transplantation compared to before transplantation. The changes in expression of RORγt was significantly varies between the two groups (P=0.01) (Figure4).
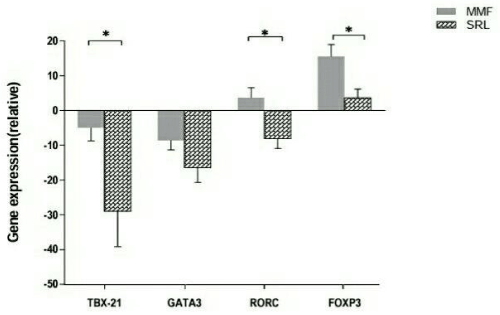
Figure 4. The expression of FOXP3, T-bet, GATA3 and RORγt associated with Treg and Th1/Th2/Th17 cells were quantified by real-time PCR, in Mycophenolate mofetil (MMF) compared to sirolimus (SRL) group. Bar shows median. *P<0.05 MMF group vs. SRL group
Discussion
Success in transplantation depends on various factors including good evaluation and matching of the recipient and donor before transplantation and good management after transplantation including precise adjustment of the immunosuppressive drugs and monitoring the clinical and para clinical test for early diagnosis of any sign and symptoms of kidney dysfunction. Allo-immune responses create an inflammatory microenvironment in transplant organ and the subsequent results mainly depends on how the immunosuppressive drugs affect the T lymphocytes. Most of the immunosuppressive drugs are selected based on their ability to control and down regulating the activity of T lymphocytes and the same time to maintain the normal function of allograft [20]. Calcineurin inhibitors such as Tacrolimus interfere with the signaling pathway of both effector T cells and Tregs by suppressing IL-2 transcription [21]. Today’s therapeutic protocols are extensively based on the minimization of the CNI dose, therefore in order to reduce the dose of CNI, using of the newer triple therapeutic immunnosupressive protocols could be effective [22]. In present study, to reduce the risk of transplant rejection, Anti- thymocyte globulin (ATG) was used for induction therapy. Marta Lopez, et al.reported that ATG has been responsible for the expansion of CD4+ CD25+ FOXP3+ regulatory T cells and maintenance of their regulatory activity in MLR assay with peripheral blood cells [23]. Our results showed that a combination therapeutic regimen containing Tacrolimus and MMF or SRL could modulate the effects of Tacrolimus on Tregs, as the frequency of CD4+ CD25+ FOXP3+Tregs, increased in both groups four months after transplantation compared to before transplantation. mTOR inhibitors such as Sirolimus and Everolimus are the new immunosuppressive drugs that unlike the tacrolimus, does not interfere with the expansion of Treg cells. Z.Q.chu, et al.in a study performed on kidney transplantation recipients showed that CNIs significantly decreases the percentage of Treg cells in Comparison to healthy subjects, while sirolimus does not change the percentage of Tregs [24]. D. Sansgundo et al. also reported that conversion the therapeutic protocol from Tacrolimus to sirolimus increases the absolute number of Treg cells [25]. However, the simultaneous use of Tacrolimus and sirolimus can suppress more effectively proliferation of alloreactive T helper cells (Th) Th1 and Th17, while maintains the population of Treg cells [26]. In line with our study combination of sirolimus with low dose of Tacrolimus increases the Tregs cells. MMF is commonly used in combination with Calcineurin inhibitors in transplantation, and significantly reduces acute rejection. There is little information about the relationship between MMF and Treg. It has been shown that MMF with vitamin D3 can induce Treg cells in the mouse model [13]. In the liver transplantation, Demirkiran et al. showed that conversion from Calcineurin inhibitors to MMF, causes an increase in the CD4+ CD25+ FOXP3+Tregs [12] while Lim et al. reported that expansion of CD4+ CD25+Treg cells isn’t so different in presence or absence of MMF [27]. Our results showed that frequency of CD4+ CD25+ FOXP3+ regulatory T cells in MMF recipients, is significantly higher than the other group. In confirmation of our findings, Zhen Wang et al. reported that low-dose Tacrolimus plus MMF, expands CD4+ CD25+ FOXP3+ regulatory cells [28]. Also, in another study on kidney transplantation recipients, the data showed that combination of Tacrolimus and MMF, increases frequencies of CD4+ CD25+ FOXP3+ T cells in Comparison with combination of Tacrolimus and Everlimus [29]. CD3+ CD8+ CD28- cells are another subsets of regulatory cells that have recently become their role are more prominent in various transplant organs. These cells have the ability to suppress alloimmune and autoimmune reactions in many animal models, and the increase can be as a predictor biomarker of better condition of the transplanted organ. It has been observed that in heart transplant recipients, the frequency of CD8+CD28- cells is higher in comparison with normal people [17]. Y.-X. Lin et al.study on liver transplantation patients, showed that combination of Tacrolimus and MMF, increased the numbers of CD3+CD8+CD28- cells [30]. while Korecka-polac et al.showed that Cyclosporine A and Rapamycin causes the suppression of CD8+CD28- cells [31]. Generally the number of CD3+CD8+CD28- cells increases in the liver transplantation and the high percentage is associated with a better graft Prognosis. However the percentage of these cells in kidney transplantation is controversial in different studies it may be related to different time point of sampling in each study as it seems to increase over time after transplantation. In the present study CD3+CD8+CD28- cells were increased after transplantation, nevertheless, we did not find a relationship between frequency of CD3+ CD8+ CD28- regulatory cells and the two different used immunosuppressive protocols. The balance between the effector T cells function and the regulatory cells is a complex mechanism that clarify the allograft outcome and survival. Tolerance or rejection are the two arms of this situation, and multiple different effective mechanisms pathways have role in these two processes. New studies are more concentrated to the plasticity of Th17 cells and the balance between the Th17 and Treg cells. It is very important to know that the Th17 and Treg cells develop from the same precursor and their development depending on the cytokine environment [32]. The imbalance of Th17/Treg in peripheral blood, is a strong predictor of CNI effects in the poor function of transplanted kidney [33]. Specific transcription factors are associated with functional evolution of various T cells such as: T-bet (Th1), GATA3 (Th2), RORγt (Th17) and foxp3 (Treg) [34]. In our study, the expression of FOXP3 and RORγt had increased in MMF group while FOXP3 has a regulatory function, RORγt were found to be elevated in inflammatory condition. In the SRL group, the expression of FOXP3 has been increased, but the expression of RORγt has decreased. It is suggested that the MMF protocol has failed to establish a balance between inflammatory and regulatory responses, while in the SRL group a safety balance is observed toward regulatory status. Heather Kopf et al.reported that sirolimus not only increases FOXP3 expression but also prevents the differentiation of the naïve T cells into Th17, and consequently, creates a more stable phenotype of CD4+ Treg cells [35]. Data from different studies have shown that Th1and TH17 cells have a profound role in induction of inflammatory transplantation process, but the role of Th2 cells are not yet fully understood [36]. The ratio of Th1 to Th2 is very important in the prediction of allograft status. In the present study, both the therapeutic protocols suppress expression of T-bet and GATA3 genes four months after transplantation compared to before transplantation, but the SRL group prevents more effectively T-bet expression and this is in favor of the allograft survival. Yi Li et al.study showed that conversion from CNI to the sirolimus reduces frequency of Th1 and Th17 [37].
In conclusion, our data demonstrated that although in the MMF group, the frequency of CD4+ CD25+ FOXP3+ Tregs increased significantly, but on the other hand the expression of RORγt has not been well inhibited, while the SRL group increase the Treg and has meaningfully decreased the expression of RORγt. It is suggested that considering the balance between alloreactivity and tolerance, using a therapeutic regimen containing SRL has a better performance in balancing and improvement of renal function.
Acknowledgments
This project (MSc thesis) is financially supported by Tehran University of Medical Sciences, Tehran, Iran. Grant number: 30721.
References
- Shan J, Feng L, Li Y, Sun G, Chen X, et al. (2014) The effects of rapamycin on regulatory T cells: its potential time-dependent role in inducing transplant tolerance. Immunol Lett 162: 74-86.
- Francis RS, Feng G, Tha-In T, Lyons IS, Wood KJ, et al. (2011) Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur J Immunol 41: 726-738.
- Salcido-Ochoa F, Tsang J, Tam P, Falk K, Rotzschke O (2010) Regulatory T cells in transplantation: does extracellular adenosine triphosphate metabolism through CD39 play a crucial role? Transplant Rev 24: p. 52-66.
- Iwase H, Kobayashi T, Kodera Y, Miwa Y, Kuzuya T, et al. (2011) Clinical significance of regulatory T-cell–related gene expression in peripheral blood after renal transplantation. Transplantation 91: 191-198.
- Chung BH, Kim KW, Kim B-M, Piao SG, Lim SW, et al. (2012) Dysregulation of Th17 cells during the early post-transplant period in patients under calcineurin inhibitor based immunosuppression. PLoS One 7: e42011.
- Net JB, Bushell A, Wood KJ, Harden PN (2016) Regulatory T cells: first steps of clinical application in solid organ transplantation. Transpl Int 29: 3-11.
- Chhabra D, Skaro AI, Leventhal JR, Dalal P, Shah G, et al. (2012) Long-term kidney allograft function and survival in prednisone-free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Clin J Am Soc Nephrol p: CJN. 06940711.
- Kim KW, Chung BH, Kim BM, Cho ML, Yang CW (2015) The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients. Immunology 144: 68-78.
- Battaglia M, Stabilini A, Roncarolo MG (2005) Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells.Blood105: 4743-4748. [Crossref]
- Wang GY, Yang Y, Li H, Zhang J, Li MR, et al. (2012) Rapamycin combined with donor immature dendritic cells promotes liver allograft survival in association with CD4+ CD25+ Foxp3+ regulatory T cell expansion. Hepatol Res 42: 192-202.
- Lu L, Qian X, Rao J, Wang X, Zheng S, et al. (2010) Rapamycin promotes the expansion of CD4+ Foxp3+ regulatory T cells after liver transplantation. Transplant Proc. Elsevier.
- Demirkiran A, Sewgobind VD, van der Weijde J, Kok A, Baan CC, et al. (2009) Conversion from calcineurin inhibitor to mycophenolate mofetil-based immunosuppression changes the frequency and phenotype of CD4+ FOXP3+ regulatory T cells. Transplantation 87: 1062-1068.
- Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, et al. (2001) Regulatory T cells induced by 1a, 25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 167: 1945-1953.
- Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, et al. (2006) Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production.Blood108: 390-399. [Crossref]
- Qu Y, Zhang B, Zhao L, Liu G, Ma H, et al. (2007) The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+T cells in mice.Transpl Immunol17: 153-161. [Crossref]
- Zheng Y, Rudensky AY (2007) Foxp3 in control of the regulatory T cell lineage.Nat Immunol8: 457-462. [Crossref]
- Colovai AI, Mirza M, Vlad G, Wang S, Ho E, et al. (2003) Regulatory CD8+ CD28- T cells in heart transplant recipients. Hum Immunol 64: 31-37.
- Liu Y, Chen N, Chen G, You P, editors (2007) The protective effect of CD8+ CD28- T suppressor cells on the acute rejection responses in rat liver transplantation. Transplant Proc. Elsevier.
- Su J, Xie Q, Xu Y, Li XC, Dai Z (2014) Role of CD8(+) regulatory T cells in organ transplantation.Burns Trauma2: 18-23. [Crossref]
- Halloran PF (2004) Immunosuppressive drugs for kidney transplantation.N Engl J Med351: 2715-2729. [Crossref]
- Furtado GC1, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ (2002) Interleukin 2 signaling is required for CD4(+) regulatory T cell function.J Exp Med196: 851-857. [Crossref]
- Hester J, Mills N, Shankar S, Carvalho-Gaspar M, Friend P, et al. (2011) Th17 cells in alemtuzumab-treated patients. The effect of long-term maintenance immunosuppressive therapy. Transplantation 91: 744.
- Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N (2006) A novel mechanism of action for anti-thymocyte globulin: induction of CD4+ CD25+ Foxp3+ regulatory T cells. J Am Soc Nephrol 17: 2844-2853.
- Chu Z-Q, Ji Q, editors (2013) Sirolimus did not affect CD4+ CD25high forkhead box p3+ T cells of peripheral blood in renal transplant recipients. Transplant Proc. Elsevier.
- San Segundo D, Fernández-Fresnedo G, Gago M, Beares I, Ruiz-Criado J, et al. (2010) Number of peripheral blood regulatory T cells and lymphocyte activation at 3 months after conversion to mTOR inhibitor therapy. Transplant Proc. Elsevier.
- Gallon L, Traitanon O, Yu Y, Shi B, Leventhal JR, et al. (2015) Differential effects of calcineurin and mammalian target of rapamycin inhibitors on alloreactive Th1, Th17, and regulatory T cells. Transplantation 99: 774-1784.
- Lim DG, Joe IY, Park YH, Chang SH, Wee YM, et al. (2007) Effect of immunosuppressants on the expansion and function of naturally occurring regulatory T cells. Transpl Immunol 18: 94-100.
- Wang Z, Shi B, Jin H, Xiao L, Chen Y, et al. (2009) Low-dose of tacrolimus favors the induction of functional CD4+ CD25+ FoxP3+ regulatory T cells in solid-organ transplantation. Int Immunopharmacol 9: 564-569.
- Fourtounas C, Dousdampanis P, Sakellaraki P, Rodi M, Georgakopoulos T, et al. (2010) Different immunosuppressive combinations on T-cell regulation in renal transplant recipients. Am J Nephrol 32: 1-9.
- Lin YX, Yan LN, Li B, Wang LL, Wen TF, et al. (2009) A significant expansion of CD8+ CD28- T-suppressor cells in adult-to-adult living donor liver transplant recipients. Transplant Proc. Elsevier.
- Korecka-Polak A, Bocian K, Pachówka M, Jalbrzykowska A, Korczak-Kowalska G (2016) Suppressor properties of human CD8+ CD28- T cells in mixed leukocyte reaction are not affected by CsA and RAPA. Arch Immunol Ther Exp (Warsz) 64: 409-416.
- Ashoor IF, Najafian N (2012) Rejection and regulation: a tight balance. Curr Opin Organ Transplant 17: 1.
- Ma L, Zhang H, Hu K, Lv G, Fu Y, et al. (2015) The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol 16: 56.
- Zhu J, Paul WE (2010) Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 238: 247-262.
- Kopf H, Gonzalo M, OMZ Howard, Chen X (2007) Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol 7: 1819-1824.
- Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, et al. (2008) Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway.Blood112: 4765-4775. [Crossref]
- Li Y, Shi Y, Huang Z, Bai Y, Niu Q, et al. (2011) CNI induced Th17/Treg imbalance and susceptibility to renal dysfunction in renal transplantation. Int Immunopharmacol 11: 2033-2038.




