Abstract
Cerebrospinal fluid is a biofluid contiguous with brain and spinal cord and a plausible tissue for measuring concentrations of central nervous system drugs and biomarkers. Lumbar puncture enables single timepoint sampling while lumbar catheterization enables repeated collection over a period of time. We report here our experience with the procedural safety of both techniques across two phase 1 studies of ESB1609, a brain penetrant sphingosine-1-phosphate receptor agonist, alongside the technical considerations, opportunities, and challenges of implementation. Across both studies, good quality samples were obtained through lumbar puncture and lumbar catheterization. Mild adverse events of post dural puncture headaches were common among participants undergoing lumbar catheterization and resolved with simple analgesics. Surprisingly we found that blood contamination was absent for single lumbar punctures but was detectable throughout the entire lumbar catheter sampling periods, although levels of hemoglobin were very low. We determined that for certain neurological therapeutics, serial cerebrospinal fluid sampling via lumber catheterization in healthy volunteers can provide informative pharmacokinetic and biomarker data but has a relatively high risk of post dural puncture headache compared to lumbar puncture. Acetaminophen co-administered with caffeine might be considered as first line management for post dural puncture headache in appropriate participants.
Keywords
CSF sampling, cerebrospinal fluid, blood brain barrier, lumbar catheterization, lumbar puncture, post dural puncture headache
Introduction
The last decade has seen significant advances in our understanding of the pathobiology of neurodegenerative diseases coupled with advances in the number of potential therapeutics and diverse mechanisms to address them [1]. Despite this, disease modifying central nervous system treatments successfully completing late-stage clinical trials remains disappointingly low. Reasons stated for clinical trial failures include inappropriate drug dosages and the selection of incorrect treatment targets because of inadequate knowledge of the underlying pathology [2]. Clinical research methods which improve dose selection decisions and enable bridging from preclinical models to biomarkers in humans are therefore highly desirable. Brain or spinal cord tissue cannot be sampled in humans but cerebrospinal fluid (CSF) can be, making it a tissue through which drug concentrations and biomarkers might be usefully measured.
The most common approach is to perform a lumbar puncture. This technique involves introducing a needle into the sub arachnoid space (commonly referred to as the intrathecal space) to obtain CSF for analysis. Potential side effects of lumbar puncture include damage (usually temporary) to nerves leading to numbness, paraesthesia or paralysis; infection; or pain at the lumbar puncture site. Post dural puncture headache however is the most frequent complication [3]. Lumbar catheterization involves the placement of an indwelling catheter into the intrathecal space and allows for the serial acquisition of CSF (e.g. hourly) to assess drug levels or biomarkers over a time course, without the need for repeated lumbar punctures. The procedure was first described by Bruce and Oldfield [4]. There are limited published data on its safety in clinical studies, side effects are expected to be similar to lumbar puncture and considered to be generally well tolerated [5].
Materials and Methods
Clinical Program Design
ESB1609 is a selective agonist of the type 5 sphingosine 1 phosphate receptor, a G protein coupled receptor expressed in the CNS and natural killer cells and was being developed for lipid driven neurodegenerative disorders such as Niemann Pick type C. Data from microdialysis studies of ESB1609 in rodents demonstrated a strong correlation between brain extracellular fluid (ECF) concentrations and CSF concentrations (data not shown) and provided a strong rationale for sampling CSF in phase 1 healthy volunteer studies to compare dose levels to anticipated therapeutic CNS concentrations using CSF concentrations as a surrogate. Additionally, confirmation of linearity of CNS penetration across multiple dose ranges and analytical validation and clinical characterization of key CSF lipid and protein biomarkers in healthy individuals could be explored.
Two phase 1 studies involving CSF collection were conducted over the time period September 2019 to May 2021. Study 1 (ESB1609-101) was a placebo controlled single ascending dose study of ESB1609 and was followed by study 2 (ESB1609-102) a placebo controlled multiple ascending dose study. Both studies were conducted at QPS, Petrus Campersingel 123, 9713 AG Groningen, The Netherlands.
Studies 1 and 2 were designed as a single program to run sequentially in a novel design that assessed the safety, tolerability, plasma pharmacokinetics (PK) and food effect of ESB1609. Additionally it enabled assessment of CSF concentration time profiles, characterization of CSF biomarkers via both lumbar puncture and serial CSF sampling, as well as tolerability of the CSF collection procedures. To better understand the quality of CSF, as well as vascular or microvascular injury safety, we developed a highly sensitive assay to detect very low concentrations of hemoglobin in CSF.
Ethics Committee Review and Approval of Studies
The original study protocols and any amendments were reviewed and approved by the Independent Ethics Committee (IEC) of the trial center (Stiching Beoordeling Ethiek Biomedisch Onderzoek Review Board, Assen, The Netherlands). The competent authority of the Netherlands (CCMO) was notified on the original protocol and amendments thereafter. Conforming to the acting regulations at that time, non-objection statements were issued by the CCMO. The studies were registered under EudraCT numbers 2019-003149-13 and 2019-004265-41.
Study Design: Study 1 (ESB1609-101)
Thirty-five male participants were enrolled into 1 of 4 dose cohorts to receive either ESB1609 or placebo (Figure 1) to study plasma and CSF PK, and safety and tolerability. CSF was collected through lumbar catheter.
The eligibility and handling of participants who received CSF sampling was designed to ensure ethical acceptability and data integrity of the study. It was not deemed acceptable to dose participants who had a lumbar catheter with placebo since CSF was only being acquired for PK, therefore the study was designed to have two sub cohorts. The first sub cohort (sub cohort A) received ESB1609 or placebo in a double blind manner for assessment of plasma PK, safety and tolerability and comprised the double blind safety and PK data set for ESB1609. B sub cohorts received ESB1609 in an open label fashion and underwent serial CSF PK sampling. To reduce the risk of post dural puncture headache, only adults aged 55-65 years were allowed to enroll in the B sub cohorts, while A sub cohorts allowed subject 18-65. Screening and eligibility for B sub cohorts included a detailed assessment of potential contradictions to lumbar catheterization including history of chronic back pain and headache, medical history of spinal surgery, assessment of risks for raised intracranial pressure and confirmation of normal blood coagulation profile and platelets one day prior to insertion of the lumbar catheter.
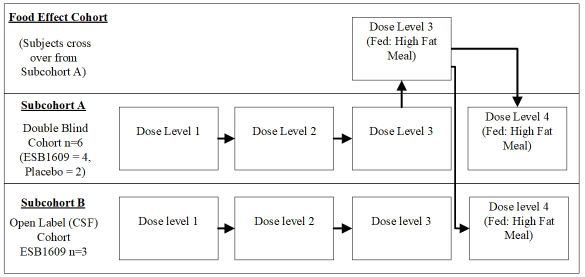
Figure 1. Study schematic for study ESB1609-101. Participants were divided into sub cohorts to allow for an open label serial CSF collection procedure which would not compromise the double-blind safety
In sub cohort B, all participants received thromboprophylaxis with subcutaneous (SC) 2850 IU of nadroparin prior to insertion of the lumbar catheter and on the 2 consecutive days after.
All participants in both sub cohorts received a single dose of ESB1609 and remained in the clinic for 7 days after dosing, during which safety assessments were performed and plasma PK samples were collected. In sub cohort B, safety assessments and plasma PK was also performed in addition to CSF PK sampling via the lumbar catheter. CSF samples were drawn at approximately the same time as plasma PK samples were obtained to enable pairing of the two data sets. The lumbar catheter was removed immediately after the 24 hours sampling timepoint and participants were prescribed bed rest for 24 hours. Participants remained in the clinic for at least 8 days in total for continued plasma PK collection and safety assessments.
Study Design: Study 2 (ESB1609-102)
Study 2 (Figure 2) was designed to characterize the safety, tolerability and plasma PK of multiple ascending doses of ESB1609 dosed up to 14 or 25 days. Additional goals included characterization of normal levels of CSF biomarkers (sterols, sphingolipids and proteins) via lumbar puncture for future studies in patients, diurnal variability of the same biomarkers using serial CSF sampling via lumbar catheter, and confirmation of linearity of CSF exposures with plasma exposures at the highest dose level (determined to be the likely therapeutic dose). Additionally, CSF samples would be used for analytical validation of biomarkers, assessment of test-retest reliability, freeze-thaw cycles, and other phenomenon on biomarker stability.
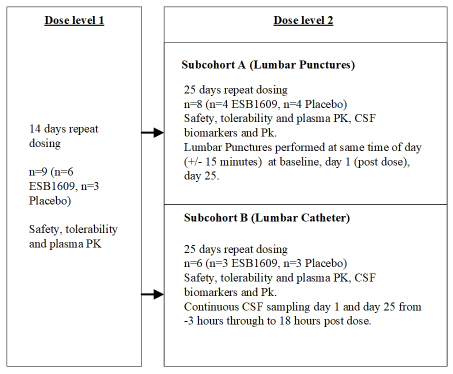
Figure 2. Study schematic for study ESB1609-102. The protocol was originally designed to study 3 dose level cohortswith the final cohort (Cohort 3) to have collection of CSF through LP and lumbar catheter in different sub cohorts. In this study,the middle dose level (cohort 2) was dropped because cohort 1 achieved exposures above those anticipated for cohort 2,resulting in 2 dose level cohorts only
The study enrolled 23 participants. Since dose level 1 was not considered to be relevant for study of CSF exposure, only safety, tolerability and plasma PK over 14 days of repeat dosing was studied. Dose level 2 included CSF sampling and consisted of two sub cohorts (a lumbar puncture sub cohort and a lumbar catheter sub cohort) receiving the same daily doses of ESB1609 or placebo over 25 days. Since this study required assessment of biomarkers it was deemed ethically acceptable to sample CSF from both placebo and ESB1609 treated participants to account for a potential, but unanticipated, drug effect on biomarkers in the ESB1609 treated arm. Sub cohort A was a lumbar puncture cohort in which the lumbar puncture was performed at 3 time points over 25 days. These cohort data were used to determine CSF levels of ESB1609 and measure key biomarkers. Sub cohort B underwent serial CSF sampling via a lumbar catheter to characterize a CSF PK time profile for confirmation of linearity of exposure at the intended therapeutic dose (the highest dose) and to characterize intraday variability of biomarkers for more accurate assessment of their clinical feasibility for later studies in patients.
Sub cohort A had CSF acquired through lumbar puncture at baseline, post dose on the first day of dosing (day 1), and on day 25. The lumbar puncture was performed at exactly the same time of day (+/15 minutes) for each timepoint to avoid diurnal variation. The protocol allowed for the day 1 CSF collection to be delayed up to 5 days in the case of a post dural puncture headache. Sub cohort B had a lumbar catheter inserted approximately 3 hours before dosing on day 1 and had samples taken at various timepoints from 3 hours before dosing to 18 hours post dose. The procedure was repeated on day 25. All sub cohort B participants received thromboprophylaxis with 2850 IU of nadroparin after insertion of the lumbar catheter and on the next day
Lumbar Puncture Procedure
A 22 Gauge Atraumatic Needle (Sprottle® Lumbar 22G, Pajunk, Ref. 321151-30C) was used for collection and the lumbar puncture was performed under aseptic conditions according to local protocol. The volume of CSF acquired at each lumbar puncture was 20 mL Participants were allowed home 48 hours after their last lumbar puncture.
Lumbar Catheter Procedure
A Pebax Catheter Epidural set (Vygon 5191.3871) was used and inserted according to local procedural standard operating procedures under aseptic conditions. The lumbar catheter was removed immediately after the 18-hour sampling timepoint and participants were prescribed bed rest for 24 hour after the catheter was removed. Participants were allowed home 48 hours after lumbar catheter removal. Volumes collected at each time point varied based on the analytes required to be measured, but the total volume for each lumbar catheter period of 22 hours was approximately 67 mL, equating to around 10% of daily CSF production.
Analysis of CSF Hemoglobin
CSF Hb levels were measured using a sandwich ELISA kit (Cat# E88-134, Bethyl labs, TX). The assay standard curve range was between 0.27 to 200 ng/mL. CSF samples were diluted 50-fold in buffer resulting in a lower limit of quantitation (LLOQ) of ~13.5 ng/mL. In some experimental runs, where the lowest standard showed %CV > 25%, the next highest standard was designated as the LLOQ (41.2 ng/mL). The concentration of hemoglobin in each CSF sample was determined by interpolation of values against the standard curve using a 4-parameter regression.
Results
Post Dural Puncture Headache and other Adverse Events
Rates of post dural puncture headache across lumbar catheter and lumbar puncture groups are summarized in Table 1.
Table 1. Summary of post dural puncture headaches across studies 1 and 2
| |
Lumbar Catheterization |
Lumbar Puncture |
Total no. of procedures performed |
25 |
24 |
Number of SAEs |
0 |
0 |
PDPH AE rate |
(14/25) 56% |
2/24 (8.3%) |
Number of subjects experiencing at least one PDPH |
13/19 (68%) |
0 |
PDPH AE grading |
Mild |
8/14 (57%) |
2/2 (100%) |
Moderate |
6/14 (43%) |
0/0 (0%) |
Severe |
0/14 (0%) |
0/0 (0%) |
AE intervention |
Acetaminophen |
10/25 (40%) |
2/2(100%) |
Opioid analgesia |
0/0 (0%) |
0/0 (0%) |
Blood patch |
0/0 (0%) |
0/0 (0%) |
Number of failed procedures |
|
1 |
0 |
Subject Withdrawals |
|
2 |
0 |
Study 2 only – number of subjects who reported a PDPH on day 25 who also had a PDPH on Day 1 |
1 |
N/A |
PDPH duration (range) |
30 minutes-27 days |
2-13 days |
*Mild AEs were defined as asymptomatic or mild symptoms, clinical or diagnostic observations only, or intervention not indicated.
**Moderate AEs are defined as minimal, local or noninvasive intervention indicated; or limiting age-appropriate instrumental daily activities.
Post dural puncture headache occurred in 14/25 (56%) of the lumbar catheterization procedures with all cases being mild or moderate and all resolved. 40% of cases required basic analgesia with acetaminophen. Only 2/24 post dural puncture headache cases occurred in the lumbar puncture group with all cases being mild and all resolved. No serious adverse events were recorded. A number of other AEs were recorded following insertion of first lumbar catheter or first lumbar puncture which might be attributed to the procedure, these included tingling in the leg, light headedness, and blurry vision. All of these events were mild in severity and are included as a complete list in the supplementary materials Table S1 and Table S2. A comprehensive overview of the post dural puncture headache events and any instituted clinical management are provided in Table S3 and Table S4.
Table S1 101 Full post procedure AE listings Study 1
Table S3::Summary of Post Dural Puncture Headaches and Management from Lumbar Catheterizationgroups
x
Subject Number |
Study no |
Study day of lumbar catheter insertions |
Severity |
Intervention |
Outcome |
Duration |
7-101 |
1 |
|
Mild |
Acetaminophen 500 mg |
Recovered |
7 days |
8-101 |
1 |
|
Moderate |
Acetaminophen 500mg increased to 1000 mg plus caffeine |
Recovered |
27 days |
16-101 |
1 |
|
Moderate |
Acetaminophen 500 mg increased to 1000mg acetaminophen plus caffeine |
Recovered |
14 days |
35-101 |
1 |
|
Moderate |
Acetaminophen 500 mg increased to 1000 mg plus caffeine |
Recovered |
10 days |
27-101 |
1 |
|
Moderate |
Acetaminophen 500 mg changed to to Acetaminophen 500 mg plus caffeine |
Recovered |
8 days |
27-102 |
2 |
25 |
Mild |
1000 mg paracetamol PRN for 1 day, changed to paracetamol 1000 mg plus caffeine PRN for 6 days total treatment |
resolved |
15 days |
28-102 |
2 |
25 |
Mild |
1000 mg paracetamol PRN for 1 day |
resolved |
7 days |
29-102 |
2 |
1 |
Mild |
None |
Resolved. Subject stayed in study to day 25 but refused Day 25 procedure |
5 days |
30-102 |
2 |
1 |
Mild |
None |
Resolved |
4 days |
31-102 |
2 |
25 |
Mild |
None |
Resolved |
30 minutes |
32-102* |
2 |
1 |
Moderate |
None for the first 4 days of AE. Then 3 days of 1000 mg paracetamol and caffeine once per day for 2 days of AE reduced to 500 mg once dose. Subject restarted paracetamol regularly for 4 days after AE resolved as prophylaxis and then stopped. |
Resolved |
11 days |
132-102
(replacement subject) |
2 |
1 |
Moderate |
1000 mg paracetamol PRN prescribed for 3 days, changed to twice daily with caffeine for 4 days |
resolved |
8 days |
129-102
(replacement subject) |
2 |
1 |
Mild |
None |
resolved |
7 days |
129-102
(replacement subject) |
2 |
25 |
Mild |
Paracentamol 1000 mg PRN changed to paracetamol 500mg plus caffeine on day 4 PRN for one day |
resolved |
12 days |
*Catheter was not draining well after insertion and removed. Subject also reported 3 other AEs all of mild severity relating to the PDPH (Nausea (7 days, moderate intensity), neck stiffness (mild intensity and lasting 24 days) and stiff upper legs ( 2 days, mild intensity) all with onset within 1-5 hours post insertion) an additional 3 days of paracetamol 1000 mg plus caffeine for neck stiffness was prescribed for days 5-7 of that AE.
Duration of headache is presented here as measured in whole days to include the full 24-hour period the PDPH started and the full 24 hour period the PDPH ended.
Table S4: Summary of Post Dural Puncture Headaches and Management from Lumbar Puncture Groups
Duration of headache is presented here as measured in whole days to include the full 24-hour period the PDPH started and the full 24 hour period the PDPH ended.
Subject Number |
Study no. |
Study Day of LP |
Severity |
Intervention |
Outcome |
Duration |
21-102 |
2 |
Day 1 |
Mild |
5 days of Paracetamol 1000 mg PRN |
Recovered |
13 days |
21-102 |
2 |
Day 25 |
Mild |
1 day paracetamol 1000 mg twice daily |
Recovered |
2 days |
Hemoglobin Contamination and Vascular or Microvascular Injury
In the lumbar puncture groups, CSF Hb levels were above the LLOQ of the assay in only 2/24 time points of samples (1 time point each in separate participants). The CSF Hb levels were 44 and 179 ng/mL, respectively in these 2 samples. After correcting for nominal blood Hb levels of 15 g/dL, this concentration of CSF Hb equates to ~30 and ~120 nL of blood contamination in 1 mL of CSF thus, individual lumbar punctures show low blood contamination and provide clean CSF samples.
In the lumbar catheter group first sampling time point (-3 hrs) CSF Hb levels were in the range of 15 to 341 ng/mL. However, at subsequent times up to the last time point (18 hrs) before removal of the catheter, CSF Hb levels were elevated in the range of 500 to 10000 ng/mL. In some participants CSF Hb levels went up at early timepoints and stayed elevated over the entire time, while in others there was an increase followed by a gradual decline towards baseline (Figure 3).
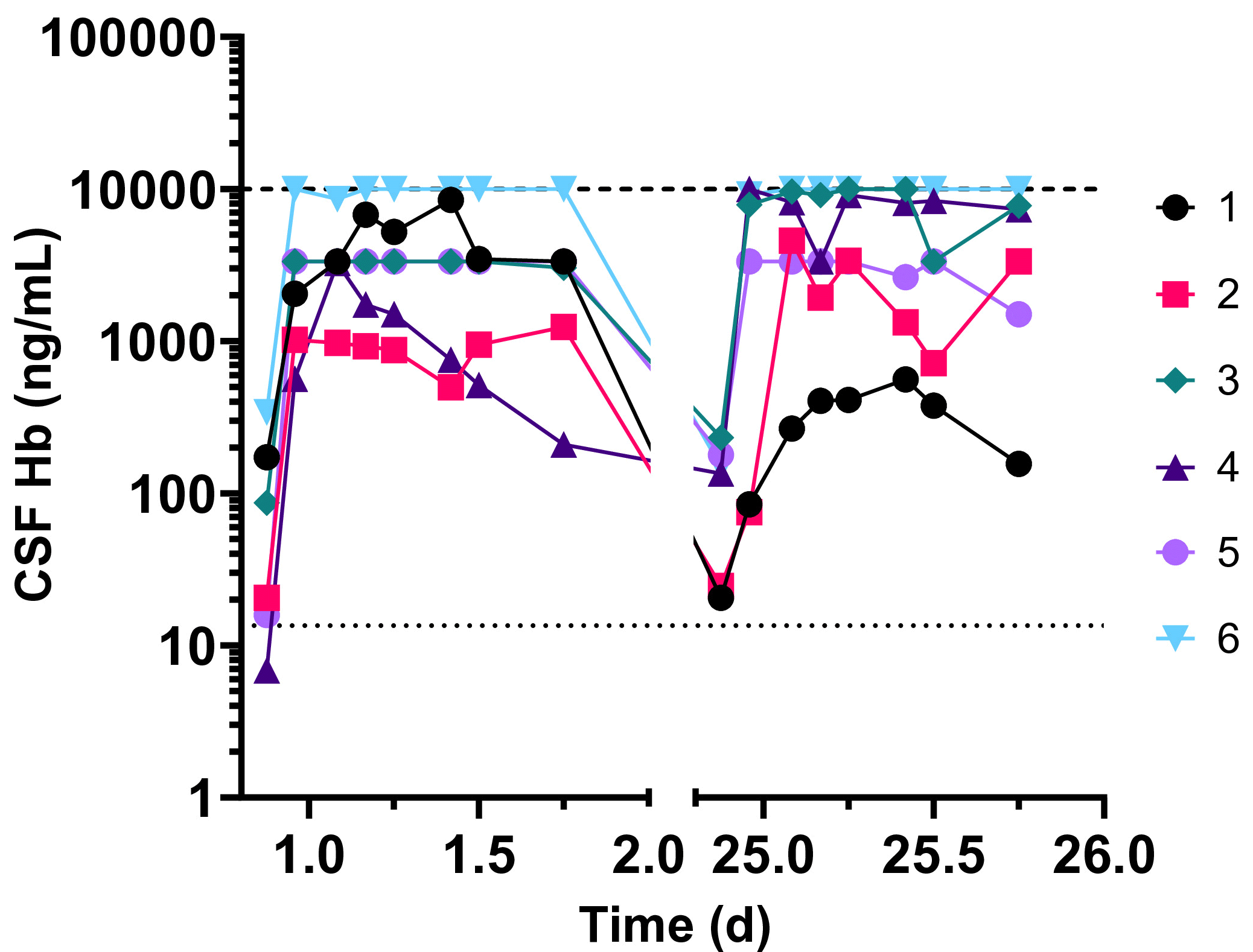
Figure 3. Change over time in CSF hemoglobin levels in the lumbar catheter cohorts in study 2 (ESB1609-102). Data are shown for each of the 6 subjects for the 2 lumbar catheter periods (day 1 and end of study)
Discussion
Rationale for CSF sampling
CSF sampling has potential utility in early clinical development including understanding CSF penetration of drugs and clinical validation and qualification of new biomarkers, but the decision to implement it should be assessed on a per study basis after a thorough evaluation of the scientific rationale which requires a solid appreciation of blood brain barrier physiology. Since multiple factors can determine the CSF concentration of systemically administered drugs, CSF concentrations may not correlate with free brain exposures. Consequently, in many cases, CSF PK sampling may provide limited information on CNS PK, demonstrating only that drug levels are detectable in CSF and therefore confirming penetration into the CNS.
Three distinct barrier layers exist in the CNS and are concerned with the maintenance of critical homeostatic parameters for CNS function and cell survival (Figure 4) therefore CSF concentrations of analytes, whether drugs or disease biomarkers may have relationships not just to the brain. The blood-brain barrier (BBB) is formed from the brain endothelium and consists of various junctional proteins which significantly reduce the permeation of molecules across it. Solutes and small polar surface area lipophilic molecules may passively diffuse across the barrier. Other molecular traffic is handled through facilitated diffusion or transporter mechanisms including solute transporters, ATP-binding cassette transporters, and vesicular transport. In addition to the BBB, plasma pharmacokinetics, protein binding, cerebral blood flow and brain tissue binding are also critical factors involved in CNS distribution [6]. The Blood-CSF barrier (the choroid plexus epithelial barrier) is the site of formation and secretion of CSF and contains comparable mechanisms to the BBB differing in some facets including the tight junction and transport proteins expressed [7]. Finally, the CSF-brain barrier (formed from the anatomical tissue barrier, the pia mater), allows passive diffusion of molecules between the CSF and brain extracellular fluid. The concentration of drug in any one of the three compartments (the cerebral blood, the CSF, and the brain ECF) is dependent on movement across these barriers and is subject to both the characteristics of the drug itself as well as critical biological processes (Figure 5 and 6). Approximately 600 ml of CSF is produced per day in adults, with a CSF volume between 140-240 ml, raising the possibility that 15-20 ml per hour could theoretically be sampled without adverse consequence. In these studies, the CSF collection procedures were justified on assumptions made from preclinical microdialysis data in which we had confidence in a model that linked CSF concentrations and brain extracellular fluid concentrations to efficacy, as well as the need to characterize and validate specific lipid and protein biomarkers associated with a potential drug effect for use in later interventional patient studies.
Accessing the CSF space via single timepoint lumbar puncture is a common procedure in clinical medicine for diagnostic sampling. Potential procedural side effects are important to take into consideration in clinical trials since the risk benefit of the procedure is likely to be different than indicated for diagnostic or approved therapeutic purposes. Post dural puncture headache is the most common complication [3] and is associated with multiple risk factors which we considered in the eligibility criteria for these studies (Table 2). It consists of a headache that is usually positional (worse when upright, better when lying flat) and may be associated with neck stiffness, photophobia, and nausea. It can usually be managed with simple analgesics, fluids, rest, and administration of caffeine. In some circumstances a blood patch procedure is required in which a small volume of autologous blood is injected into the epidural space to stop the CSF leak.
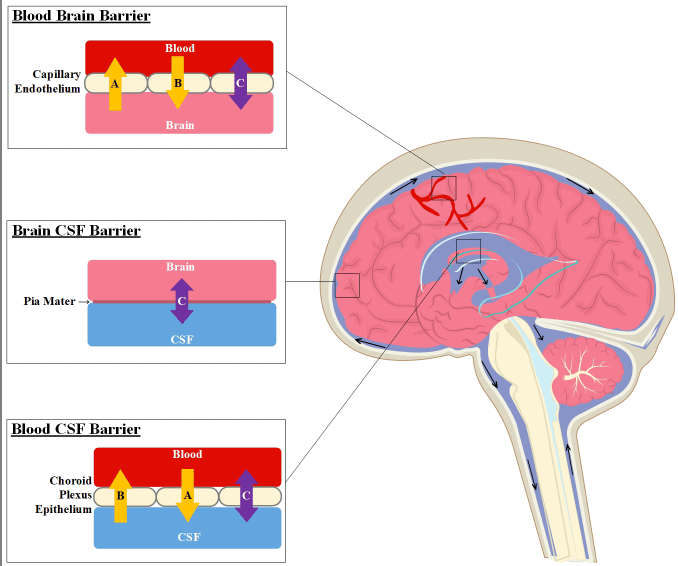
Figure 4. The membranes and physiological systems governing drug concentrations in the CNS and CSF of systemically delivered drugs. CNS drugs may cross these membranes via active uptake (A), active efflux (B), and passive or facilitated diffusion (C) systems depending on the drug characteristics. The relationships between these processes, alongside other drug characteristics including protein binding and free plasma concentration, determines the concentration of drug in the brain extra-cellular fluid and CSF and as such how CSF concentrations relate to free brain concentrations
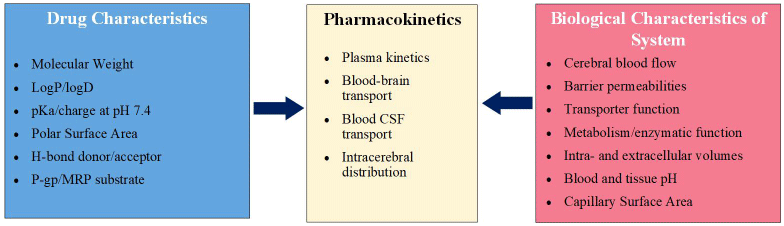
Figure 5. The relationship between CNS compartments and their governing factors
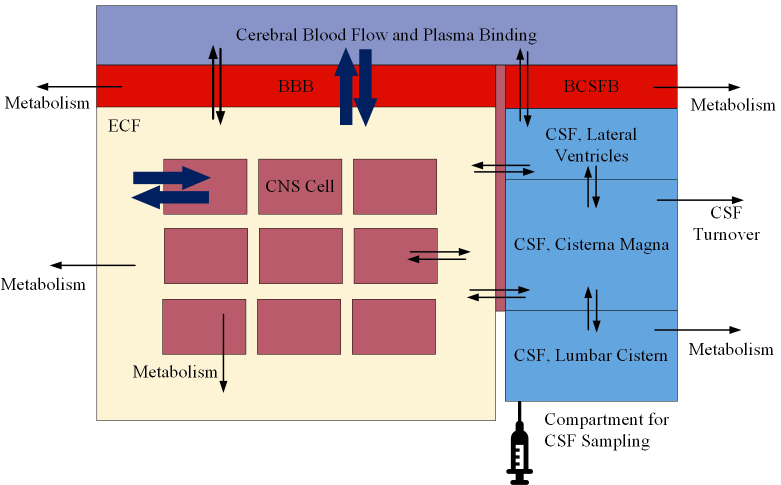
Figure 6. Brain ECF drug concentrations and its relationship to CSF and cerebral blood drug concentrations are governed by several factors including drug characteristics and the characteristics of the biological system involved, the later being a source of variability within participants, e.g. throughout the course of the day and between participants. Of particular importance is the consideration of potential differences between healthy normal controls and patients, between sexes and between adult and pediatric individuals. BBB = blood brain barrier, BCSFB= blood CSF barrier, ECF= extracellular fluid,
Table 2. Demographic and Operational Considerations for Eligibility Criteria for Clinical Trials Involving Lumbar Puncture or Lumbar Catheterization
Increased Risk of PDPH |
Reduced Risk of PDPH |
Young age |
Experienced operator |
Female |
Use of atraumatic needles |
Low body mass index |
|
Multiple dural puncture procedures |
|
Past medical history of chronic headache |
|
Single timepoint lumbar punctures have limitations. They can only be performed a limited number of times in the same subject due to the risk of side effects, and selecting the optimal time for PK sampling after dosing is also challenging. Furthermore, if a CSF PK time course curve is desired, many participants would need to be enrolled to enable collection of enough samples, but inter-subject variability could introduce significant noise. In these circumstances, serial CSF sampling via lumbar catheter might be considered.
The potential advantages of serial CSF sampling over lumbar puncture in drug development depends on the drug characteristics but include establishing linearity of CNS exposures at different dose levels and after multiple dosing has achieved steady state, direct comparison to plasma PK, and confirming absence of accumulation of drug in the CNS. Additionally, the CSF/ECF drug concentration relationship via microdialysis in animal studies may be translated to the clinic to estimate human brain ECF concentrations and guide dose selection. In addition to the post dural puncture headache risks outlined for lumbar puncture, serial CSF sampling may require bed confinement during and after lumbar catheterization (to avoid post dural puncture headache after catheter removal). Risks associated with periods of immobility have been well documented in the medical literature and include the risk of decubitus ulcers and venous thromboembolism.
Major advances in biomedical sciences over the past years have also enabled us to dissect molecular signatures associated with disease severity, progression, and drug effect in neurological diseases, some of which, such as protein, lipids, neuroinflammatory markers, and neurotransmitters, are present in CSF [8]. Some biomarkers of disease progression have been relatively well validated (e.g. amyloid species and tau) [9] however, new and emerging clinical biomarkers require validation.
Biomarker validation is a time and resource intensive, and highly complex process and should contain an assessment of clinical feasibility. Serial CSF sampling can have a major role in determining the utility and feasibility of pursuing new CNS biomarkers in clinical trials. In later stage clinical trials involving patients, infrequent lumbar punctures are a preferred method for collection of CSF since it is less invasive than lumbar catheterization, but the single time point collections may provide misleading data if the biomarkers of interest display considerable variability. Using serial CSF sampling to understand biomarker characteristics in healthy participants can help support the rationale for CSF collection via lumbar punctures in specific later stage studies in patients. Identifying significant variability in healthy participants, whether due to circadian biorhythm or other sources of variability throughout the day or night (e.g., posture and feeding, sampling, handling etc.,) may indicate that such biomarkers are not suitable to pursue in patients. However, identifying biomarkers with acceptable variability can support their selection for interventional pharmacodynamic studies in patients using single lumbar puncture sampling. Such data can provide increased confidence that any changes seen over time represent real change rather than variability, and may support statistical sample size planning for such studies.
In the studies presented here, the procedural safety risks were minimized as best as possible by specific eligibility criteria in which participants at a higher risk of post dural puncture headache were excluded, but the narrower characteristics of this enriched population introduces some limitations on translatability of findings. Notably, the enrollment of only older men may result in a dose exposure relationship specific to that age and sex. However, if the relationship between plasma PK and CSF can be assumed approximate across sex and age groups then extrapolation of the data may be possible.
With respect to interpretation of biomarker data, the issues of age and sex become increasingly important, particularly with respect to biomarkers that may be used in pediatric or older populations, however these effects would not impact the value of analytical validation and other exploratory measures such as stability assessments of CSF samples. Additionally, extrinsic sources of variability may be different in an in-clinic environment compared to a real-world environment, such as food consumption, time spent in a supine position, and external stressors from procedures such as blood tests.
Enrolling only healthy participants also limits the choice of biomarkers to those measurable in healthy humans (such as sphingolipids, sterols, certain proteins etc.,). Other caveats are likely to exist depending on the disease, the biomarker and the age range of the intended treatment population (e.g. pediatric or adult).
All but one of the lumbar catheter procedures were performed successfully with visually clear CSF drawn from the catheters at all time point. All participants who received a lumbar catheter were prescribed bed rest for 24 hours after catheter removal and then advised to mobilize based on their subjective symptoms if they had any.
Post dural puncture headache was associated with more than half of all lumbar catheter procedures and were all of mild or moderate severity and responded to acetaminophen if needed. Of note, many of the participants who had a moderate post dural puncture headache who received a lower dose (500 mg) of acetaminophen went on to receive 1000 mg of acetaminophen with 1000 mg of caffeine. It might therefore be suggested that a post dural puncture headache of moderate intensity due to lumbar catheterization is best managed initially with a full adult dose of acetaminophen and caffeine if not contraindicated, recognizing that caffeine can increase heart rate as a side effect.
Unexpectedly, lumbar catheterization was associated with consistently higher Hb contamination throughout the entire sampling window compared to lumbar puncture, indicating that placement of the lumbar catheter may result in mild trauma at the site of insertion and can result in sustained blood leakage into the CSF compartment despite care being taken to reduce movement and keep the subject comfortable. This finding may have implications for the interpretability of biomarker data obtained from CSF in this manner [10].
These studies were operationally complex. It was not deemed ethically acceptable to over enroll the study to account for potential withdrawals due to the risk of the procedure, consequently the study timelines were extended considerably due to the need to replace two participants. Additional factors which cannot be easily predicted include the 24 hours of bed rest following removal of the catheter, with mobilization after this period being determined by the participants based on subjective symptoms. The operational complexity of the study required increased inpatient confinement, experienced operator staff availability and recruitment from a narrower pool of participants with lower risk of post dural puncture headache than a standard phase 1 study, all adding to the timelines.
In summary serial CSF sampling via lumber catheterization in healthy volunteers can provide informative PK data for certain CNS drug development programs as well as biomarker data but has a relatively high risk of mild post dural puncture headache compared to lumbar puncture. A thorough assessment of the potential risks against the value of acquired data should be undertaken as part of clinical development planning.
Funding
The study was funded by ESCAPE Bio, Inc.,
Competing Interests
NF, MM, EL and SS and CB were employees of ESCAPE Bio during the execution of the study and were paid employees during the period of the study conduct.
References
- Fish PV, Steadman D, Bayle ED, Whiting P (2019) New approaches for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett29: 125-133. [Crossref]
- Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH (2019) Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomed 7: 97. [Crossref]
- Almeida SM de, Shumaker SD, LeBlanc SK, Delaney P, Marquie‐Beck J, et al. (2011) Incidence of Post‐Dural Puncture Headache in Research Volunteers. Headache 51: 1503-1510. [Crossref]
- Bruce JN, Oldfield EH (1988) Method for Sequential Sampling of Cerebrospinal Fluid in Humans. Neurosurgery 23: 788–790. [Crossref]
- Daas I den, Wemer J, Farha KA, Tamminga W, Boer T de, et al. (2013) Serial CSF sampling over a period of 30 h via an indwelling spinal catheter in healthy volunteers: headache, back pain, tolerability and measured acetylcholine profile. Eur J Clin Pharmacol 69: 1083-1090. [Crossref]
- Lange ECM de, Danhof M (2002) Considerations in the Use of Cerebrospinal Fluid Pharmacokinetics to Predict Brain Target Concentrations in the Clinical Setting: Implications of the Barriers Between Blood and Brain. Clin Pharmacokinet41: 691-703.
- Liddelow SA, Temple S, Møllgård K, Gehwolf R, Wagner A, et al. (2012) Molecular characterisation of transport mechanisms at the developing mouse blood-CSF interface: a transcriptome approach. Plos One7: e33554. [Crossref]
- Gaetani L, Paoletti FP, Bellomo G, Mancini A, Simoni S, et al. (2020) CSF and Blood Biomarkers in Neuroinflammatory and Neurodegenerative Diseases: Implications for Treatment. Trends Pharmacol Sci41: 1023-1037. [Crossref]
- Hansson O (2021) Biomarkers for neurodegenerative diseases. Nat Med27: 954-963. [Crossref]
- SchwenkenbecherP, Janssen T, Wurster U, Konen FF, Neyazi A, et al.(2019) The Influence of Blood Contamination on Cerebrospinal Fluid Diagnostics. Front Neurol10: 584. [Crossref]






