Inguinal hernia repair is the most frequently performed operation in General Surgery. Complications such as chronic inguinal pain (12%) and recurrence rate (11%) significantly influence the surgical results. The 4 main impacting factors affecting hernia repair results are: mesh material and integration biology; mesh fixation; tissue healing and regeneration and, the surgical technique. All these factors have been analysed in this article. Then a new procedure, L-PRF-Open Mesh Repair, has been introduced with the aim of improving both short and long term results. We are presenting in a case report the feasibility of the technique.
Statistics show that the most common hernia site is inguinal (70-75% cases) [1].
Hernia symptoms include local discomfort, numbness and pain which, sometimes can be severe and worsen during bowel straining, urination and heavy lifting [2]. Occasionally, complications such as incarceration and strangulation can lead to bowel obstruction, bowel ischemia, necrosis of the omentum and eventually become a life-threatening condition [3].
Inguinal hernia open mesh repair is one of the most frequently performed surgical procedures. Literature indicates over 20 million hernia repair operations per year worldwide [4].
The use of hernia mesh products to surgically repair or reconstruct anatomical defects has been adopted and developed over the last 30 years. In their International Guidelines, the HerniaSurge Group strongly recommends a mesh-based repair technique for patients with inguinal and/or femoral hernia [5].
The surgical mesh definitely helps to close the abdominal wall defects and to reinforce the weakened area through the incorporation of fibro-collagenous tissue.
A “tension-free” repair with mesh is associated with less postoperative pain and faster recovery [6].
Open mesh repair for inguinal hernia is the most common technique.
This relies on the placement of a mesh to seal the hernia defect and reinforce the surrounding tissue. The Lichtenstein technique requires stitches to fix the located mesh whereas other procedures fix it using either fibrin or cyanoacrylate glue [1].
The results of hernia surgery are mainly measured by postoperative pain, time to return to work, short-term complications, chronic groin pain, and hernia recurrence.
The expected rate of recurrence following inguinal hernia repair is currently 11%.
Only 57% of all inguinal hernia recurrences occurred within 10 years after the hernia operation. Some of the remaining 43% of all recurrences happened only much later, even after more than 50 years [7].
A further complication after inguinal hernia repair is chronic groin pain lasting more than 3 months, occurring in 10-12% of all patients. Approximately 1-6% of patients have severe chronic pain with long-term disability, thus requiring treatment [5,8].
PRF-Open Mesh Repair: the rationale supporting the method
There are 4 main impacting factors affecting hernia repair results:
mesh material and integration biology; mesh fixation; tissue healing and regeneration; surgical technique.
Mesh material properties: Billroth T. in 1890 firstly suggested that the ideal way to repair hernias was to use a prosthetic material to close the hernia defect [9].
Many materials were tried, but all failed due to complications mostly infections and rejections [10].
In 1955, Usher F. started using a new material, the polyolefin (Marlex) and developed a woven mesh. In 1958, Usher published a new surgical technique using a knitted polypropylene mesh which could be autoclaved and also more rapidly incorporated by tissue [11].
The Usher experience showed that optimal tissue growth through the large mesh pores was the main difference when compared to previous materials. In the days following surgery he noticed improved mesh incorporation and that fibroblast activity and collagen production progressed suitably with less giant cells [12].
Lichtenstein, 30 years later, popularized the technique as “tension-free hernioplasty” for inguinal hernia [13].
In recent decades researchers have investigated various materials to optimise this aspect of mesh repair surgery. The fundamental material characteristics resulted to be biocompatibility, resistance to infection, maintenance of adequate long-term tensile strength, rapid incorporation into the host tissue, sufficient flexibility and non-carcinogenic response [6,14].
Currently, most of the surgical meshes are chemically and physically biocompatible, non-toxic, and non-immunogenic [1].
Mesh chemical and physical properties are designed to improve the integration process, optimise immune reaction and wound healing [15].
Therefore, the main properties required by a mesh are as follows:
a) Elasticity and Tensile Strength.
b) Pore size.
Porosity plays a key role in the reaction of the tissue to the prostheses. Macroporous meshes have been found to facilitate the colonization of macrophages, fibroblasts and EMC collagen fibres formation [16].
c) Weight (density).
Lightweight meshes contain less material so reduce the impact of the foreign body reaction [17].
d) Structure.
Surgical meshes are made using monofilament or multifilament systems. A surgical mesh formed of monofilament fibres provides good tissue reinforcement but is stiff and less pliable.
A surgical mesh formed of multifilament yarns is soft and malleable. However, multifilament fibres mesh tends to retain infectious and inflammatory substances, increasing the erosion rates by 20–30% and increasing the risk of recurrence [18].
f) Material absorption.
Non-absorbable meshes have good mechanical qualities; they are intra-operatively easy to shape and have long-term stability. However, mesh deterioration and distortion: stiffness, erosion, shrinking and adhesions have been widely reported to promote recurrence in mid-long term.
Absorbable meshes were developed to reduce these long-term complications. These meshes promote regulated postoperative fibroblast colonisation. Nevertheless, after mesh absorption is completed, the resulting scar tissue may be too weak and insufficient to provide required support resulting in increased hernia recurrence rate. For this reason, mixed meshes made with both absorbable and non-absorbable material have been introduced. [1].
The “ideal” mesh should promote a fast and organized integration process supporting tissue regeneration together with a minimal inflammatory reaction [2].
For inguinal hernia surgery the most recommended are the macroporous, non-absorbable, monofilament, lightweight soft mesh. Polypropylene has been the material of choice for our study.
Mesh integration biology: After the mesh has been implanted, an extraordinarily complex series of biological events start and mark the initiation of the healing process.
First stage: the acute Inflammation: The coagulum is the aggregation of albumin, fibrinogen, plasminogen, complement factors, immunoglobulins around the mesh [19,20]. This is the starting process for platelets to gather and adhere to the coagulum and to activate the classical and alternative complement pathways. The chemotactic factors C3a and C5a generated by the platelets activation convey polymorphonucleocytes (PMNs), macrophages, fibroblasts and smooth muscle cells to the wound area in an ordered sequence [21].
During this first stage, migrated PMNs phagocyte microorganisms and necrotic material. PMNs death cause the release of their cytoplasmic and granular contents near the mesh generating an additional inflammatory response [22].
Second stage: chronic inflammation. In this stage, monocytes attracted to the wound site differentiate into macrophages. Further to macrophages, other primary cellular components such as plasma cells and lymphocytes actively contribute to the advanced inflammatory response. Macrophages start the phagocytosis of dead cells, necrotic tissue and consume foreign bodies and generally prepare the way for fibroblasts settlement [23].
Third stage: foreign body reaction. In response to the presence of large indigestible foreign bodies, macrophages fuse into a foreign body giant cell in the attempt to seal the extraneous material in an epithelioid granuloma [24- 26].
Foreign body reaction is a complex defence response involving foreign body giant cells, fibroblasts, and angiogenesis formation in variable amounts depending on the nature, form and structure of the implanted material [24].
Fourth stage: scar formation. This is characterized by the replacement of damaged tissue with a new extracellular matrix (EMC) produced by fibroblasts and myofibroblasts which, generates the scar.
Wound healing and scar formation can be affected by persistent inflammation and the severity of the primary injury [27].
Fibroblasts are the cells that mediate the wound healing progression. These cells enter the wound site once the acute inflammatory response has receded. The main function of fibroblasts is to synthesize extracellular matrix (EMC) and its collagen components are essential to regenerate the connective tissue [28].
Fibroblasts play a key role for the success of mesh integration and their optimum amount (density) at the wound is achieved approximately two weeks after surgery [29].
At first, fibroblasts synthesize an immature, frail collagen type III. A fragile collagen ECM network is produced for around the first 21 days, and then there is a modification in the ratio of collagen type III and I.The collagen type III reduces and the type I, stronger and stable, arises. The mechanical strength increases progressively until 6 months after surgery [29].
Therefore, an altered Type I/III collagen ratio results in decreased tensile strength and mechanical stability. Thus, the alterations of collagen subtypes play a central role in the pathophysiology of hernia repair, mesh integration and recurrence [30-32].
Recent Literature highlights the responsibility of enzymes like Matrix Metallo-Proteinases, MMPs and the lack of their inhibitors Tissue Inhibitors of Metalloproteinases, TIMPS to be the possible cause for the altered ratio of collagen subtypes. [33,34]. Specifically, MMP-1 and MMP-13 are the principal matrix enzymes responsible for the type I, II, III collagen turn over [35,36]. Therefore, the alterations in MMP-1 and MMP-13 protein expressions could have a role in the derangement of the ratio type I/III collagen [29,37,38].
Prolonged inflammation can jeopardize the mesh integration; fibroblasts and myofibroblasts overexpression prolong the inflammatory phase resulting in increasing fibrosis and this can cause contraction and shrinkage of the mesh, eventually resulting in fibrosis, adhesions and fistulas. This can lead to prosthesis rejection and short term recurrence [39,40].
Mesh Fixation: Mesh fixation is a controversial area in inguinal hernia surgery.
The most common methods used to fix the hernia mesh are stitches, fibrin and cyanoacrylate glues. A minority of surgeons suggest for a non-fixation technique (mostly in TEP); however, reports about the rate of recurrence are incomplete and controversial.
Stitch fixation: The Lichtenstein technique is currently the most popular technique to repair unilateral primary groin hernias [13].
In the Lichtenstein technique the mesh fixation is provided by a Prolene (polypropylene) overrunning or single stitch suture to sew the mesh firmly to the surrounding structures: the pubic tubercle, inguinal ligament and muscles.
Some surgeons opt for absorbable stitch material such as polyglycolic acid (Vicryl) in the hope of reducing the risk of long-term nerve entrapment in the suture line.
Lichtenstein technique has a generally good reported outcome in Literature: easy to perform, low morbidity and good long-term results [41].
Nonetheless, several recent articles showed high incidence of chronic inguinal pain, with an average incidence of 12%, and sometimes reported as high as 53% [42- 50].
Many studies considered chronic postoperative pain as a surgical primary outcome but only a few of them evaluated the social impact of post-hernia repair chronic pain. This chronic groin pain has been reported to affect the social, sexual and work life of up to 6% of patients [8,42,44,47,49].
Specifically, in the Lichtenstein operation, chronic groin pain can be due to nerve entrapment in the suture either in the scar tissue or neuroma development, inflammation of the periosteum of the pubic tubercle (traditionally taken into the first stitch), and persistent inflammation with foreign body over-reaction to the mesh [51,52].
Lichtenstein technique has been always described as a “tension free technique”. However, stitches do generate tension and stiffness in the inguinal area particularly during muscle activity. This tension may cause postoperative mechanical pain, persistent inflammation and delay in recovery time.
Glue sealant: To reduce the risk of nerve entrapment and chronic pain, different methods of mesh fixation have been considered and most of all tissue-compatible glues. The goal is to provide a suture-less fixation.
The ideal adhesive material should be:
a. Biocompatible
b. Cheap
c. Easy to store and use.
The advantages promoted by glue fixation are reduced postoperative acute and chronic pain, improved haemostasis, and speed up operation time.
There is no report in Literature of any difference in recurrence when comparing glue with stitch fixation.
Fibrin glue: The main components of Fibrin glueare concentrated fibrinogen, thrombin, and calcium chloride, thus duplicating the final stage of the coagulation cascade. Fibrin acts as a haemostatic barrier, adheres to the surrounding tissue, and operates as a scaffold for migrating fibroblasts [53].
Fibrin glue is used as a tissue adhesive for a variety of surgical procedures.
The main advantages of fibrin glue are tissue compatibility, biodegradability, and efficacy when applied to wet surfaces. Potential contamination by transmissible blood-borne pathogens has been criticised by some authors [54].
Fibrin glue has given very good results in tension free mesh fixation both in open and laparoscopic approaches. Nonetheless, it does not fulfil the requirements (b) and (c) because it is expensive and difficult to store and to prepare for the surgical application. Further, fibrin glue can potentially transmit blood-related infections [55-57].
Cyanoacrylates (CAs) glue: Some surgeons consider cyanoacrylates the best choice for mesh fixation in open mesh repair for inguinal hernia.
CAs is biocompatible, inexpensive and easy to store and use [8].
CAs is an efficient way to seal the mesh to the nearby tissue and works as haemostatic although does not provide a scaffold or facilitate tissue regeneration.
The main problem of the CAs has been recognised in the general increase of macrophage response when compared to absorbable sutures [58].
However, this excessive inflammatory reaction induced by the CAs does not seem, in experimental models, to alter significantly the collagen maturation process or delay the mesh integration, which seems very similar to absorbable sutures [58].
Platelet rich fibrin – PRF: Platelet rich fibrin (PRF) has been described as a second-generation autologous platelet concentrate because it does not require any biochemical additives like anticoagulants or bovine thrombin for fibrin polymerisation such as PRP.
However, PRF biological properties are rather different from PRP and cannot be considered a PRP development but rather a different biostimulator.
L-PRF typically is made with 9 ml fresh blood in glass-coated plastic tubes, immediately centrifuged at 2700 rpm for 12 minutes [59].
After centrifugation, through the activation of autologous thrombin, a fibrin clot is created. Three distinct layers can be seen in the tube (Figure 1). The clot by itself contains a great amount of exudate, which is rich in growth factors, and this exudate can be pressed out by gentle compression of the clot in order to obtain PRF membranes.
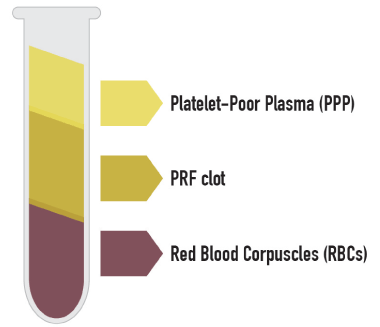
Figure 1. Three distinct layers can be seen in the tube: red blood corpuscles RBCs at the bottom of the tube, platelet-poor plasma PPP on the top of the tube, and the PRF clot in the middle of the tube
Serum squeezed out from the PRF clot, called hyper-acute serum, has a greater cell proliferative effect on different connective cell lineages such as bone marrow mesenchymal stem cells (BM-MSCs), adipose stem cells (ASCs), fibroblasts, osteoblasts and chondroblasts cells [60,61].
PRF membrane is a three-dimensional, adhesive, biocompatible and biodegradable scaffold. The membrane surface facilitates contact and cell interactions. Furthermore, PRF membranes are able to slowly release bioactive molecules that facilitate migration, adhesion and proliferation of local MSCs [62-64].
During centrifugation, the soluble fibrinogen contained in the plasma transforms to fibrin polymerizing to a three-dimensional structure. The activated platelets and some leukocytes are entrapped in the fibrin net. Consequently, a storage pool of growth factors is formed from platelets and leukocytes upon activation [65].
Autologous platelet concentrates are widely used as a bioactive surgical component to decrease inflammation and increase the speed of the healing process.
L-PRF is made of a strong fibrin matrix that variably contains:
- High concentration of vital and non-vital: platelets, leucocytes and circulating MSCs
- Variable pool of cytokines
- An elevated concentration of long releasing growth factors (GFs). These include platelet derived growth factor (PDGF A-B), vascular endothelial growth factor (VEGF), transforming growth factor (TGF β -1,2), insulin-like growth factor (IGF-I), epidermal growth factor (EGF); connective tissue growth factor (CTGF); bone morphogenetic protein 2 (BMP-2) [59; 66].
- An elevated concentration of fibrin, fibronectin, vitronectin, and thrombospondin
- A variable pool of heat shock proteins HSPs (not well studied yet)
For its regenerative properties, PRF has been successfully used for the treatment of non-responding skin ulcers including diabetic foot ulcers (DFU), pressure ulcers (PU), acute surgical wounds, and venous leg ulcers (VLUs) [66,67]. In dentistry and maxillofacial surgery, the application of PRF membrane is widespread. There are numerous described procedures including the treatment of periodontal bony defects and regeneration, ridge preservation, sinus-floor elevation, implant surgery, and the creation of the PRF bone block [68,69].
PRF increases new bone formation and has a positive effect on early bone healing [70].
In dermatology and plastic surgery have been reports of dermal fibroblasts migration and activation resulting in the increase of collagen synthesis of the skin exposed to PRF treatment [71,72].
We have recently started using L-PRF clot and its constituents PRF membranes and hyper-acute serum to fixing the mesh in inguinal hernia repair.
Regenerative surgery technique: PRF-open mesh repair: PRF-open mesh repair applies some basic rules to achieve the best clinical result: minimally invasive approach, tension-free technique and regenerative surgery principles. It will be described in Materials and Methods [73].
These basic principles reduce oedema, collection and postoperative acute inflammation. These are the main factors that hinder the integration process and trigger postoperative pain.
We used L-PRF to combine the benefits showed by fibrin glue sealant with the PRF properties to streamline the integration of the mesh by optimising connective tissue regeneration. Moreover, PRF regenerative capacities should substantially prevent chronic fibrotic inflammation, mesh retraction, hard and painful scars and chronic nerve entrapment syndrome [73].
PRF-Mesh Repair clinical study was approved by the scientific-ethical board of Villa Aurora Hospital-Foligno-Italy. The study was temporarily stopped last February 2020 due to the Coronavirus pandemic.
We describe a case report to explain the feasibility and safety of the procedure.
On November 2019, we admitted in day surgery, a 67 years old male with a non-complicated bilateral inguinal hernia.
PMH: blood hypertension, prostate hypertrophy. BMI:23
ASA 2
The patient was consented for the PRF-open mesh repair technique and for the use of his own blood to extract the L-PRF. The patient agreed to have his data collected and recorded into an electronic database to be duly analysed.
The operation was performed in general anaesthetic.
For the right inguinal hernia was performed the PRF-open mesh repair technique
A small (5-6 cm)transverse incisionmade in the inguinal region.
A self-retaining retractor with smooth non-traumatic branches positioned.
Findings: direct hernia.
Tissues were sharply cut avoiding any stretching or shredding during the dissection. Minimal manipulation used to prepare the sac from the cord and to make the space where the mesh will be located. It is important to minimise the detachment of tissues and in particular to respect as much as possible the nerves that cross the area. Haemostasis checked step by step.
After the sac was isolated and content examined, it was repositioned into the abdomen and the trasversalis fascia approximated with Vicryl 2-0 stitches.
The mesh was then customised to be suitable for the patient’s inguinal region. We choose a soft, light, macroporus, monofilament, polypropylene mesh BARD.
The mesh was designed according to the shape and size of the inguinal canal and fixed in place with a 2 cm overlap of the mesh above the tubercle.
Mesh fixation. We used L-PRF clot (Leukocyte-Platelet Rich Fibrin) with both components: membrane and hyper-acute serum to fix the mesh and secure a tension free technique.
The L-PRF clot was prepared in theatre with IntraSpine centrifuge using 9 ml fresh patient’s blood in glass-coated plastic tubes and immediately centrifuged at 2700 rpm for 12 minutes.
After centrifugation, three distinct layers were in the tube (Figure 1): red blood corpuscles RBCs at the bottom of the tube, platelet-poor plasma PPP on the top of the tube, and the PRF clot in the middle of the tube.
The L-PRF clot was removed from the tube with surgical tweezers (Figure 2).
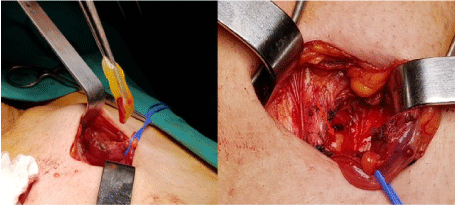
Figure 2. PRF membrane positioning. The arrow indicates the PRF membrane and hyper-acute serum positioned onto the transversalis (posterior) fascia
Serum squeezed out from the PRF clot generates PRF membranes and the hyper-acute serum. We have used 3-5 L-PRF clots to fix the mesh.
Both components membranes and hyper-acute serum were applied on the posterior fascia (trasversalis) and the mesh attached over them (Figure 2).
Only one single stitch Vicryl 2-0 used to close the mesh tails.
Anterior fascia closure. Fascia sutured below the spermatic cord using Vicryl 2-0 stitches to press the mesh between the anterior and the trasversalis fascia.
Fat sutured with Vicryl 2-0 and skin intradermic suture with monocryl 3-0.
For the left inguinal hernia was performed the Lichtenstein technique.
Findings: direct hernia.
The operation was made following the same patterns described for the right side.
Unlike the right side, the mesh fixation was accomplished with Vicryl 2-0 stitches to the pubic tubercle, inguinal ligament and surrounding muscles structures.
Bupivacaine 20 ml 0.25% local anaesthetic infiltration on both sides at the end of the procedure.
Oral paracetamol was prescribed after discharge 1 gr TDS per 5 days and then as needed.
Pain measurement
Postoperative pain was measured with VAS (Visual Analog Scale) by direct interview or by phone call at 3 hours, 24 hours, 48 hours, 7 days, 15 days, 1 month, 3 months and 6 months after the operation.
Clinical follow up was set at 1 month and 6 months.
Chronic groin pain would have been diagnosed whether still present at the 6th month follow-up.
The time needed to recover daily activities was recorded.
Data were anonymised, stored and elaborated in electronic database.
The aim of this case report was to explain the technique and evaluate the feasibility.
No acute short-term complications such as collection, hematoma, wound infection, oedema of the scrotum, DVT have been reported in both inguinal sides.
The results of hernia surgery are mainly measured by postoperative pain, time to return daily activities and work (when applicable), chronic groin pain and, recurrence.
Post-operative pain was the primary outcome to be assessed after PRF-open mesh repair procedure.
VAS (Visual Analog Scale) is a subjective pain scale numerically expressed from 0 to 10. VAS was discussed with patient before the surgery.
Table 1 has been reported the intensity of pain (VAS) at 3 hours, 24 hours, 48 hours, 7 days, 15 days, 1 month, 3 months and 6 months after the operation.
Table 1. has been reported the intensity of pain (VAS) at 3 hours, 24 hours, 48 hours, 7 days, 15 days, 1 month, 3 months and 6 months after the operation
|
3h |
24h |
48h |
7days |
15days |
1month |
3months |
6months |
Right Hernia |
1 |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
Left Hernia |
1 |
6 |
5 |
4 |
4 |
2 |
0 |
0 |
Post-operative VAS score reported during follow up for the Right (PRF-open mesh repair) and the Left inguinal hernia (Lichtenstein technique).
Post-operative VAS score reported during follow up for the Right (PRF-open mesh repair) and the Left inguinal hernia (Lichtenstein technique).
The peak was 24h after surgery. This peak had several explanations: the post-operative infiltration of Bupivacaine ended its effect and the patient was pushed to move and walk. However, there was a clear difference in pain perception and intensity between the right and the left side as reported in Table 1.
Furthermore, due to the intermittent left sided discomfort, the patient requested occasional pain killer consumption up to 15 days after the operation.
The patient came back to normal daily activities after 7 days from surgery.
At three and six months follow up, the patient did not complain of any pain, symptoms or discomfort.
No recurrence detected after 6 months follow up.
Lichtenstein technique promotes a rigid fixation of the mesh, recommending non-absorbable overrunning stitches (Prolene) to sew the mesh to the inguinal ligament and surrounding muscles structures; this may generate tension, inflammation and pain [43;46].
In addition, the risk that a permanent suture would trap a nerve is reasonably high (average 11- 12% in Literature) causing chronic inguinal pain, which quite often requires prolonged pain killer consumption, local infiltrations or unreliable nerve-lysis operations [44;48].
We have analysed the use and performance of glue sealant such as Fibrine glue and Cyanoacrylates (CAs) to reduce the pain and risk of nerve entrapment syndrome related to the stitch fixation.
In 2009, De Hingh IHJT, et al. described the use of autologus P-RFS (Platelet-Rich Fibrin Sealant) on 22 patients. The aim was fixing the mesh replacing the human/bovine fibrin glue. The advantages claimed focused on the P-RFS haemostatic and antibacterial effects. The preparation of the blood required a large amount of patient’s own blood (120 ml) and stored into a designated preparation unit containing sodium citrate for anticoagulation. Afterwards, the container was placed in the centrifuge for 23 min to produce an average of 6 ml of P-RFS. With a spray applicator, the P-RFS was applied along the ligament and the medial and cranial side of the mesh [74].
On the base of the above experiences, we deemed that L-PRF provided by the patient’s own blood might become another glue option to fix the mesh.
L-PRF is an autologous platelet-rich fibrin centrifuge product capable of glue and scaffolding performance besides tissue regeneration properties.
In Literature, PRF resulted to be able to minimise the local inflammatory acute response and promote optimal fibroblast colonization and efficient collagen production. These PRF features may be central to streamline the mesh integration and support the effective wound healing process [73].
Furthermore, PRF resulted to be less expensive than either fibrin glue or cyanoacrylate. It provides similar scaffolding properties to fibrin glue but eliminates the risk of transmission of blood pathogens.
In addition, PRF has beneficial anti-inflammatory properties as opposed to the characteristic of cyanoacrylate which, seems to be inclined to promote inflammatory tissue reactions.
Most important, PRF exhibits both good fixation capacity and unique tissue regeneration properties when compared with other glue sealants [58; 73].
The clinical perspectives of the PRF-mesh repair procedure are the reduction of postoperative pain, accelerate the patient's recovery, prevent the chronic inguinal pain, reduce the rate of recurrence and reduce financial costs of the operation [73].
Limited to this case report experience, L-PRF showed: 1) “tension free” fixation of the mesh; 2) less local inflammation and pain; 3) easy prevention of nerve entrapment complication.
It would not be unexpected that further clinical trial show improvements in mesh integration and tissue regeneration.
This case report showed an impressive difference in pain and time of recovery between the two inguinal hernias operated with the two different techniques. The VAS score indicated that only after 3 months there was an equalization of symptoms amid two sides.
Covid 19 pandemic has abruptly interrupted our clinical experience after first 6 cases. The study will hopefully be authorised to restart as soon as pandemic declines.
PRF-open mesh repair is a physiologic and genuinely "tension-free technique" that follows sound regenerative surgery principles.
The surgical technique, the biological method of fixation and the regenerative properties of PRF seem to minimise wound site inflammation and assist the correct integration of the mesh for a prompt and painless recovery after inguinal hernia surgery.
In this case-report, PRF-open mesh repair offered some short-term benefit comparing to the Lichtenstein technique resulting in less pain and shorter recovery time.
Covid 19 pandemic has abruptly interrupted our clinical experience, nonetheless, we believe to be worth to publish this case report.
The authors declare that they do not have financial or nonfinancial competing interests.
- Baylón K, Rodríguez-Camarillo P, Elías-Zúniga A, Robert Gilkerson JA, Lozano (2017) Past, Present and Future of Surgical Meshes: A Review. Membranes (Basel) 7: 47. [Crossref]
- Bendavid R, Abrahamson J, Arregui ME, Flament JB, Phillips EH (2001) Abdominal Wall Hernias: Principles and Management. (1st Edn) Springer; New York, NY, USA.
- Heniford BT (2015) Hernia Handbook. (1st Edn) Carolinas HealthCare System, Charlotte, NC, USA:
- Kingsnorth A (2004) Treating inguinal hernias: Open mesh Lichtenstein operation is preferred over laparoscopy. BMJ 328: 59-60. [Crossref]
- HerniaSurge Group (2018) International guidelines for groin hernia management. Hernia 22:1-165. [Crossref]
- Pandit AS, Henry JA (2004) Design of surgical meshes—An engineering perspective. Technol. Heal. Care. 12: 51-65. [Crossref]
- Köckerling F, Koch A, Lorenz R, Schug-Pass C, Stechemesser B, et al. (2015) How long do we need to follow-up our hernia patients to find the real recurrence rate? Front Surg 2: 24. [Crossref]
- Tebala GD, Tognoni V, Tristram Z, Macciocchi F, Innocenti P (2015) Cyanoacrylate glue versus suture fixation of mesh in inguinal hernia open repair a randomized controlled clinical trial. Gastroenterol Hepatol 2: 5.
- Billroth T (1924) In: The Medical Sciences in the German Universities: A Study in the History of Civilization. Welch WH (Ed) Macmillan; New York, NY, USA.
- Chowbey P (2012) Endoscopic Repair of Abdominal Wall Hernias. (2nd Edn) Byword Books, Delhi, India.
- Usher FC, Hill JR, Ochsner JL (1959) Hernia repair with Marlex mesh. A comparison of techniques. Surgery 46: 718-728. [Crossref]
- Klinge U, Klosterhalfen B, Birkenhauer V, Junge K, Conze J, et al. (2002) Impact of polymer pore size on the interface scar formation in a rat model. Surg Res 2002; 103: 208-214. [Crossref]
- Lichtenstein IL, Shulman AG, Amid PK (1989) The tension free hernioplasty. Am J Surg 157: 188-193. [Crossref]
- Melero Correas H (2008) Master Thesis. Universitat Politècnica de Catalunya; Barcelona, Spain. Caracterización Mecánica de Mallas Quirúrgicas Para la Reparación de Hernias Abdominales.
- Brown CN, Finch JG (2010) Which mesh for hernia repair? Ann R Coll Surg Engl 9: 2272-278. [Crossref]
- Zogbi L (2008) The Use of Biomaterials to Treat Abdominal Hernias. In: Pignatello R (Ed) Biomaterials Applications for Nanomedicine. (1st Edn) 18: 359-382.
- Bilsel Y, Abci I (2012) The search for ideal hernia repair; mesh materials and types. Int J Surg 10: 317-321. [Crossref]
- Winters JC, Fitzgerald MP, Barber MD (2006) The use of synthetic mesh in female pelvic reconstructive surgery. BJU Int 98: 70-76. [Crossref]
- Tang L, Ugarova TP, Plow EF, Eaton JW (1996) Molecular determinates of acute inflammatory response to biomaterials. J Clin Invest 97: 1329-13234. [Crossref]
- Busuttil SJ, Ploplis VA, Castellino FJ, Tang L, Eaton JW, et al. (2004) A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost 2: 1798-1805. [Crossref]
- Earle DB, Mark LA (2008) Prosthetic Material in Inguinal Hernia Repair: How Do I Choose? Surg Clin North Am 88: 179-201. [Crossref]
- Schaechter M (2009) Encyclopedia of Microbiology. (3rd Edn) Academic Press, Cambridge, MA, USA.
- Greenberg JA, Clark RM (2009) Advances in suture material for obstetric and gynecologic surgery. Rev Obstet Gyneco 2:146-158. [Crossref]
- Anderson JM, Rodriguez A, Chang DT (2008) Foreign Body Reaction to Biomaterials. Semin Immunol 20: 86-100. [Crossref]
- Murch AR, Grounds AD, Marshall CA, Papadimitriou JM (1982) Direct evidence that inflammatory multinucleate giant cells form by fusion. J Pathol 137: 177-180. [Crossref]
- Woloson SK, Greisler HP (2001) Biochemistry, immunology, and tissue response to prosthetic material. In: Bendavid R, Abrahamson J, Arregui ME, Flament JB, Phillips EH (Eds) Abdominal wall hernias. Principles and management. New York, pp: 201–207.
- Chu C, Von Fraunhofer JA, Greisler HP (1997) Wound Closure Biomaterials and Devices. (1st Edn) CRC Press LLC, Boca Raton, FL, USA.
- Anderson JM (2001) Biological Response to Materials. Annu Rev Mater Res 31: 81-110.
- Elango S, Perumalsamy S, Ramachandran K, Vadodaria K (2017) Mesh materials and hernia repair. Biomedicine (Taipei) 7: 16. [Crossref]
- Junge K, Rosch R, Klinge U, Schwab R, Peiper C, et al. (2006) Risk factors related to recurrence in inguinal hernia repair: a retrospective analysis. Hernia 10: 309-315. [Crossref]
- Si Z, Bhardwaj R, Rosch R, Mertens PR, Klosterhalfen B, et al. (2002) Impaired balance of type I and type III procollagen mRNA in cultured fibroblasts of patients with incisional hernia. Surgery 131: 324–331. [Crossref]
- Rangaraj A, Harding K, Leaper D (2011) Role of collagen in wound anagement. Wounds 7: 54-63.
- Abci I, Bilgi S, Altan A (2008) Role of TIMP-2 in Fascia Transversalis on Development of Inguinal Hernias. J Invest Surg 18: 123-128. [Crossref]
- Guillen-Marti J, Diaz R, Quiles MT, Lopez-Cano M, Vilallonga R, et al. (2009) MMPs/TIMPs and inflammatory signalling de-regulation in human incisional hernia tissues. J Cell Mol Med 13: 4432-4443. [Crossref]
- Bellön JM, Bajo A, Ga-Honduvilla N, Gimeno MJ, Pascual G, et al. (2001) Fibroblasts From the Transversalis Fascia of Young Patients With Direct Inguinal Hernias Show Constitutive MMP-2 Overexpression. Ann Surg 233: 287-291. [Crossref]
- Donahue TR, Hiatt JR, Busuttil RW (2006) Collagenase and surgical disease. Hernia 10: 478-485.
- Ashcroft GS, Herrick SE, Tarnuzzer RW, Horan MA, Schultz GS, et al. (1997) Human ageing impairs injury-induced in vivo expression of tissue inhibitor of matrix metalloproteinases (TIMP)-1 and -2 proteins and mRNA. J Pathol 183: 169-176.
- Lau H, Fang C, Yuen WK, Patil NG (2007) Risk factors for inguinal hernia in adult males: A case-control study. Surgery 141: 262–266. [Crossref]
- Klinge U, Zheng H, Si ZY, Schumpelick V, Bhardwaj R, et al. (1999) Synthesis of type I and III collagen, expression of fibronectin and matrix metalloproteinases-1 and -13 in hernial sac of patients with inguinal hernia. Int J Surg Investig 1: 219-227.
- Bendavid R (2004) The unified theory of hernia formation. Hernia 8: 171-176. [Crossref]
- Amid PK, Lichtenstein IL (1998) Long-term results and current status of the Lichtenstein open tension-free hernioplasty. Hernia 2: 89-94.
- Bay-Nielsen M, Kehlet H, Strand L, Malmstrom J, Andersen FH, et al. (2001) Quality assessment of 26,304 herniorrhaphies in Denmark: a prospective nationwide study. Lancet 358: 1124-1128. [Crossref]
- Erhan Y, Erhan E, Aydede H, Mercan M, Tok D (2008) Chronic pain after Lichtestein and preperitoneal (posterior) hernia repair. Can J Surg 51: 383-387. [Crossref]
- Franneby U, Sandblom G, Nordin P, Nyern O, Gunnarson U (2006) Risk factors for long-term pain after hernia surgery. Ann Surg 244: 212-219. [Crossref]
- Kumar S, Wilson RG, Nixon SJ, Macintyre IM (2002) Chronic pain after laparoscopic and open mesh repair of groin hernia. Br J Surg 89: 1476-1479. [Crossref]
- Pagane H (2002) Do absorbable mesh sutures cause less chronic pain than nonabsorbable sutures after Lichtenstein inguinal herniorraphy? Hernia 6: 26-28.
- Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH (2001) Chronic pain and quality of life following open inguinal hernia repair. Br J Surg 88: 1122-1126. [Crossref]
- Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH (2003) A review of chronic pain after inguinal herniorraphy. Clin J Pain 19: 48-54. [Crossref]
- Kehlet H (2008) Chronic pain after groin hernia repair. Br J Surg 95: 135-136. [Crossref]
- Bay-Nielsen M, Nilsson E, Nordin P, Kehlet H (2004) Chronic pain after open mesh and sutured repair of indirect inguinal hernia in young males. Br J Surg 91: 1372-1376. [Crossref]
- Helbling C, Schlumpf R (2003) Sutureless Lichtenstein: first results of a prospective randomised clinical trial. Hernia 7: 80-84. [Crossref]
- Klosterhalfen B, Klinge U, Hermanns B, Schumpelick V (2000) Pathologie traditioneller chirurgischer Netze zur Hernienreparation nach langzeitimplantation in Menschen. Chirurg 71: 43-51.
- Brennan M (1991) Fibrine Glue. Blood Rev 5: 240-244. [Crossref]
- Canonico S (2003) The use of human fibrin glue in the surgical operations. Acta Biomed 2: 21-5. [Crossref]
- Campanelli G, Champault G, Pascual MH, Hoeferlin A, Kingsnorth A (2008) Randomized controlled blinded trial of Tissucol/Tisseel for mesh fixation in patients undergoing Lichtenstein technique for primary inguinal hernia repair: rationale and study design of the TIMELI trial. Hernia 12: 159-165. [Crossref]
- Lovisetto F, Zonta S, Rota E, Mazzilli M, Bardone M (2007) Use of human fibril glue (Tissucol) versus staples for mesh fixation in laparoscopic transabdominal preperitoneal hernioplasty: a prospective, randomized study. Ann Surg 245: 222-231. [Crossref]
- Schwab R, Schumacher O, Junge K, Binnebosel M, Klinge U (2006) Fibrin sealant for mesh fixation in Lichtestein repair: biomechanical analysis of different techniques. Hernia 11: 139-145. [Crossref]
- Pascual G, Mesa-Ciller C, Rodríguez M, Pérez-Köhler B, Gómez-Gil V, et al. (2018) Pre-clinical assay of the tissue integration and mechanical adhesion of several types of cyanoacrylate adhesives in the fixation of lightweight polypropylene meshes for abdominal hernia repair. Plose one 13: e0206515. [Crossref]
- Doan Ehrenfest DM, Pinto NR, Pereda A, Jiménez P, Corso MD, et al. (2018) Impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 29: 171-184. [Crossref]
- Simon M, Major B, Vácz G, Kuten O, Hornyák I, et al. (2018) The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int 2018: 4854619. [Crossref]
- Kuten O, Simon M, Hornyak I, De Luna-Preitschopf A, Nehrer S, et al. (2018) The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng Part A 24: 1011-1021. [Crossref]
- Kardos D, Hornyák I, Simon M, Hinsenkamp A, Marschall B, et al. (2016) Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int J Mol Sci 19: 3433. [Crossref]
- Di Liddo R, Bertalot T, Borean A, Pirola I, Argentoni A, et al. (2018) Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J Cell Mol Med 22: 1840-1854. [Crossref]
- Aulino P, Costa A, Chiaravalloti E, Perniconi B, Adamo S, et al. (2015) Muscle Extracellular Matrix Scaffold Is a Multipotent Environment. Int J Med Sci 12: 336-340. [Crossref]
- Varela HA, Souza JCM, Nascimento RM, Araujo RF, Vasconcelos RC, et al. (2019) Injectable platelet rich fibrin: Cell content, morphological, and protein characterization. Clin Oral Investig 23:1309-1318. [Crossref]
- Dhurat R, Sukesh MS (2014) Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg 7: 189-197. [Crossref]
- Pinto NR, Ubilla M, Zamora Y, Del Rio V, Dohan Ehrenfest DM, et al. (2017) Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: a prospective cohort study. Platelets 29: 468-475. [Crossref]
- De Almeida Barros Mourao CF, Calasans-Maia MD, de Mello Machado RC, de Brito Resende RF, Alves GG (2018) The use of platelet-rich fibrin as a hemostatic material in oral soft tissues. Oral Maxillofac Surg 22: 329-333. [Crossref]
- Bakhtiar H, Esmaeili S, Fakhr Tabatabayi S, Ellini MR, Nekoofar MH, et al. (2017) Second-generation Platelet Concentrate (Platelet-rich Fibrin) as a Scaffold in Regenerative Endodontics: A Case Series. J Endod 43: 401-408. [Crossref]
- Miron RJ, Pikos MA (2018) Sinus Augmentation Using Platelet-Rich Fibrin With or without a Bone Graft: What Is the Consensus? Compend Contin Educ Dent 39: 355-361. [Crossref]
- Desai CB, Mahindra UR, Kini YK, Bakshi MK (2013) Use of Platelet-Rich Fibrin over Skin Wounds: Modified Secondary Intention Healing. J Cutan Aesthet Surg 6: 35-37. [Crossref]
- Crisci A, Marotta G, Licito A, Serra E, Benincasa G, et al. (2018) Use of Leukocyte Platelet (L-PRF) Rich Fibrin in Diabetic Foot Ulcer with Osteomyelitis (Three Clinical Cases Report). Diseases 6: 30. [Crossref]
- Di Nicola V (2020) Regenerative Surgery in Open Mesh Repair for Inguinal Hernia. J Regen Med 9: 1.157.
- De Hingh IHJT, Nienhuijs SW, Overdevest EP, Scheele K, Everts PAM (2009) Mesh Fixation with Autologous Platelet-Rich Fibrin Sealant in Inguinal Hernia Repair. Eur Surg Res 43: 306-309. [Crossref]


