Abstract
Sevelamer (SV) is frequently used to control hyperphosphatemia in CKD patients. Relatively mild gastrointestinal (GI) intolerance was reported in a minority of patients in preclinical trials. More serious GI morbidity has been reported with general clinical use, including GI bleeding and colonic complications including colitis and perforation. Use of phosphate binders including SV may be required in the setting of delayed graft function (DGF) early post transplant.
We report 3 cases of significant GI morbidity in association with SV occurring in transplant recipients within the first 2 weeks after kidney transplantation. One patient had a perforated colonic diverticulum associated with SV crystals. Two patients had severe esophagitis and esophageal ulcerations associated with SV crystals. Two patients had long-term use of SV prior to transplantation; one patient was treated with SV for a short time only after transplantation.
SV use may have more serious GI adverse effects in CKD patients than noted in the original clinical trials, and should be considered as a potential cause of GI symptoms in this patient population.
Keywords
sevelamer, gastrointestinal adverse events, chronic kidney disease, kidney transplant complications, esophagitis, colonic perforation
Introduction
Sevelamer and sodium polystyrene (Kayexelate) are anion-exchange resins commonly used in the chronic kidney disease (CKD) population for control of hyperphosphatemia and hyperkalemia respectively. The gastrointestinal (GI) toxicity of kayexelate has been well described in multiple reports and has been ascribed to the use of sorbitol, however evidence of toxicity seen with kayexelate use in the absence of sorbitol suggests direct injury due to resin crystals themselves [1,2].
Sevelamer hydrochloride was approved for clinical use by the Federal Drug Administration (FDA) in 1998 (and sevelamer carbonate in 2007) for treatment of hyperphosphatemia; subsequently sevelamer has become a commonly prescribed phosphate binder in CKD patients in part to avoid use of calcium based phosphate binders and minimize the risk of developing vascular calcifications.
In pre marketing clinical trials, GI complaints were the most common side effects of sevelamer and lead to drug discontinuation in a minority of patients [3]. In post marketing experience, there have been reports of more significant GI pathology including gastrointestinal bleeding, colonic ulceration, colitis, and perforated diverticulum in association with the use of sevelamer in CKD patients as well as the more benign side effects seen in premarketing studies [4-16]. GI symptoms and complications occur with some frequency in kidney transplant recipients with a complex spectrum of causes of which side effects of immunosuppression medications and infectious disease problems are contributing factors. We describe three cases of clinically significant gastrointestinal complications occurring in kidney transplant recipients in the early post transplant period associated with sevelamer. This suggests that sevelamer GI toxicity should be considered a contributing cause in GI problems in recent renal transplant recipients.
Case 1
A 28-year-old male admitted to our center for deceased donor renal transplant (DDRT). His cause of CKD was C1q nephropathy documented by biopsy and he was on hemodialysis for 8 years prior to transplantation. His past medical history was significant for ventriculoperitoneal shunt for pseudotumor cerebri and a hospitalization for diverticulitis one year prior to this admission without further episodes. A colonoscopy performed after clinical resolution of a prior episode of diverticulitis demonstrated diverticulosis of the ascending, transverse and descending colon, moderate diverticulosis of the sigmoid colon, but without any signs of infection or inflammation associated with the diverticular disease. At the time of admission for transplantation, he was taking sensipar 30 mg daily for management of secondary hyperparathyroidism and had been on sevelamer carbonate for more than 5 years with a current dose of 2,400 mg three times daily (TID) in conjunction with sucroferric oxyhydroxide (Velphoro) 500 mg TID for control of hyperphosphatemia. On admission, intact parathyroid hormone level (iPTH) was 157 pg/ml, serum calcium 9.9 mg/dl and serum phosphorus 6.1 mg/dl. He received induction immunosuppression using basiliximab and methylprednisolone. On post-operation day 2 he developed worsening abdominal pain, distension and clinical signs of sepsis. Computed Tomography (CT) of the abdomen revealed ileus, suspected fluid collection adjacent to the ventriculoperitoneal shunt and pneumoperitoneum. The shunt was externalized because of concern for infection followed by exploratory laparotomy for diffuse peritonitis, appendectomy for possible appendicitis and incidental resection of a normal appearing Meckel’s diverticulum. Close inspection of the sigmoid colon did not show evidence of diverticulitis or bowel perforation. Pathological evaluation of the specimens from his first abdominal exploration including the appendix and Meckel’s diverticulum were negative for inflammation or perforation. Subsequent recurrence of clinical abdominal sepsis and hemodynamic instability led to another urgent abdominal exploration during which feculent peritonitis and thickened sigmoid colon with inflamed pericolic fat without focal site of perforation were found, and the rectosigmoid was resected for a presumed microperforation. Due to the amount of intraperitoneal contamination, the fascia was left open for twenty-four hours with a temporary closure device applied. A planned abdominal washout, end colostomy, abdominal wall debridement and closure was subsequently performed with resulting clinical improvement. Pathology of the sigmoid colon showed diverticulitis with sevelamer-related injury, abscess formation, perforation, and acute serositis without infectious agents (Figure 1). The VP shunt was removed in its entirety due to resolution of papilledema. His transplanted kidney function improved with good urine output and normalizing serum creatinine at the time of his discharge at post-operative day 49.
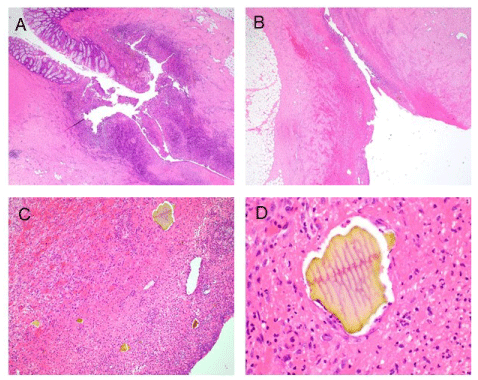
Figure 1. (A) Diverticulitis showing abscess formation and bowel perforation. Hematoxylin-eosin (H&E) stain. Original magnification x20. (B) Perforation site showing acute serositis with necroinflammatory exudate and granulation tissue. H&E. Original magnification x20. (C) Acute serositis with numerous Sevelamer crystals in granulation tissue and necroinflammatory exudate. H&E stain. Original magnification x100. (D) A rectangular-shaped Sevelamer crystal embedded in the necroinflammatory debris showing fish scales and 2-toned colors with pink in the center and yellow at edges. H&E stain. Original magnification x400
Case 2
A 49-year-old male with CKD due to membranoproliferative glomerulonephritis was on peritoneal dialysis for six years prior to undergoing DDRT at our center. Before the transplant, he was treated long term with calcium acetate 2668 mg TID for control of hyperphosphatemia. One month prior to transplantation, Velphoro 750 mg TID was added for inadequate control of hyperphosphatemia. He did not have any GI symptoms or documented GI disease prior to transplantation. At the time of his admission, serum phosphorus was 4.2 mg/dl, calcium 8.2 mg/dl and iPTH 234 pg/ml. Antithymocyte globulin and methylprednisolone were given for induction immune suppression. His post transplant course was complicated by delayed graft function requiring dialysis for control of metabolic abnormalities and volume excess. Three days after transplantation, sevelamer carbonate 1600 mg TID was started for control of hyperphosphatemia. Calcium acetate 1334 mg TID was added on post-operative day 5, and sevelamer dose increased to 2400 mg tid post-operative day 7 for continued hyperphosphatemia. On post-operative day 6, he complained of severe dysphagia and odynophagia which did not significantly improve with combination therapy with oral H2-blockers and proton-pump inhibitor (PPI). He had received oral mycophenolate mofetil (MMF) for two days prior to its discontinuation for his upper gastrointestinal symptoms. Esophagogastrodudenoscopy (EGD) on post operative day 8 showed severe esophagitis with exudate noted between the Z-line (38cm) and 25cm; biopsies of abnormal area were obtained. No specific abnormalities were noted in the stomach or duodenum on EGD. He was started on sucralfate and intravenous PPI with slow improvement in his symptoms and ability to tolerate oral diet. Esophageal biopsy revealed active esophagitis with sevelamer resin crystals within a mucopurulent exudate (Figure 2A and 2B). Sevelamer was discontinued at the time esophageal biopsy findings were available. Renal graft function had improved by the time of hospital discharge and he was no longer requiring dialysis. He was continued on high dose oral PPI with eventual resolution of his symptoms.
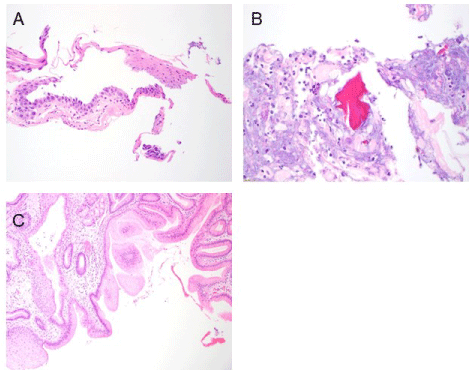
Figure 2. (A) Active esophagitis with erosion of the squamous epithelium. H&E stain. Original magnification x200. (B) Mucopurulent exudate with pink-red sevelamer resin crystals. H&E stain. Original magnification x400. (C) Post-treatment biopsy of the gastroesophageal junction mucosa showing mild hyperplasia of the gastric foveolar epithelium. No active inflammation or crystals are present. H&E stain. Original magnification x100
He underwent a second endoscopy approximately 2 months after his initial symptoms which confirmed healing of the esophageal ulceration, but probable inflammation of the esophageal mucosa and possible Barrett’s esophagus and nodularity just below the apparent Z-line. Biopsies of nodular and non-nodular areas showed mild hyperplasia of the gastric foveolar epithelium without active inflammation or crystals (Figure 2C).
Case 3
A 41 year-old man with CKD of unknown etiology was on peritoneal dialysis for four years prior to admission for DDRT. He had one episode of peritonitis six months after initiating dialysis. Past medical history was significant for hyperparathyroidism and gastroesophageal reflux disease treated with long term PPI therapy. Medications at admission included cinacalcet 30 mg daily and sevelamer 800 mg three times daily. He was taking sevelamer for the past four years. Laboratory values at the time of transplant included calcium 7.2 mg/dL, ionized calcium 0.69 mmol/L, phosphorus 6.0 mg/dL and parathyroid hormone level >1,700 pg/ml. He received induction therapy with antithymocyte globulin and methylprednisolone, and placed on PPI therapy per standard protocol. Post-transplant course was complicated by delayed graft function requiring peritoneal dialysis. Calcium acetate was started post-operatively for hyperphosphatemia, and cinacalcet was not restarted. MMF1000 mg twice daily was started on post-operative day number four when he was tolerating a diet. On post-operative day 8 Sevelamer 1600 mg three times daily was started for hyperphosphatemia not adequately controlled with calcium acetate alone. On post-operative day 10, he complained of dyspepsia and burning chest pain. At this time MMF was transitioned to mycophenolic acid at a reduced dose of 540 mg twice daily, and pantoprazole dose increased to 40 mg twice daily. EGD showed esophageal ulcers and a single linear gastric ulcer. PPI therapy was converted from oral to intravenous and sucralfate added. Mycophenolic acid and both oral phosphate binders were discontinued. His symptoms improved over the next few days, and he was able to tolerate oral intake and medications. Esophageal biopsy results showed ulceration, granulation tissue, fibropurulent exudate, and bacterial overgrowth; special stains were negative for fungal organism. CMV and HSV IHC and AFT stains were negative. Stomach biopsy showed active gastritis with fibropurulent exudate and bacterial overgrowth with sevelamer crystal present (Figure 3). His kidney function improved and he was no longer requiring dialysis at time of hospital discharge on post-operative day 15. He was continued on pantoprazole twice daily, and his symptoms resolved four weeks post-transplant at which time he re-started MMF 750 mg twice daily.
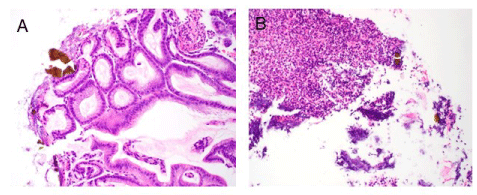
Figure 3. (A) Active gastritis with brown-orange sevelamer crystals surrounded in a fibropurulent exudate on the gastric mucosal surface. H&E stain. Original magnification x200. (B) Proximal esophageal biopsy specimen showing only fibropurulent exudate with multiple sevelamer crystals trapped within. H&E stain. Original magnification x200
Discussion
Sevelamer hydrochloride (Renagel) was approved by the FDA in 1998, and in 2007, sevelamer carbonate (Renvela) as non-calcium containing phosphate binders. Sevelamer carbonate was developed to minimize possible negative effect of sevelamer hydrochloride on metabolic acidosis and minimize gastric intolerance. The most common adverse effects in pre marketing clinical trials of sevelamer carbonate were GI complaints including nausea, vomiting, diarrhea, dyspepsia, abdominal pain, flatulence, and constipation occurring in 8-22% of patients and led to drug discontinuation in 3-16% of patients [3]. The most frequent serious side effect reported in clinical trials leading to drug discontinuation was peritonitis occurring in 8% of peritoneal dialysis patients receiving sevelamer (vs 4% control patient). Cases of fecal impaction were reported less commonly, and bowel obstruction and bowel perforation reported rarely. Since FDA approval and widespread use of sevelamer as a phosphate binder in CKD patients there have been several case reports of more severe GI complications associated with the use of sevelamer including gastrointestinal bleeding, colonic ulceration, colitis, and perforated diverticulum. The majority of reports are of colonic pathology associated with sevelamer crystals, but also several cases involving upper GI or small bowel pathology [4-16].
Two centers have reported small case series of their experience with sevelamer associated GI pathology: Swanson, et al. [17] was the earliest report in which specimens with novel, histologically similar crystals were collected; the crystals determined to be those of sevelamer by comparing them with crushed sevelamer tablets submitted to histologic processing similarly to patient tissue samples. They report 7 CKD patients (5 male, 2 female; age 38 to 81) with sevelamer crystals found in GI specimens. Four of the seven pts had GI biopsies performed to evaluate symptoms (3 for upper GI symptoms, one for rectal bleeding); three of seven had biopsies performed during routine colon examination. Sevelamer dose ranged from 800 mg TID to 3200 mg TID. Three of the reported cases had a history of transplantation: one with a history of stem cell transplant, one post liver transplant, and one post kidney transplant. Length of CKD duration and whether receiving renal replacement therapy was not reported.
George, et al. [18] reported 15 cases of resin associated GI injury, 8 of which were associated with sevelamer (patient age 45-77; 2 female, 6 male). All of the cases involved the lower GI tract; sevelamer crystals were associated with abnormal findings including ulcerations of the colon or anal canal, pancolitis, inflammatory polyps, or diverticulitis with perforation. In three of the cases, patients were also treated with kayexelate, and kayexelate crystals were also seen in pathology specimens.
Non-absorbable resins in clinical use, which include sevelamer, sodium polystyrene sulfonate (Kayexelate), and bile acid sequestrants (which include cholestyramine and cholestipol) can be identified on gastrointestinal biopsy specimens [17,19]. Recent reports have emphasized the importance of awareness of typical and atypical appearance of the resin on biopsy specimens by the pathologist assessing the specimen, as well as clinicians providing information regarding patient exposure to these medications. Bile acid sequestrants are considered biologically inert and as such have not been shown to cause GI injury. Kayexelate is not infrequently used in CKD patients to treat hyperkalemia and its use has been associated with bowel ulceration, necrosis and perforation [1]. Bowel injury has been attributed to hyperosmotic sorbitol in which the drug is often diluted, but animal studies have demonstrated colonic necrosis with the use of kayexelate alone, and there are reports of adverse bowel events with the use of kayexelate alone in clinical use [1,2].
Crystals seen on GI biopsy specimens have been reported to have a ‘classic morphology’ for each resin based on crystal morphology and color felt to aid identification [19]. Both Sevelamer and kayexelate crystals typically have an internal structure resembling ‘fish scales’, but stain differently on hematoxylin-eosin (H&E) stain with kayexelate crystals generally appearing purple and sevelamer 2-toned pink and yellow. Bile acid sequestrants lack the internal fish scale appearance and appear bright orange on H&E stain. With acid-fast bacillus (AFB) stain, kayexelate crystals appear black and sevelamer magenta. However crystals may not always have the typical appearance which makes clinical correlation and documenting drug exposure important. In particular, sevelamer crystals usually have typical coloration when associated with areas of mucosal injury, but may stain differently if seen in non-injured areas. Though sevelamer crystal color has been reported to vary depending on extent of background mucosal injury, the ‘fish scale’ pattern remained consistent [17].
It has been questioned whether sevelamer is the culprit inducing GI mucosal injury or simply a bystander [20]. In fact, in two cases described by Swanson, et al. where lower GI biopsy specimens were obtained during routine colon cancer screening in asymptomatic patients, sevelamer crystals were seen in the absence of mucosal injury [17]. However in numerous other reports of patients evaluated for GI symptoms, sevelamer crystals are seen intimately associated or embedded in areas of mucosal injury, suggesting that sevelamer is at least a contributing factor to the pathology.
The prevalence of severe GI complications associated with sevelamer use is not known, since published experience is based on case reports and two small case series. GI symptoms are not uncommon in CKD patients and can have numerous causes, and patients may not undergo endoscopy to identify specific etiology. In initial drug trials, GI side effects related to sevelamer were dose dependent; case reports of GI pathology are associated with a wide range of sevelamer dosages. It is not clear whether duration of use of sevelamer is associated with risk of GI pathology. Patient duration of use is often not reported, but when reported is most often several years
Most of the patients with GI pathology in association with sevelamer use were not reported to have preexisting GI disease, however a subset of reported patients had bowel perforation occurring in context of preexisting colonic diverticular disease.
Use of sevelamer is contraindicated with bowel obstruction (Renvela prescribing information), with warnings for patients with swallowing disorders or bowel motility disorders. It should be noted that patients with dysphagia, swallowing disorders, severe GI motility disorders including severe constipation, or major GI tract surgery were not included in the initial sevelamer clinical studies performed for FDA approval. Reassessment of the use of sevelamer in patients with known underlying bowel abnormalities should be considered.
We report 3 patients with clinically significant GI complications in the early post transplant period with pathology associated with sevelamer crystals. To our knowledge, sevelamer related GI pathology has not been reported in the early post transplant setting. There was one patient with a history of a kidney transplant in the report of Swanson, et al. but although not specifically stated, the presumption is that this patient had a failed graft with long term CKD. Yuste, et al. [4] describe two patients with sevelamer associated GI complications with a history of a history of a kidney transplant, but they were described as having failed grafts and were on renal replacement therapy for 5 and 6 years respectively.
All three of our patients had GI complications severe enough to markedly lengthen their hospital course and cause significant morbidity. Two of the 3 patients had a history of GI problems prior to transplantation: one with known diverticular disease and history of diverticulitis; the second with history of GERD but with good control of symptoms on PPI therapy. These two patients also were receiving long term treatment with sevelamer for phosphorus control. The third patient had no documented history of GI problems and denied any prior symptoms suggesting disease. This patient was requiring two phosphate binders for control of hyperphosphatemia while on dialysis, but had not been treated with sevelamer. He received sevelamer for about 3 days prior to development of UGI complaints. None of the three patients had any GI symptoms at the time of admission for transplant, and GI complications occurred within the first 1-2 weeks after transplantation. Two patients were asymptomatic before transplantation in the context of long term use of sevelamer on dialysis, while one patient became symptomatic and was found to have UGI pathology associated with sevelamer after taking it for only a few days. This suggests that additional factors present in the early post-transplant setting may contribute to the risk of sevelamer related GI injury. These factors could include surgical stress and abnormalities of integrity of bowel mucosal barrier related to high dose corticosteroids, uremia in the context of delayed graft function, or mycophenolate. This may have been an important mechanism in the patient who did not have any prior history of GI problems. It is unclear whether MMF was a significant contributor to symptoms and pathology in our patients since our reported patients either did not receive any MMF prior to their GI complication or received MMF for a very brief time before it was discontinued. UGI pathology occurred in our patients despite the routine use of prophylactic high dose PPI therapy. Two of the reported patients were requiring cinacalcet for control of CKD associated hyperparathyroidism; two required more than one oral phosphate binder for control of hyperphosphatemia, the patient not receiving sevelamer during CKD care was managed with high dose oral calcium binder. It is conceivable that small vessel calcification played a role in our patient’s GI complications.
In the two patients with upper GI pathology, symptoms improved and then resolved with high dose PPI therapy. Case 2 had endoscopic documentation of healing of his esophageal pathology and sevelamer crystals were not seen on follow up. Case 1 had the most serious morbidity requiring partial colectomy and treatment for intraabdominal infection. All 3 patients recovered well with no ongoing GI problems, resolution of delayed graft function with eventual good function of the renal allograft.
Adverse GI effects were seen in significant proportion of CKD patients in premarketing trials of sevelamer, but were usually mild. Case reports and case series have reported more severe GI complications related to use of sevelamer in CKD, particularly in those patients with significant GI pathology and/or clinical characteristics that would have excluded them from the initial trials. Avoidance of sevelamer use in CKD patients with significant underlying GI problems not included in the drug contraindications should be considered. Performance of endoscopy and biopsy of abnormal findings in addition to empiric treatment for GI symptoms in CKD patients receiving sevelamer should be considered to document whether sevelamer may be related to the etiology of the symptoms. Sevelamer related GI pathology can occur in patients early after transplantation, even in patients not previously treated with sevelamer, and should be added to spectrum of GI complications that can occur in this setting. Avoidance of sevelamer to control hyperphosphatemia post transplant in the setting of delayed allograft function, or in the setting of the failing allograft, should be considered.
Support
None.
Financial disclosure
None.
References
- Harel Z, Harel S, Shah P, Wald R, Perl J, et al. (2013) Gastrointestinal Adverse Events with Sodium Polystyrene Sulfonate (Kayexelate) Use: A Systematic Review. Am J Med 126: e9-24 [Crossref]
- Ayoub I, Oh M, Gupta R, McFarlane M, Babinska A, et al. (2015) Colon Necrosis Due to Sodium Polystyrene Sulfonate with and without Sorbitol: An Experimental Study in Rats. PLOS One 10: e0137636. [Crossref]
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022127s011lbl.pdf
- Yuste C, Merida E, Hernandez E, García-Santiago A, Rodríguez Y, et al. (2017) Gastrointestinal complications induced by sevelamer crystals. Clin Kidney J 10: 539-544 [Crossref]
- Tieu C, Moreira R, Wong Kee Song L, Majumder S, Papakakis K, et al. (2016) A case report of sevelamer-associated recto-sigmoid ulcers. BMC Gastroenterol 16: 20. [Crossref]
- Okwara C, Choi C, Park J (2015) Sevelamer-Induced Colitis Presenting as a Pseudotumor. Clin Gastroenterol Hepatol 13: A39-40. [Crossref]
- Kim J, Olson K, Butani L (2016) Sevelamer crystals in the mucosa of the gastrointestinal tract in a teenager with end-stage renal disease. Pediatr Nephrol 31: 339-341. [Crossref]
- Oka Y, Miyasaki M, Monobe Y (2018) Sevelamer Crystals Found in Necrotic Mucosa of a Perforated Diverticulum. Ther Apher Dial 22: 411-412. [Crossref]
- Chintammaneni P, Das R, Kuan S, Kermanshahi T, Hashash J (2014) Hematochezia Associated with Sevalamer-Induced Mucosal Injury. ACG Case Rep J 1: 145-147 [Crossref]
- Magee J, Robles M, Dunaway P (2018) Sevelamer-Induced Gastrointestinal Injury Presenting as Gastroenteritis. Case Reports in Gastroenterology 12: 41-45.
- Nambiar S, Pillai U, Devasahayam J, Oliver T, Karippot A, Colonic Mucosal Ulceration and Gastrointestinal Bleeding Associated with Sevelamer Crystal Deposition in a Patient with End Stage Renal Disease. Case Rep Nephrol 2018: 4708068. [Crossref]
- Uy P, Guerrero Vinsard D, Hafeez S (2018) Sevelamer-Associated Rectosigmoid Ulcers in an End-Stage Renal Disease Patient. ACG Case Rep J 5: e83. [Crossref]
- Keri K, Veitla V, Samji N (2019) Ischemic Colitis in Association with Sevelamer Crystals. Indian J Nephrol 29: 191-193 [Crossref]
- Modi R, Swanson B, Duggirala V (2017) Long-Standing Diarrhea Associated With Sevelamer Crystalopathy in Colonic Mucosa. Clin Gastroenterol Hepatol 15: xxvi-xxvii [Crossref]
- Yamaguchi T, Ohyama S, Furukawa H, et al., (2016) Sigmoid colon diverticula perforation associated with sevelamer hydrochloride administration: A case report. Ann Med Surg (Lond) 10: 57-60 [Crossref]
- Okwara C, Gulati R, Rustagi T, Birg A, Hanson J, et al. (2018) Upper Gastrointestinal Bleeding of Unusual Causation. Dig Dis Sci 63: 2541-2546 [Crossref]
- Swanson B, Limketkai B, Liu T, Montgomery E, Nazari K, et al. (2013) Sevelamer Crystals in the Gastrointestinal Tract (GIT): A New Entity Associated With Mucosal Injury, Am J Surg Pathol 11: 1686-1693 [Crossref]
- George S, Francis I (2019) Pathology of Resin-Induced Gastrointestinal Damage: Report of 15 Cases and Review of Literature. Turk Patoloji Derg 35: 221-227. [Crossref]
- Gonzalez R, Lagana S, Szeto O, Arnold C (2017) Challenges in Diagnosing Medication Resins in Surgical Pathology Specimens: A Crystal-Clear Review Guide. Arch Pathol Lab Med 141:1276-1282 [Crossref]
- Arriola A, Martin N, Tondon R (2017) Crystals in Perforated Bowel: Culprits or Innocent Bystanders, Int J Surg Pathol 26: 238-239. [Crossref]



