A bone scaffold was intraoperatively prepared from the same region and in the same form as the excised element without the need for shaping using a freeze-and-thaw method. In twenty-four rabbits (n = 48), part of the mandibular angle was excised and made acellular by repeated freezing and thawing; it was histologically examined at 2, 4, and 12 weeks after surgery. Less than five cells per high-power field (cphf) were found in the samples immediately after freezing and thawing. The cells were degenerated; the matrix was severely cluttered. After 2 weeks, the matrix seemed more organized with few undifferentiated cells (30–50 cphf). After 4 weeks, connective fibers and fibroblasts were identified, indicating matrix regeneration (70–90 cphf). After 12 weeks, the matrix was reconstructed and connective-tissue fibers were condensed. A significant portion of the cells were osteoblast-like and osteoclast-like (90–100 cphf, 20%–30% osteoblasts, analysis by one-way analysis of variance (ANOVA), p ≤ 0.001). Differentiated fibroblasts were found in the bone graft in the vicinity of new blood vessels. The bone scaffold prepared by repeated freezing and thawing with an autologous source along the vascular bone is a reliable source of bone tissue needed for craniofacial bone repair.
recycling, bone, craniofacial defect
Providing bone tissue for the repair of bone defects has always been a problem [1]. From a variety of metal plaques, which pre-Incas in South America used during 2000 BC, to titanium mesh, which is used today, and on the other hand, the use of bone grafts of autologous to free flaps of bone and allograft have been used to provide bone tissue. Each of these methods has its own pros and cons. Different methods have been proposed for the preparation of bone grafts; however, methods for the reuse of diseased bone tissue are limited. Pasteurization and freezing are the two methods that have been proposed for reusing bone tissue in some clinical cases [2-4].
In the present study, repeated freeze-and-thaw method is used to create acellular samples from rabbit’s mandibular bone.
We shaved the mandibular angles of twenty four rabbits (n = 48) after the induction of general anesthesia via intramuscular injections of 5 mg/kg xylazine 2% and 50 mg/kg ketamine 10%. After prep and drape, we incised the skin parallel to the lower mandibular border, dissected it subperiosteally, excised a bone fragment with approximate dimensions of 2 × 1 × 0.5 cm from the mandibular angle, and passed a 4-0 stainless steel wire through it. The dimensions and location of the biopsy were selected keeping possible future problems with the nutrition of the rabbits in mind. We placed the fragment in a sterile metal container and let it float in liquid nitrogen for 1 minute; subsequently, the container with the bone was placed in water of 55°C for 5 minutes. This cycle of freezing and thawing was repeated five times. We cut a piece of processed bone of approximately 2–3 mm to ensure the acellularity. Next, we placed the bone fragment back at the original site of biopsy at the mandibular angle using the same 4-0 wire and repaired the muscle and skin using 3-0 vicryl.
This surgery was performed on both sides of each rabbit’s mandible at different times. We excised the re-implanted bone fragments at 2, 4, and 12 weeks after surgery, under the same general anesthesia. Sixteen pieces of the mandible were excised 2 weeks after the surgery, sixteen pieces from the other mandibles at 4 weeks after the surgery, and sixteen pieces from the other mandibles at 12 weeks after the surgery, making a total of 12 samples. No rabbits were executed after the second biopsy.
A board-certified pathologist examined the samples quantitatively for the number of cells per high-power field (cphf) (400× magnification) and the extracellular matrix qualitatively using hematoxylin and eosin and trichrome staining. The number of cells in the high-power field was analyzed statistically by SPSS 16 software using a one-way analysis of variance (ANOVA) test.
The ethics committee of Tehran University of Medical Sciences approved the study.
Samples taken immediately after the freeze-and-thaw method exhibited acellular tissue with a cphf < 5; many of the existing cells were degenerated. Though the acellularity was acceptable, the matrix was highly irregular; the connective fibers did not have the necessary coherence and seemed to be disrupted (Figure 1).
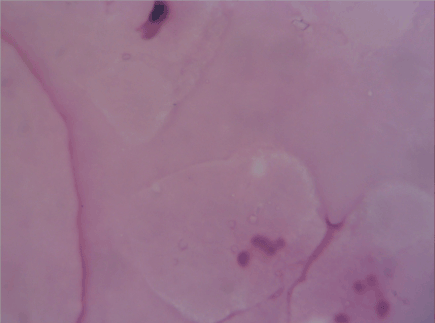
Figure 1. Immediately after freeze and thaw procedure though the acellularity was acceptable, the matrix was highly irregular
The matrix was somewhat organized 2 weeks after the surgery; however, the fibers still did not have the necessary coherence. Some undifferentiated cells were implanted among the regenerative fibers, but there was no differentiation (cphf was 30–50) (Figure 2).
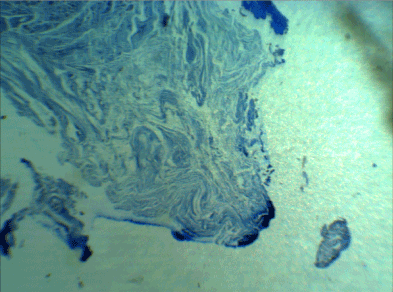
Figure 2. Two weeks later, the fibers still did not have the necessary coherence. Some undifferentiated cells were implanted among the regenerative fibers, but there was no differentiation (cphf was 30–50) (Masson's trichrome staining, 400X)
In the samples taken after 4 weeks, the extracellular fibers were more complete, and Masson's trichrome staining confirmed the regeneration of the matrix. More fibroblast-like cells were diagnosable among them. However, some cells were histomorphologically closer to bone-making cells. The cphf was 70–90, and 1%–2% of the cells were osteoblast-like (Figure 3).
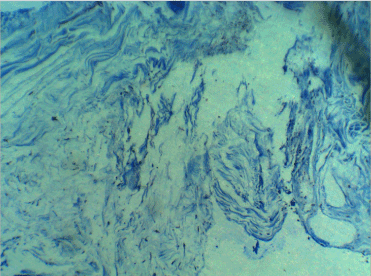
Figure 3. Four weeks later, Masson's trichrome staining confirmed the regeneration of the matrix. More fibroblast-like cells were diagnosable among them
In the samples taken after 12 weeks, the matrix was completely restored and the arrangement of fibers was similar to that of the dense tissues. Less number of undifferentiated cells were observed, and most cells were similar to connective-tissue cells. However, the fusion of several osteoblast-like cells had also created a quasi-osteoclast cell. In addition, fibrocytes were completely differentiated. The cphf was 90–100, and 20%–30% of the cells were osteoblasts, osteoclast-like cells, and fibroblasts (Figure 4).
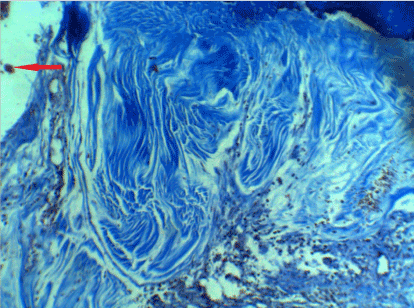
Figure 4. After 12 weeks, the matrix was completely restored and the arrangement of fibers was similar to that of the dense tissues and most cells were similar to connective-tissue cells. However, the fusion of several osteoblast-like cells had also created a quasi-osteoclast cell (red arrow)
Analysis of the cellular data using one-way ANOVA indicated a significant increase in the cell population in the re-implanted scaffold statistically and histologically, both in total cell count and osteoblast proportion (ANOVA, F=2.34; df =3,46; p ≤ 0.001)
Providing bone tissue for bone defects due to tumor resection, infection, or trauma is not easy, particularly in craniofacial surgery. Therefore, the recycling of disease-affected bone could be a step forward for providing bone structure, particularly if the gross structure of the diseased bone is not affected by major tumoral or traumatic distortion. This recycling is not a new method, and clinical use has been reported.
Noguchi et al. reported two patients with femoral osteosarcoma in whom, after resection and removal of a considerable length of their femoral bone, the resected tumoral bone had been pasteurized and subsequently re-implanted along with a free fibula at the site of the resected bone [2]. After a few months, the patients, who were adolescents, had returned to their normal activity and there was no sign of local recurrence or bone loss. Gede et al also reported four cases in which this treatment was performed on the distal tibia using frozen and thawed tumoral bone with osteosarcoma, Ewing sarcoma, adamantinoma, and recurrent giant cell tumor [5]. However, these studies did not conduct a histological examination and only performed a radiological examination postoperatively. Tanzawa et al (2008,2009). histologically examined a frozen recycled bone autograft for human limb osteosarcoma. Interestingly, they found that the recycled tumoral bone contained many new osteoblast and osteoclast cells 5 months after the surgery [6,7].
Different connective-tissue scaffolds have been prepared by the freeze-and-thaw method. In the aforementioned studies, the extracellular structure was largely preserved (even the tendon preserved its collagen structure) [8-10]; however, in our study, we found a collapsed structure that became organized over time. Of course, none of the papers mentioned examining the cell population of the tissue after replacement.
Sirinoglu et al studied freezing and thawing in a different way in the mandible of rats and found that a bone graft will survive after freezing in liquid nitrogen but this method of sterilization is not suitable for tumor treatment [11]. Their method comprised one cycle of freezing and thawing but at a different speed of thawing than used in the present study.
In the present study, in addition to the structure, the cellular characteristics of the samples were checked and had acceptable results. One explanation for this structural and cellular regulation seems to be that fibroblasts have been implanted inside the initially collapsed matrix and then have been converted to osteoblast and even osteoclast cells; the structure may eventually be further improved, particularly in the vicinity of new blood vessels.
On the other hand, both the previous experimental and clinical studies had been conducted on cortical bone while the examination in the present study was on the rabbit’s mandibular bone, which has a spongy structure that possibly affects the structure of its connective tissue and scaffold initially.
However, the present study has a limited number of samples and also limitation of size. Acellular bone tissue produced using repeated freezing and thawing can be considered an important source of bone in craniofacial surgery, particularly near vascularized bone.
The authors are grateful to all academic staff in department of pathology Emam Khomeini Hospital for help and support.
- 1. Eastman BL (1895) Two cases of bone-grafting for cranial defect. Ann Surg 22(6): 787-91.
- 2. Noguchi M (2008) An intramedullary free vascularized fibular graft combined with pasteurized autologous bone graft in leg reconstruction for patients with osteosarcoma. J Reconstr Microsurg 24(7): 525-30.
- 3. Takeuchi A (2015) Successful correction of tibial bone deformity through multiple surgical procedures, liquid nitrogen-pretreated bone tumor autograft, three-dimensional external fixation, and internal fixation in a patient with primary osteosarcoma: a case report. BMC Surg 15: 124. [Crossref]
- 4. Abdel Rahman MA, Bassiony, H Shalaby (2009) Reimplantation of the resected tumour-bearing segment after recycling using liquid nitrogen for osteosarcoma. Int Orthop 33(5): 1365-70. [Crossref]
- 5. Gede EWI (2017) Outcome of bone recycling using liquid nitrogen as bone reconstruction procedure in malignant and recurrent benign aggressive bone tumour of distal tibia: A report of four cases. J Orthop Surg 25(2): 2309499017713940. [Crossref]
- 6. Tanzawa Y (2009) Histological examination of frozen autograft treated by liquid nitrogen removed after implantation. J Orthop Sci 14(6): 761-8. [Crossref]
- 7. Tanzawa Y (2008) Histological examination of frozen autograft treated by liquid nitrogen removed 6 years after implantation. J Orthop Sci 13(3): 259-64. [Crossref]
- 8. Yamamoto NH, Tsuchiya K, Tomita (2003) Effects of liquid nitrogen treatment on the proliferation of osteosarcoma and the biomechanical properties of normal bone. J Orthop Sci 8(3): 374-80. [Crossref]
- 9. Jung HJ (2011) The effects of multiple freeze-thaw cycles on the biomechanical properties of the human bone-patellar tendon-bone allograft. J Orthop Res 29(8): 1193-8. [Crossref]
- 10. Huang H (2011) Effects of repetitive multiple freeze-thaw cycles on the biomechanical properties of human flexor digitorum superficialis and flexor pollicis longus tendons. Clin Biomech (Bristol, Avon) 26(4): 419-23. [Crossref]
- 11. Sirinoglu H (2015) The effect of liquid nitrogen on bone graft survival. Facial Plast Surg 31(4): 401-10.




