In this work, the self-assembly of molecular aggregates of two different compounds representing monomethyncyanine and merocyanine dyes, which differ by location, size, and orientation of their dipole moments arisen due to intramolecular polarity, respectively, is compared. It is demonstrated that the structure of molecular associates strongly dependent not only on the inner molecular structure, but also on the nature of the surface they absorb to, and that molecular association can further be changed with the film thickness. On gold substrate, the molecules tend to lie flat on the surface, while on glass surface they adsorb with the random orientation, which play an important role in formation of further aggregates as film thickness increases.
Self-assembly; Electronic absorption; Aggregate; Substrate
The development of electronic devices based on organic materials, i.e., small molecules or polymers with conjugated bonds, whose films have semiconducting properties, demonstrates continuous adaptation of new materials that provide various advantages such as cheapness, plasticity, flexibility, ease of chemical modification, production on large scale from solutions, etc. [1-6]. Specific self-assembly of molecules into molecular films largely determines electronic properties of the corresponding devices, such as electron excitation, energy transfer, charge transport, electronic absorption, redox activity, etc. [7-13]. For instance, the optical properties of molecular films to some extent correlate with the structure of molecular aggregates that are formed as a result of intermolecular interaction, and the analysis of the aggregate structure through optical features is still largely based on the Kasha model, which was developed half a century ago [14,15]. However, preparation of structures based on the above materials is always associated with solid substrates onto which the organic materials are self-assembled [16]. In contrast to the strong coupling of atoms in inorganic crystals, molecules in a condensed state have much smaller forces of interaction, which causes the fluidity and variability of their film structure, which is vulnerable to the action of various factors such as the chemical composition of the substrate surface, the electron affinity of the substrate material, the deposition rate of molecules on the surface, the temperature of the environment, etc. [17,18]. All these factors, as well as the competition between the molecule-substrate and molecule-molecule interactions will determine specific structure of the film, which can be particularly undergone to variations at the very first layer on the surface. Therefore, it is very important to develop new models and presentations about the structure of aggregates on the surface of a solid substrate, as well as to deeper understand their evolution depending on the distance to the surface, as well as influence of other factors.
In this work, we compare self-assembly of molecular aggregates of two different compounds representing monomethyncyanine and merocyanine dyes, respectively (Figure 1), which differ by location, size, and orientation of their dipole moments arisen due to intramolecular polarity, in respect to the molecular plane, and which are able to undergo intramolecular conformational changes [19,20]. We show that the structure of molecular associates is strongly dependent not only on the inner molecular structure, but also on the nature of the surface they absorb to, and such an association can further be changed with the film thickness.
Two compounds representing monomethyncyanine and merocyanine dyes, i.e., 2-[(3-methyl-3H-benzothiazol-2-ylidene)methyl]benzo[c,d]indole (BT) and 2,6-Di(tert-butyl)-4-[2-(1-methyl-1H-quinolin-4-ylidene)-ethylidene]-cyclohexa-2,5-dienone (Qu-Me) (Figure 1) have been synthesized at the Institute of Organic Chemistry, National Academy of Sciences of Ukraine.
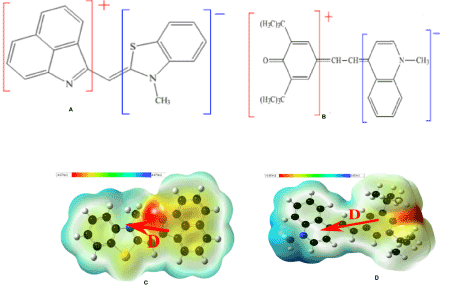
Figure 1. Chemical structure and corresponding map of the electrostatic potential of (a) BT and (b) Qu-Me.
Solutions were prepared by dissolving the dye molecules in dioxane with concentration of 10-3M. Dye aggregation was provided by adding a small amount (1 vol%) of the dioxane solution to the distilled water, where the compounds are not soluble.
Films were deposited using a VUP-5 vacuum camera equipped with optical spectrometers Polytec to control the spectral changes in-situ. Thin films of dyes were fabricated at the deposition rate of 0.1-1.6 Е/s, then the samples were heated at 250 to 300 °C under the residual pressure of 5x10–3 Pa. Two series of films were deposited in the same process. In the first series, the dye molecules were thermally evaporated onto the glass and in the second series onto the semitransparent gold layers (thickness ~ 30 nm) preliminary deposited on the glass substrate.
Additionally, two methods of film deposition have been used in order to confirm reproducibility of the results. In the first method, the film was grown successively on the same substrate and formation of the filmstructureas a function of film thickness was monitored in-situ by the spectrometer through recording its spectra every 30 seconds. In the second method, films of different thickness were deposited by gradient variation of thickness onto the row of samples which were removed from the evaporation source in the camera at the different distance followed by measurement of their spectra after the deposition process. The results of the two methods were similar, so we will not distinguish them further.
Molecular simulation was performed with Gaussian 09 and Materials Studio 8.0 (Forcite Module) software, using the density functional method (DFT/ B3LYP 6-31+(d,p)) and method of molecular dynamics (MD).
Association in solutions
First, self-assembly of the compounds were studied in the solutions. In aqueous media where the compounds are not soluble, their spectra immediately changed. BT, whose monomer spectrum demonstrated S0-S1 transition at about 570 nm followed by vibronic overtones at ~530 and 500 nm, showed blue shift and splitting of the absorption band in water into components at ~530 and 495 nm (Figure 2a). Such a change indicated in favor of a dimer formation in the form of a stack where the molecules are inclined to each other by a certain angle. Molecular simulation confirmed this suggestion (Figure 3). First, the dipole moment of the unaffected molecule was calculated to be about 8 degrees out of the molecular plane. In the dimer, the molecules experience torsional distortion by about 20 degrees, which results in mutual dipole orientation of the adjacent molecules to be inclined by approximately 145 degrees in respect to each other (Figure 3).
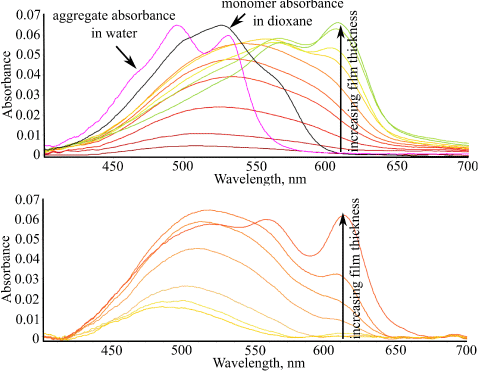
Figure 2. Electronic absorption spectra of BT films on (a) glass and (b) gold substrates as a function of the film thickness. Spectra of monomer and dimer solution in dioxane and water, respectively, are shown in (a) for comparison.
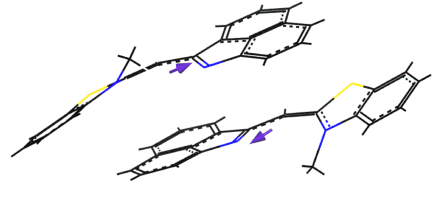
Figure 3. Simulated BT dimer structure in an aqueous medium.
Qu-Me showed more complex behavior in the solutions. Its monomer spectrum demonstrated S0-S1 transition at about 670 nm followed by vibronic overtones at ~610, 560 nm, etc. (Figure 4a). In water, the spectrum became abnormally broad, with two maxima at 545 and 740 nm, indicating formation of two types of dimers of H- and J-type, respectively. Molecular simulation showed that the dimer structure of H-type should be in the form of a stack, with antiparallel dipole orientation of the adjacent molecules (Figure 5a), while in J-aggregate the molecules are located ‘head-to-tail’ where the dipoles are parallel (Figure 5b). In the observed broad spectrum, a nonzero absorption near 670 nm (Figure 4a) can indicate the presence of some monomers in the solution.
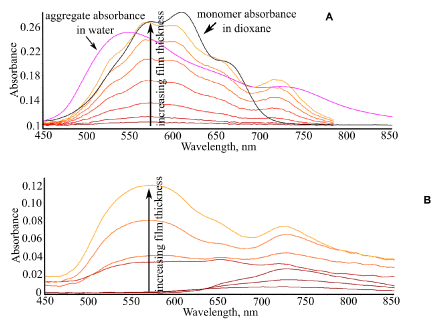
Figure 4. Electronic absorption spectra of Qu-Me films on (a) glass and (b) gold substrates as a function of the film thickness. Spectra of monomer and dimer solution in dioxane and water, respectively, are shown in (a) for comparison.
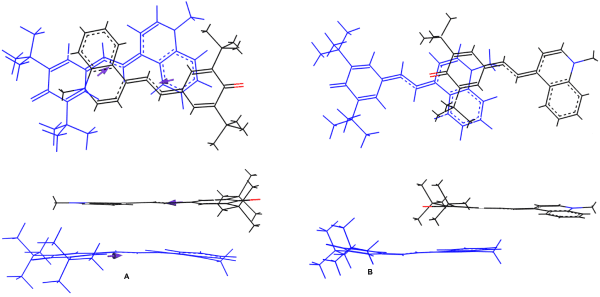
Figure 5. Simulated Qu-Me dimer structures in an aqueous medium.
Formation of BT films
Aggregate formation was monitored directly through changes in electronic absorption spectra of growing films. It was found that self-assembly of BT molecules occurs differently on glass and gold surfaces from the very beginning (Figure 2). On gold, spectra of thin layers of BT were similar to its monomer spectrum, with clear S0-S1 transition seen at ~570 nm, indicating that BT molecules are present in the first layers on the gold surface in the monomer form. Due to the relatively weak molecular dipole, the intermolecular aggregation here is weak and the influence of the metal substrate through image forces [21] dominates, which promotes more flat conformation of the molecules (Figure 6).
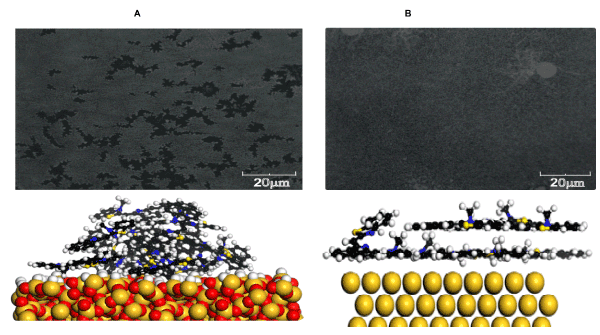
Figure 6. Optical micrographs of films and simulated intermolecular arrangement in thin layers of BT on (a) glass and (b) gold substrates.
On glass substrates, spectra of thin layers of BT were also in the spectral range of the monomer absorption, although these were more broad and structureless, indicating stronger disorder in the first molecular layers on the glass. With increasing film thickness, BT showed formation of aggregates both on glass and gold surfaces, whose structure however was different from that in water. The aggregate spectrum indicated red shift in respect to the monomer absorption and band splitting. Simulation of the dimer structure of BT aggregate in films showed that the molecular conformation is flat, however, the mutual orientation of their dipoles is more than 90 degrees (Figure 7).
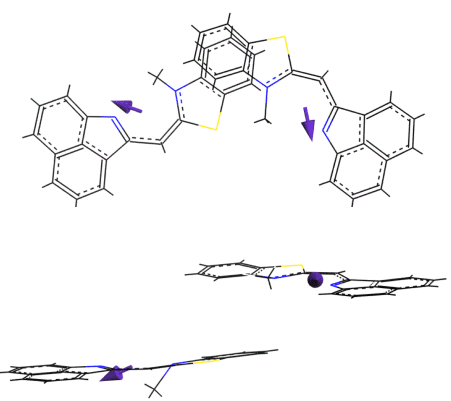
Figure 7. Simulated BT dimer structure in thick films.
Formation of Qu-Me films
It was found that self-assembly of Qu-Me molecules occurs differently on glass and gold surfaces as well (Figure 4). On glass, spectra of thin layers of Qu-Me were in the spectral range of monomer absorption, indicating that these layers are composed presumably as monomers. In thicker films, however, the aggregate features at ~725 nm can be seen, which is related to the J-type dimer observed in aqueous solution as well (Figure 5b). It should be noted that thick films still indicated absorption features of the monomer (Figure 4a), suggesting that the film structure is a superposition of the monomers and J-type dimers/aggregates.
On gold substrates, thin layers of Qu-Me demonstrated formation of J-type aggregates with a typical band at ~720 nm from the very beginning, followed by formation of H-type aggregates with maximum absorption around 550 nm in thicker films (Figure 4b). Formation of J-type dimers on the gold surface can be explained by competition of two factors: first, interaction with the metal substrate which tends to flatten the molecule due to compensation of its dipole through image forces; second, strong dipole-dipole intermolecular interaction due to significant dipole in Qu-Me molecule itself (Table 1) which promotes their dimer formation. As a result of the above two factors, the J-type dimer is formed. In thicker films, where the substrate influence becomes weaker the molecules which are far from the substrate surface tend to aggregate as well, but this aggregate is of different, H-type, structure due to the intermolecular dipole-dipole interaction only.
Table 1. Major geometric characteristics and dipole moment of the dye used.
Dye |
height, Å |
width, Å |
length, Å |
α°* |
Dipole moment, debye |
BT |
8.3 |
4.2 |
15.3 |
8.5 |
1.58 |
Qu-Me |
10.4 |
5.6 |
14.8 |
0 |
11.02 |
*- torsion angle of the central chromophore
It has been found that aggregate structure of the compounds studied is largely dependent not only on their molecular structure, but also on the environment used. In solid films the aggregate structure is different from that in the solution, and that structure is additionally influenced by the substrate on which the film was grown. It was revealed a significant influence of gold surface on self-assembly of both types of molecules. Both BT and Qu-Me tended to adopt a more flat conformation on gold surface due to compensation of their intramolecular dipole by image forces, however, strong dipole in Qu-Me nevertheless promoted J-type dimer formation with slight overlap of the molecules in the dimer even in the first molecular layer on the metal. With increasing film thickness the molecular self-assembly of Qu-Me was changing due to the shift of the substrate-molecule and molecule-molecule competition in favor of the latter. At the same time, BT with its small dipole, showed higher contribution of the molecule-substrate interaction on gold, but did not show significant variation of the aggregate structure with the film thickness. The obtained results are believed to promote better understanding of formation of molecular films in relevant applications.
The authors are grateful to Dr. Yu. Slominski for granting the dyes and to Prof. O. Kachkovsky for fruitful discussions.
- Tour JM1 (2000) Molecular electronics. Synthesis and testing of components. Acc Chem Res 33: 791-804. [crossref]
- Moth-Poulsen K, Bjørnholm T, (2009) Molecular electronics with single molecules in solid-state devices. Nature Nanotechnology 4: 551-556.
- de la Torre G1, Claessens CG, Torres T (2007) Phthalocyanines: old dyes, new materials. Putting color in nanotechnology. Chem Commun (Camb): 2000-2015. [crossref]
- Coe B, Curati M, (2004) Metal complexes for molecular electronics and photonics. Comments on inorganic chemistry 25: 147-184.
- Astruc D, Boisselier E, Ornelas C, (2010) Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem Rev 110: 1857-1959.
- Hagfeldt A1, Grätzel M (2000) Molecular photovoltaics. Acc Chem Res 33: 269-277. [crossref]
- Lim II1, Goroleski F, Mott D, Kariuki N, Ip W, et al. (2006) Adsorption of cyanine dyes on gold nanoparticles and formation of J-aggregates in the nanoparticle assembly. J Phys Chem B 110: 6673-6682. [crossref]
- Hlawacek G1, Teichert C (2013) Nucleation and growth of thin films of rod-like conjugated molecules. J Phys Condens Matter 25: 143202. [crossref]
- EtienneM, Quach A, Grosso D, Nicole L, Sanchez C, Walcarius A, (2007) Molecular Transport into Mesostructured Silica Thin Films:? Electrochemical Monitoring and Comparison between p6m, P63/mmc, and Pm3n Structures. Chem Mater 19: 844-856.
- Brede J, Linares M, Kuck S, Schwobel J, Scarfato A, et al. (2009) Dynamics of molecular self-ordering in tetraphenylporphyrin monolayers on metallic substrates. Nanotechnology 20: 275602.
- Cai K1, Xie J1, Zhang D1, Shi W1, Yan Q2, et al. (2018) Concurrent Cooperative J-Aggregates and Anticooperative H-Aggregates. J Am Chem Soc 140: 5764-5773. [crossref]
- Matsuura A, Obayashi T, Kondoh H, Ohta T, Oji H, Kosugi N, Sayama K, Arakawa H, (2002) Adsorption of merocyanine dye on rutile TiO2 (1 1 0). Chem Phys Lett 360: 133-138.
- Sayama K, Tsukagoshi S, Mori T, Hara K, Ohga Y, Shinpou A, Abeb Y, Suga S, Arakawa H, (2003) Efficient sensitization of nanocrystalline TiO2 films with cyanine and merocyanine organic dyes. Solar Energy Materials and Solar Cells 80: 47-71.
- Kasha M, Rawls H, Ashraf El-Bayoumi M, (1965) The exciton model in molecular spectroscopy. Pure and Applied Chemistry 11: 371-392.
- Hestand NJ1, Spano FC1 (2018) Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem Rev 118: 7069-7163. [crossref]
- Amabilino D, (2016) Supramolecular Chemistry at Surfaces. Cambridge: The Royal Society of Chemistry, USA.
- Forrest SR1 (1997) Ultrathin Organic Films Grown by Organic Molecular Beam Deposition and Related Techniques. Chem Rev 97: 1793-1896. [crossref]
- Love J, Proctor C, Liu J, Takacs C, Sharenko A, Poll T, Heeger A, Bazan G, Nguyen T (2013) Film Morphology of High Efficiency Solution-Processed Small-Molecule Solar Cells. Adv Funct Mater 23: 5019-5026.
- Tolmachev O, Pilipchuk N,. Kachkovsky O, Slominski Yu, Gayvoronsky V, Shepelyavyy E, Yakunin S, Brodyn M, (2007) Spectral and non-linear optical properties of cyanine bases’ derivatives of benzo[c,d]indole. Dyes and Pigments 74: 195-201.
- Coe B, Foxon S, Harper E, Harris J, Helliwell M, et al. (2009) The syntheses, structures and nonlinear optical and related properties of salts with julolidinyl electron donor groups. Dyes and Pigments 82: 171-186.
- Gabovich A, Li M, Szymczak H, Voitenko A, (2012) Image forces for a point-like dipole near a plane metal surface: An account of the spatial dispersion of dielectric permittivity. Surface Science 606: 510-515.







