Abstract
Objective: Nasal polyps are, benign growths outgrowths of sinonasal tissue, affecting approximately 1-4% of the population. They have been correlated to the presence of chronic inflammation, but the molecular underlying mechanisms has not been completely defined. Defective epithelial barrier has been correlated with chronic rhinosinusitis (CRS) and nasal polyps (NPs) Moreover, a variation of ΔNp63 isoform expression has been noted in human airway epithelial cells and in the NPs epithelium. The benign lesion in some patients can have a recurrence, and the difference between recurrent and not recurrent patients is still unclear.
The aim of the study was to molecularly characterise NPs patients, distinguishing between patients presenting NP recurrence after surgical treatment respect to those in which recurrence has been not reported.
Methods: In the present work we analysed a cohort of patients affected by nasal polyposis, all submitted to Functional Endoscopic Sinus Surgery (FESS). The patients have been six-monthly clinical, checked in the two years following surgery. In all patients we analysed keratin 5, and p63 isoform expression, by RTqPCR using fresh NPs tissues taken after surgery. Moreover, confocal imunnofluorescence analysis again for p63 and Keratin 5 has been performed on fixed sections.
Results and conclusion: The results show high ∆Np63 expression and keratin 5, in nasal polyps of patients. The analysis of the expression level of the TAp63 isoform, shows a differential expression between patients with recurrence, respect to the not recurring. A more comprehensive marker could be considered the ∆N/TAp63 ratio. In fact, even though ∆Np63 is expressed in non-recurrent patients the ratio ∆N/TAp63 result significantly lower in these patients. This clearly indicates that the status of TA63 expression, and represented by ∆N/TAp63 ratio, could be considered a prognostic markers of low recurrence probability. Since TAp63 is also upregulated by HDAC inhibitors could be the consideration of local treatment of nasal polyps with these molecules.
key words
chronic rhinosinusitis, nasal polyps, endoscopic examinations, sinonasal tissue
Background
Chronic rhinosinusitis (CRS) is a widespread clinical condition that affects approximately 5-15% of the European and American population. A phenotype classification, based on endoscopic examinations of the nasal cavity or imaging procedures, differentiates CRS into chronic rhinosinusitis with nasal polyps (CRSwNP) and chronic rhinosinusitis without nasal polyps (CRSsNP) [1].
Nasal polyps are inflammatory outgrowths of sinonasal tissue, which affects approximately 1-4% of the general population, more likely male patients and the typical age of diagnosis goes from 40 to 60 years. Usually nasal polyps present as bilateral inflammatory lesions originating in the ethmoid sinuses and projecting into the nasal airway beneath the middle turbinate [2].
In addiction of obstruction/congestion or nasal discharge (anterior/posterior rhinorrea), reduction or loss of smell and facial pain/pressure, CRSwNP patients often comorbid other important medical conditions that can influence disease severity, such as atopy, asthma, Aspirin Exacerbated Respiratory Disease (AERD), Wegener’s granulomatosis and pathologies characterized by excessive inspissated mucus (Cystic Fibrosis) and mucociliar-transport alterations (e.s. Kartagener syndrome). The percent of allergic rhinitis patients with nasal polyps is similar to that of the general population (0.5-4.5%) [3]. On the other hand, 51-86% of CRSwNP patients are sensitized to at least one aeroallergen.
Sharing similar features of inflammation and remodelling, asthma and CRSwNP frequently coexist: CRSwNP is estimated to occur in 7% of all asthmatics while asthma is reported in 26-48% of patients with CRSwNP [4]. AERD (originally Samter’s triad: nasal polyps, asthma and aspirin sensitivity) is defined as a clinical condition characterized by development of upper and/or lower respiratory tract symptoms following the ingestion of medications that inhibit the cycloygenase-1 (COX-1) enzyme. Among 10-30% of patients with CRSwNP present AERD; in those cases, nasal polyps are multiple, characterized by rapid growth and in the absence of medical management – universal recurrence after surgery [5].
To date, the underlying mechanisms that contribute to the chronic inflammation of the sinonasal mucosa have not completely defined, but the interactions between epithelial cells, the host immune system and pathogens certainly play an important role in CRSwNP pathogenesis [1].
A recent classification of chronic rhinosinusitis with nasal polyposis has been described by Dennis et al. [6] (Table 1). Basing on endotype approach, there are four subtypes: type-2 cytokine characterized by a high presence of eosinophils, mast cells, basophils, and T-helper 2 (Th2) cells with a high level of IL-5, typically in nasal polyposis [7], eosinophil-based approach with a predominant presence of eosinophils; IgE-based approach with elevated levels of IgE (except AERD) and cysteinyl-based approach characterized by a high level of cystenyl leukotriene (CysLT), especially in AERD patients [6]. Because CysLT is metabolized and excreted through the urine, the 24-hour urinary measurement of leukotriene C4 synthase (LTC4) has been suggested to identify CRSwNP patients who have the AERD variant [8]. To date, no large-scale studies have been performed to identify genetic linkages and polymorphisms related to CRS pathogenesis [9], but data on the expression levels (mRNA signature) of specific genes are now available. The airway epithelium of the human nasal mucosa represents a barrier that protects against inhaled substances and pathogens via tight junctions, defective epithelial barrier with decreased expression of TJ proteins is found in patients with chronic rhinosinusitis (CRS) and nasal polyps (NPs) [10,11]. Tumor protein p63 (TRP63) is one of the regulators of various cell–matrix and cell–cell adhesion complexes in the epidermis [12]. Loss of ΔNp63 isoform significantly reduces epithelial proliferation and increases E-cadherin expression in human airway epithelial cells [13]. The human p63 genes express at least three alternatively spliced C-terminal isoforms (a, b, g), and it is transcribed from two different promoters [14], giving rise to two proteins that either contain (TAp63) or do not contain (ΔNp63) the N-terminal TA domain, [14,15]. The ΔNp63 isoform can exert dominant-negative effects over p53, p73 by either competing for DNA binding sites or by direct protein interaction. ΔNp63 isoforms were also shown to directly activate specific gene targets not induced by TA isoforms [16,17]. ΔNp63 is the predominant isoform is crucial for the maintenance of epithelial cells regenerative potential [18-22].
Table1. Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP) endotypes classification by Dennis et al. [6].
Endotypes approach |
CRSwNP subtypes |
Cellular markers |
Molecular markers |
Targeted treatment |
Type 2 cytokine-based |
CRSwNP associated with asthma and atopy |
Eosinophils
Mast-cells
Basophils
T-helper 2 |
IL-5
IL-4
IL-13 |
Anti-IL5 (Reslizumab, mepolizumab)
Anti-IL4/13 (Dupilumab) |
Eosinophil-based |
CRSwNP in
AFRS (Allergic Fungal RhinoSinusitis)
AERD (Aspirin-Exacerbated Respiratory Disease)
EMCRS (not-otherwise-categorized eosinophilic rhinosinusitis) |
Eosinophils |
AFRS: Fungi, IL-4
AERD: IL-4
EMCRS: IL-5, IL-13 |
Anti-IL5
Ligands for Siglec-8 |
Immunoglobulin IgE-based |
All CRSwNPs except AERD |
Lymphocytes B |
Ig-E |
Anti-Ig E (Omalizumab)
Anti-GATA 3 |
Cysteinyl-based |
AERD (polyps+asthma+intolerance to aspirin and other inhibiting COX1) |
Eosinophils
Mast-cells |
CysLT |
Leukotriene receptor-antagonists (montelukast)
5-lipoxiygenase inhibitor (zileuton)
Platelet-targeted |
An increase in p63-positive cells is observed in the epithelium of NPs and the expression of p63 in multiple cell layers is an important pathologic phenomenon in the epithelial remodelling seen in NPs [23,24]. Inhibitors of NF-κB, HDACs and p38 MPAK, and RSV infection, prevented p63 expression and induced TJ proteins, p63-negative regulation of the epithelial barrier and ciliogenesis of the nasal epithelium [25].
Histone deacetylase (HDACs) are a class of enzymes that remove acetyl groups from the lysine residues of target proteins, thereby promoting chromatin condensation and reduced transcription. The inhibition of HDAC activity reconstitutes a defective barrier by increasing TJ proteins expression [26]. ΔNp63, HDAC1 and HDAC6 seems to be upregulated and CLDN-1 and -4 downregulated in the epithelium of sinusitis and probably in NPs. Knockdown of ΔNp63 induced expression of CLDN-1 and -4, enhancing barrier and fence functions and increased the number of microvilli on the cell surface.
Unlike p53, p63 is rarely mutated in human cancers, but it is involved in these pathological conditions, controlling cell cycle arrest and apoptosis [15,17]. The balance between TAp63 and ΔNp63 isoforms appears to dictate the different cellular endpoints, survival and transformation versus cell death, although their precise roles in tumorigenesis remain unclear. The majority of tumours maintain ΔNp63 expression and in many cases, it appears to be over expressed or its locus is amplified, consistent with a potential p63 pro-proliferative or oncogenic role [27,28]. p63 is a target of genomic amplification and/or over expression in >80% of primary head and neck squamous cell carcinomas (HNSCC) as well as other squamous epithelial malignancies [29-32]. Recent reports show that ΔNp63α expression directly correlates with a poor clinical response to cisplatin in patients with head and neck tumors [33]. TAp63 has been found to exerts critical functions in the development and function of the heart [34] and oocytes [35,36], in the differentiation of cochlear neuroepithelium via the regulation of Notch pathway [37]. ΔNp63 proteins are involved in the early stages of skin developmentand being rapidly degraded when keratinocytes are induced to differentiate [38]. The hypothesis is that TAp63 and ΔNp63 isoforms work in competition, and the Notch signalling pathway is very important in epidermal stratification and in keratinocyte differentiation [39].
In a recent study it has been demonstrated that one of the transcriptional activators of TAp63 is OTX2. OTX2 can transactivate TAp63, via a responsive element located into the intron 1 of the gene, whereas there is not a transcriptional regulation of ∆Np63. These results can identify a regulatory cascade going from OTX2 to TAp63, arriving to the Notch pathway [40].
OTX proteins, are important class of Homeodomain-containing transcription factors which the main role is in embryonic morphogenesis. Analysing the role of OTX’s gene in the cancerogenesis OTX1 has been demonstrated to be involved in breast cancer physiology, being able to interact with wild type p53, suggesting that p53 and OTX1 overexpression represent an attempt to force the neoplastic cells to differentiate [41]. Importantly, it has been demonstrated the presence of OTX1 and OTX2 proteins in normal sinonasal mucosa and in different epithelial and neuroectodermal nasal neoplasms of adult subjects [42]. There is a significant modulation in the expression of OTX1 and/or OTX2 in neoplastic tissue, compared with normal tissue, suggesting that the activation/inactivation of OTX factors is a significant event in response to sinonasal neoplasms development [42].
Nasal polyps’ recurrence after surgery is well known and documented. However, prevention and prediction recurrency is still a subject of research and debate; in fact, a potential relationship between clinical, radiological, immunologic, molecular factors and polyp’s recurrence remain undetermined [43-45]. Patients presenting with extensive disease, suggested by CT scan staging, seem to have a higher risk for the development of recurrences after endonasal surgery for nasal polyps [46]. Various studies show that patients with both CRSwNP and AERD are at risk for the development of recurrences after endonasal surgery for nasal polyposis [46,47].
The aim of this work has been to understand the underlying mechanism or markers useful to characterise patients with different clinical outcomes. We evaluated the expression of the two isoforms of the transcription factor p63 in cases of recurrence and non-recurrence.
Methods
All patients have been treated according to European Position Paper on Rhinosinusitis and Nasal Polyps [48], all patients were preliminary studied with clinical and radiological investigation before surgery, including nasal endoscopy and axial and coronal computed tomography (CT) scanning. All patients underwent in surgical treatment by FESS under general anaesthesia after obtaining informed consent. Standard surgical steps were applied in each case according to the extent of disease.
Immunofluorescence analysis
For immunofluorescence analysis, we used a previous described protocol [40,49,50]. The following primary antibodies: mouse polyclonal anti-p63 (Abcam Ab735,) and rabbit polyclonal anti-K5 (Covance PRB-160P). The following secondary antibodies: Alexa fluor®488 goat anti-rabbit igG (H+L) (Invitrogen, Carlsbad, CA) and Alexa fluor®568 goat anti-mouse igG (H+L) (Invitrogen, Carlsbad, CA,). DAPI for nuclei visualization. Primary and secondary antibodies were prepared in blocking buffer. Sections were covered by Prolong Antifade reagent (Invitrogen, USA) and observed using A1 Nikon confocal laser microscope system, and software NIS Element AR4.00.04 (Nikon).
QPCR
Analysis of Tap63, DeltaNp63, keratin 5 and OTX2 expression levels was performed through Real-Time PCR, quantitative technique that allows a selective amplification and the visualization of PCR products in real time. We use GoTaq® qPCR Master Mix (Promega) and specific primers to amplify each gene: TAp63 forward 5’- GGACTGTATCCGCATGCAG-3’, reverse 5’-GAGCTGGGCTGTGCGTAG-3’, DNp63 forward 5’-GAAGAAAGGACAGCAGCATTGAT- 3’, reverse 5’-GGGACTGGTGGACGAGGAG-3’, Keratin 5 forward 5’-TGGACCTGGATAGCATCATC- 3’, reverse 5’- CATTGTCAATCTCGGCTCTC -3’ and OTX2 forward 5’-TCGAAGAGCTAAGTGCCGCC-3’, reverse 5’-GGCAATGGTCGGGACTGAGG -3’. Analysis was performed using Sybr-Green reagent, molecule binding the minor groove of DNA, results were normalized to actin endogenous control (forward 5’-CTGGCACCACACCTTCTACAATG-3’, reverse 5’-GTTGCTATCCAGGCTGTGCTA-3’). The amplification was performed by 7500 Real Time PCR System (Applied Biosystems) and data analysed with 7500 Software v2.3.
Results
Patients
Our study project analysed a group of 28 patients affected by chronic rhinosinusitis (Figure 1) and referred to our Otolaryngology Unit from 2014 to 2016. All these patients underwent in surgical treatment by FESS. The clinical diagnosis was confirmed by the definitive histopathological examination and in 10 cases it was nasal polypoid rhinosinusitis, while in 5 cases it was an anthro-choanal polyp and in the remaining 13 a simple hyperplastic rhinosinusitis. Therefore, this group of 13 patients with polyposis was subsequently subjected to six-monthly clinical checks in the two years following surgery. From the follow up it emerged that 5 cases of nasal polyposis were relapse.
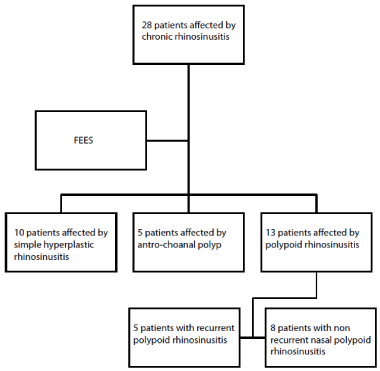
Figure 1. Schematic layout of the study
Analysing this group of patients affected by nasal polypoid rhinosinusitis, further distinguishing features emerged between the two groups of recurrent and non-recurrent cases. In particular, the group of patients suffering from recurrent polyposis had a mean age of 63 years, they were non-smokers, not allergic. The group of non-recurrent patients was characterized by an average age of 67 years, non-allergic. In both groups the patients were not affected by relevant comorbidities.
Based on the knowledge of the transcription factor p63 and its role in the development of epithelial cell, we have expanded the study analysing the OTX2 factor too, which, although currently demonstrated in the context of cochlear and macular development, has a fundamental role in the regulation of p63 and in particular of the TAp63 isoform [37,40], and recently found to be expressed in nasal polyps [42].
Molecular analysis
To achieve the analysis of the expression of both isoform of p63 in polyps of our patients, we used firstly a molecular approach consisting in extracting RNA from part of fresh polyps’ biopsies, followed by an RTqPCR. Using this method, we amplified selectively TAp3, ∆Np63, Keratin5. The results have been analysed as fold over control respect to the normal nasal mucosa.
In our study sample, the patients with nasal polyposis recurrence are characterized by higher expression levels of ∆Np63, respect to those that not relapsed (Figure 2A). Furthermore, in these cases high level of expression of the epithelial basal cytokeratin K5 has been detected. The expression levels of the latter are concordant with the ∆Np63 expression observed (Figure 2B). On the contrary in the group without recurrence of polyposis history the levels of ∆Np63 remain lower, similar to that observed in the turbinate epithelium used as normal control (Figure 2A). The indicator of basal epithelial cytokeratin K5 remain also lower expressed (Figure 2B). Since the ∆Np63 action seem to be counteracted by the TA isoform, we analysed its expression also by RTqPCR. The results showed a variable TAp63 expression in both group but analysing the ∆N/TA ratio, again the patient is distinguished into two class of relapsing and not-relapsing (Figure 2C). Indeed, this ratio is higher in the groups of relapsing patients, confirming that the balance between these two transcriptions factor characterize the character of polyps. The box plot organization of the data well clarify the differences between the two class of patients (Figure 2B,D,F). Moreover, according to recent data published respect the possible driving of TAp63 expression by OTX2, we also tested the expression of the latter. Again, data interpretation, due to the expression variability and for the small number of patients analysed are difficult to be graphically represented but looking to the raw data (Figure 2F), the indication is that OTX2 is concordant with TAp63 expression. These results confirm the previous proposed pathway [40], in which the expression of OTX2 can transactivate TAp63, probably to counteract the ∆N isoform.
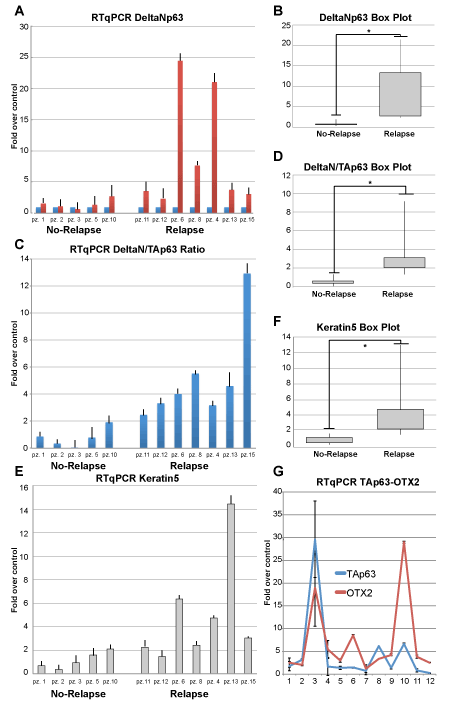
Figure 2. Expression analysis. A, RTqPCR analysis showing the expression level of ∆Np63 evaluated as fold over control respect to the normal sinonasal epithelium. B, Box-plot representation of the data. Data have been clustered into two class, relapsing and not relapsing. The differences between the two class are significantly different (*, T-test, P= 0,033). B, ∆Np63/TAp63 ratio using RTqPCR expression data. D, Box plot showing that the ratio between the two p63 isoform is different in patients presenting recidivation respect to those that not (*, T-test, P= 0,013). E, F, the same analysis for Keratin 5 expression, showing a behaviour similar to ∆Np63 (*, T-test, P= 0,032). G, Expression level, in fold over control, of OTX2 and p63, showing a similar tendency in patients.
Histological analysis
To analyse the expression of p63 in specimen from patients with diagnosis of polyposis, we performed on some surgical samples an immune fluorescence confocal analysis, using antibodies against p63 and keratin 5. In specimen from patients with relapse events, the primary polyp shows a strong ∆Np63 staining, the number of positive cells are disposed in multiple layers (Figure 3 panels A,B, asterisks in D, E). The analysis of polyps from patients without relapse shows a characteristic one-layer disposition of p63 (Figure 3 panels C, F), indicating the presence of active regenerating cells only in the basal compartment. This situation is confirmed by the staining with K5 antibody, showing the presence of this keratin again in multiple cell layers (Figure 2 panels G, H and L, M), where in the patients with no relapse the distribution is more concentrated in the basal layer (Figure 2 panels I, N), as in the normal epithelia compartment. The distribution of K5 clearly resemble that of p63 according the well-known mechanism of driving the expression of K5 by p63∆N isoform.
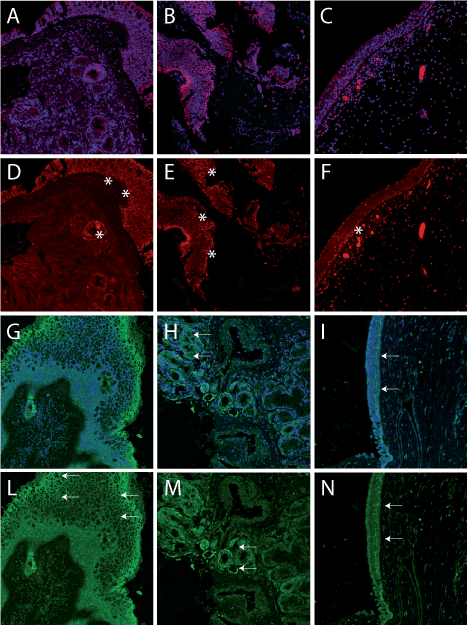
Figure 3.Histological immunofluorescence confocal analysis of polyps. A, B examples of polyps from relapsing patients. C, example of polyp from not relapsing patient, (Blue is DAPI and red is p63 staining). D,E, the staining with anti p63 antibody show the presence of multilayer positive cells. The epithelium result thicker respect to that is visible in F, from a non-relapsing patient, in which a lower number of positive, mainly in the basal compartment, is visible. G, H, Keratin 5 staining (green) from two selected relapsing patients (blue is DAPI). I, Keratin 5 staining (green) from a selected non-relapsing patient (blue is DAPI). J-L, Keratin 5, normally present only in the basal layer is in an evident multilayer distribution, indicating the presence of proliferate cells, whereas in M, the expression is limited in a more basal distribution.
Discussion
As it is known, most t2021 Copyright OAT. All rights reservharacterised by high ΔNp63 expression indicating a potential p63 pro-proliferative or oncogenic role [27,28]. In squamous cell carcinoma and ΔNp63 was the predominant isoform expressed at the protein level [51]. Recent data also indicate ΔNp63 as capable of sustaining the production of the hyaluronic acid (HA) the major component of the extracellular matrix (ECM) in basal-like breast carcinoma (TNBC) favouring a HA-rich microenvironment, which can sustain epithelial cell proliferation [52]. An increase in p63-positive cells has been previously reported also in the epithelium of rhinosinusitis patients [23,24]. The expression of p63 in multiple cell layers, has been found in our recurring NPs patients, representing an important possible pathologic signature.
In fact, in our experiments, patients with recurrence show high ∆N expression and high ∆N/TAp63 ratio. This clearly indicates that the TAp63 expression level, is important in modify the absolute ∆Np63 expression. The ratio between the two transcription factors can be considered a possible prognostic markers of high recurrence probability, even if a more extensive study should be performed to define its critical level. Indeed, the status of TAp63 expression is important since it influence directly the balance and can well reflect the status of polyp’s proliferation in patients. It is conceivable, that OTX2 expression in nasal polyps [42], driving specifically TAp63 expression [40] may represent a response mechanism to the cell proliferation.
The hypothesis underlying these findings is that the role of OTX2 is to transactivate TAp63 and therefore to promote ∆Np63 inhibition and cell differentiation, thus reducing their proliferation potential. This should bring to a kind of recovery of the disease. In patients in which this pathway is low or not active, the ratio between the two isoform of p63 (∆N/TA p63) is too high, this control is missing and the patients gone into recurrence.
Regarding to the treatment of NPs, according to European and US guidelines, intranasal corticosteroids are recommended as initial medical treatment [53]. These topical drugs can improve patient’s quality of life, by reducing sinonasal symptoms and decreasing polyps’ size, and can be managed better than oral corticosteroids [54,55], but are not resolutive.
Sinus surgery approach (usually Functional Endoscopic Sinus Surgery, FESS) should be considered for patients with severe sinonasal disease and those who failed medical treatments. However, as shown, nasal polyps can reoccur after surgery, especially in patients having both CRSwNP and asthma [45,56]. In recent years, several biologics (such as Omalizumab, Mepolizumab and Dupilumab) underwent clinical trials evaluating their safety and efficacy in CRSwNP. Although recent studies are promising, currently Omalizumab, Mepolizumab and Dupilumab are not yet approved for the treatment of nasal polyps [57-59].
The possible role of the OTX2/TAp63 axis in the differentiation between relapsing or not of NPs in patients opens the possibility to the use of new drugs. HDAC inhibitors, largely used in carcinoma therapy are able to induce TAp63 expression, [37,60], their therapeutic importance is due to the inhibitory effect of this isoform on the ∆Np63. Furthermore, as reported, the inhibition of HDAC1 and HDAC6 induces also the expression of TJ proteins [26], ameliorating the inflammation end restoring the normal basal lamina barrier.
This paper is a first step of analysis, and more work should be done to characterise the pathway. This is important because if confirmed, the treatment with HDAC inhibitors such as hydroxamic acids, and benzamides could became one possibility in the topic treatment of nasal polyps to ameliorate the disease and counteract the recurrence.
References
- Koennecke M, Klimek L, Mullol J, Gevaert P, Wollenberg B (2018) Subtyping of polyposis nasi: phenotypes, endotypes and comorbidities. Allergo J Int 27: 56-65. [Crossref]
- Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, et al. (2015) A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immun Inflamm Dis 3: 14-22. [Crossref]
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, et al. (2012) European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 23: 3 p preceding table of contents, 1-298.
- Langdon C, Mullol J (2016) Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy 9: 45-53. [Crossref]
- Steinke JW, Borish L (2015) Factors driving the aspirin exacerbated respiratory disease phenotype. Am J Rhinol Allergy 29: 35-40. [Crossref]
- Dennis SK, Lam K, Luong A (2016) A Review of classification schemes for chronic rhinosinusitis with nasal polyposis endotypes. Laryngoscope Investig Otolaryngol 1: 130-134.
- Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, et al. (2016) Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 137: 1449-1456.e4. [Crossref]
- Divekar R, Hagan J, Rank M, Park M, Volcheck G, et al. (2016) Diagnostic Utility of Urinary LTE4 in Asthma, Allergic Rhinitis, Chronic Rhinosinusitis, Nasal Polyps, and Aspirin Sensitivity. J Allergy Clin Immunol Pract 4: 665-670. [Crossref]
- Schleimer RP (2017) Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol 12: 331-357. [Crossref]
- Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, et al. (2012) Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol 130: 1087-1096.e10. [Crossref]
- Kojima T, Go M, Takano K, Kurose M, Ohkuni T, et al. (2013) Regulation of tight junctions in upper airway epithelium. Biomed Res Int: 947072. [Crossref]
- Arason AJ, Jonsdottir HR, Halldorsson S, Benediktsdottir BE, Bergthorsson JT, et al. (2014) deltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS One 9: e88683. [Crossref]
- Li CW, Shi L, Zhang KK, Li TY, Lin ZB, et al. (2011) Role of p63/p73 in epithelial remodeling and their response to steroid treatment in nasal polyposis. J Allergy Clin Immunol 127: 765-772.e1-e2. [Crossref]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, et al. (1998) p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2: 305-316. [Crossref]
- Melino G, Lu X, Gasco M, Crook T, Knight RA (2003) Functional regulation of p73 and p63: development and cancer. Trends Biochem Sci 28: 663-670. [Crossref]
- Dohn M, Zhang S, Chen X (2001) p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 20: 3193-3205. [Crossref]
- Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, et al. (2003) DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res 63: 2351-2357. [Crossref]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, et al. (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398: 708-713. [Crossref]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, et al. (1999) p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398: 714-718. [Crossref]
- Candi E, Terrinoni A, Rufini A, Chikh A, Lena AM, et al. (2006) p63 is upstream of IKK alpha in epidermal development. J Cell Sci 119: 4617-4622. [Crossref]
- Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, et al. (2006) Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ 13: 1037-1047. [Crossref]
- Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, et al. (2006) p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 8: 551-561. [Crossref]
- Warner SM, Hackett TL, Shaheen F, Hallstrand TS, Kicic A, et al. (2013) Transcription factor p63 regulates key genes and wound repair in human airway epithelial basal cells. Am J Respir Cell Mol Biol 49: 978-988. [Crossref]
- Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, et al. (2009) Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med 180: 122-133. [Crossref]
- Kaneko Y, Kohno T, Kakuki T, Takano KI, Ogasawara N, et al. (2017) The role of transcriptional factor p63 in regulation of epithelial barrier and ciliogenesis of human nasal epithelial cells. Sci Rep 7: 10935. [Crossref]
- Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, et al. (2017) Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol 139: 93-103. [Crossref]
- Mills AA (2006) p63: oncogene or tumor suppressor? Curr Opin Genet Dev 16: 38-44. [Crossref]
- Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, et al. (2007) TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle 6: 274-285. [Crossref]
- DeYoung MP, Johannessen CM, Leong CO, Faquin W, Rocco JW, et al. (2006) Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res 66: 9362-9368. [Crossref]
- Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, et al. (2000) AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci U S A 97: 5462-5467. [Crossref]
- Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, et al. (2003) Gonzalez,Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res 63: 7113-7121. [Crossref]
- Sniezek JC, Matheny KE, Westfall MD, Pietenpol JA (2004) Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope 114: 2063-2072. [Crossref]
- Zangen R, Ratovitski E, Sidransky D (2005) DeltaNp63alpha levels correlate with clinical tumor response to cisplatin. Cell Cycle 4: 1313-1315. [Crossref]
- Rouleau M, Medawar A, Hamon L, Shivtiel S, Wolchinsky Z, et al. (2011) TAp63 is important for cardiac differentiation of embryonic stem cells and heart development. Stem Cells 29: 1672-1683. [Crossref]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, et al. (2006) p63 protects the female germ line during meiotic arrest. Nature 444: 624-628. [Crossref]
- Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, et al. (2009) Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 15: 1179-1185. [Crossref]
- Terrinoni A, Serra V, Bruno E, Strasser A, Valente E, et al. (2013) Role of p63 and the Notch pathway in cochlea development and sensorineural deafness. Proc Natl Acad Sci U S A 110: 7300-7305. [Crossref]
- Romano RA, Smalley K, Magraw C, Serna VA, Kurita T, et al. (2012) DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 139: 772-782. [Crossref]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, et al. (2002) Jagged1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ 9: 842-855. [Crossref]
- Palombo R, Porta G, Bruno E, Provero P, Serra V, et al. (2015) OTX2 regulates the expression of TAp63 leading to macular and cochlear neuroepithelium development. Aging (Albany NY 7: 928-936. [Crossref]
- Terrinoni A, Pagani IS, Zucchi I, Chiaravalli AM, Serra V, et al. (2011) OTX1 expression in breast cancer is regulated by p53. Oncogene 30: 3096-3103. [Crossref]
- Pirrone C, Chiaravalli AM, Marando A, Conti A, Rainero A, et al. (2017) OTX1 and OTX2 as possible molecular markers of sinonasal carcinomas and olfactory neuroblastomas. Eur J Histochem 61: 2730. [Crossref]
- Bruno E, Mohamed EI, Alessandrini M, Russo S, Schiaroli S, et al. (2002) Long-term follow-up of cellular proliferation as a predictive index for the relapse of nasal polyposis. Am J Rhinol 16: 237-241. [Crossref]
- Bruno E, Bonmassar E, Di Girolamo S, Adamo R, Alessandrini M, et al. (2004) Evaluation of telomerase activity in nasal polyps. Am J Rhinol 18: 197-201. [Crossref]
- Young J, Frenkiel S, Tewfik MA, Mouadeb DA (2007) Long-term outcome analysis of endoscopic sinus surgery for chronic sinusitis. Am J Rhinol 21: 743-747. [Crossref]
- Akhtar S, Ikram M, Azam I, Dahri T (2010) Factors associated with recurrent nasal polyps: a tertiary care experience. J Pak Med Assoc 60: 102-104. [Crossref]
- Albu S, Tomescu E, Mexca Z, Nistor S, Necula S, et al. (2004) Recurrence rates in endonasal surgery for polyposis. Acta Otorhinolaryngol Belg 58: 79-86. [Crossref]
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, et al. (2012) EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50: 1-12. [Crossref]
- Terrinoni A, Didona B, Caporali S, Chillemi G, Lo Surdo A, et al. (2018) Role of the keratin 1 and keratin 10 tails in the pathogenesis of ichthyosis hystrix of Curth Macklin. PLoS One 13: e0195792. [Crossref]
- Palombo R, Savini I, Avigliano L, Madonna S, Cavani A, et al. (2016) Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis 7: e2344. [Crossref]
- Cui R, He J, Mei R, de Fromentel CC, Martel-Planche G, et al. (2005) WITHDRAWN: Expression of p53, p63, and p73 isoforms in squamous cell carcinoma and adenocarcinoma of esophagus. Biochem Biophys Res Commun 336: 339-345. [Crossref]
- Compagnone M, Gatti V, Presutti D, Ruberti G, Fierro C, et al. (2017) DeltaNp63-mediated regulation of hyaluronic acid metabolism and signaling supports HNSCC tumorigenesis. Proc Natl Acad Sci U S A 114: 13254-13259. [crossref]
- Stevens WW, Schleimer RP, Kern RC (2016) Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol Pract 4: 565-572. [Crossref]
- Lund VJ, Flood J, Sykes AP, Richards DH (1998) Effect of fluticasone in severe polyposis. Arch Otolaryngol Head Neck Surg 124: 513-518. [Crossref]
- Rudmik L, Schlosser RJ, Smith TL, Soler ZM (2012) Impact of topical nasal steroid therapy on symptoms of nasal polyposis: a meta-analysis. Laryngoscope 122: 1431-1437. [Crossref]
- Bhattacharyya N (2007) Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope 117: 1834-1838. [Crossref]
- Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, et al. (2013) Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 131: 110-116.e1. [Crossref]
- Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, et al. (2011) Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol 128: 989-995.e1-e8. [Crossref]
- Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, et al. (2016) Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients with Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 315: 469-479. [Crossref]
- Giacobbe A, Bongiorno-Borbone L, Bernassola F, Terrinoni A, Markert EK, et al. (2013) p63 regulates glutaminase 2 expression. Cell Cycle 12: 1395-1405. [Crossref]



