The production of xylo-oligosaccharides from xylan using an optimal immobilized catalyst of xylanase from Aspergillus niger is presented. The enzyme extract has several xylanases with different properties doing necessary the development of cheap and simple methods to purify them at industrial scale. The enzyme was successfully purified, immobilized and highly stabilized using a simple protocol. The principal purified xylanase was a 34 KDa protein corresponding with 50% of the endo-xylanase activity of the total strain. Among the different immobilization assayed protocols, the use of aldehyde support allowed the complete immobilization of this fraction keeping 80% of its initial catalytic activity. An optimization of this method promoted a stabilization factor of around 1100-fold more stable than soluble enzyme. The use of the optimal catalyst allowed a maximum hydrolysis degree of 73.4%. The optimization of the reaction conditions (different time and temperature) allowed producing 62% (11.93 mg/mL) of interesting xylooligo-saccharides (XOS2-XOS6) (Figure 1).
Figure 1. Graphical abstract.
Xylanase, Aspergillus niger, biocatalysis, enzyme thermostabilization, XOS production
The xylan represents the most abundant natural polysaccharide in nature after cellulose. The xylan consists of a linear polymer of β-(1-4)-D-xylopyranosyl units which can be substituted with 4-O-methyl-α-D-glucuronopyranosyl units, acetyl groups or α-L-arabinofuranosyl in variable proportions [1].
Xylan is mainly hydrolyzed by endo-xylanases to produce xylo-oligosaccharides (XOS) mixtures, which are considered as emerging prebiotics. Among the health benefits attributed to XOS, the increase of bifidobacteria and Lactobacillus, the decrease of pathogenic and putrefactive bacteria, the improvements in bowel function and calcium absorption, the immunological properties, and antioxidant , anti-inflammatory or antiallergic activities are included [2]. The conversion of the oligosaccharides into xylose can be catalyzed by β-xylosidases. Xylose can be used such as carbon source in ethanol production or can be chemically or enzymatically transformed into other products such as xylitol, which is an important non-caloric sweetener, using a xylose reductase [3].
There are reported endo-xylanases from different sources such as fungi or bacteria and usually can be inducted when microorganisms are grown using xylan as carbon source. An industrially attractive alternative is the use of abundantly available and cost-effective agricultural residues (wheat bran, corn cobs, corn stover, rice bran, rice husk, etc.) to obtain the enzyme with reduction of the overall manufacturing cost [4].
The crude strains obtained after culture of different carbon sources are usually composed of different endo-, exo-xylanases and other enzymes that can catalyze the transformation of the desired products into other different compounds; even the use of a mixture of endo-xylanases difficult the control of the process because the properties of the global catalyst (activity, stability and selectivity) are a mixture of those of all the enzymes.
On the other hand, Aspergillus niger is able to produce up to 15 different xylanases depending on the fermentation conditions [5]. This makes necessary the design of easy purification processes. Considering that the use of soluble enzyme at industrial level is in many cases expensive, it is usually convenient the use of the enzyme as immobilized preparation in order to reuse the catalyst improving the productivity and permitting an easier design of the reactors, avoiding expensive purification processes. Due the importance of immobilizing the enzyme, it is also highly interesting using the immobilization processes to produce more stable biocatalysts. This may permit catalyzing the hydrolysis of agricultural wastes into prebiotics with added value minimizing the cost of the process.
Especially in the last decade, xylanases from different sources have been immobilized using different protocols allowing different improvements on their operational properties (Table 1) [6-32].
Table 1. Immobilization of endo-xylanases reported in the literature
Xylanase source |
Support / activation |
Molecular
mass (kDa) |
Type of
immobilization |
Recovered
activitya (%) |
Immobilization
yieldb(%) |
Stability factorc |
References |
Armillaria gemina |
Silicon oxide nanoparticles |
47 |
Covalent |
117 |
69.2 |
4.5 (50°C) |
[6] |
Aspergillus niger |
Eudragit L-100 |
24 |
Adsorption |
60 |
93 |
nd |
[7] |
Aspergillus niger |
Chitosan treated with dialdehyde starch |
nd |
Covalent |
60.8 |
71.2 |
3 (55°C) |
[8] |
Aspergillus niger |
Alginate beads treated with glutaraldehyde |
32 |
Covalent |
nd |
94.6 |
1.5 (55°C) |
[9] |
Aspergillus niveus expressed in
Aspergillus nidulans |
Glyoxyl-agarose |
36 |
Covalent |
83 |
100 |
8.5 (70°C) |
[10] |
Aspergillus sp. (strain 44) |
Eudragit S-100 |
nd |
Adsorption |
80 |
100 |
2.5 (60°C) |
[11] |
Aspergillus sp. (strain 5) |
Eudragit S-100 |
nd |
Adsorption |
70 |
99 |
2 (60°C) |
[11] |
Aspergillus tamarii |
Duolite A147 treated with glutaraldehyde |
nd |
Covalent |
54.2 |
48.4 |
1.3 (60°C) |
[12] |
Aspergillus terreus F-413 |
Porous glass beads treated with
3-aminopropyltriethoxysilane |
nd |
Covalent |
nd |
100 |
nd |
[13] |
Aspergillus versicolor |
Glyoxyl-agarose |
21 |
Covalent |
84 |
88 |
700 (60°C) |
[14] |
Bacillus halodurans |
Lewatit MonoPlus MP64 |
45 |
Adsorption |
nd |
60.2 |
nd |
[15] |
Bacillus pumilus MK001 |
Q-Sepharose |
nd |
Adsorption |
45 |
15.8 |
nd |
[16] |
Bacillus pumilus MK001 |
HP-20 beads |
nd |
Covalent |
42 |
18 |
nd |
[16] |
Bacillus pumilus SV-205 MTCC 9862 |
Aluminum oxide treated with glurataldehyde |
nd |
Covalent |
83.6 |
nd |
1.2 (70°C) |
[17] |
NS50014 (Novozymes) |
Epoxy-chitosan |
nd |
Covalent |
64 |
100 |
1.3 (75°C) |
[18] |
NS50014 (Novozymes) |
Glyoxyl-agarose (after enzyme amination) |
nd |
Covalent |
nd |
100 |
40 (70°C) |
[18] |
Pholiota adiposa |
Silicon oxide nanoparticles |
37 |
Covalent |
144 |
66 |
nd |
[19] |
Pulpzyme HC (Novozymes) |
Cellulose acetate membranes |
nd |
Covalent |
nd |
nd |
nd |
[20] |
Streptomyces halstedii |
Glyoxyl-agarose |
32.6 |
Covalent |
65 |
95 |
200 (60°C) |
[21] |
Streptomyces olivaceoviridis E-86 |
Eudragit S-100 |
47 |
Adsorption |
92 |
99.5 |
1.6 (60°C) |
[22] |
Talaromyces thermophilus |
Gelatin treated with glutaraldehyde |
nd |
Covalent |
100 |
98.8 |
1.5 (100°C) |
[23] |
Thermomyces lanuginosus |
Nanoporous gold |
25 |
Covalent |
nd |
nd |
nd |
[24] |
Thermomyces lanuginosus SSBP |
Eudragit S-100 |
24 |
Adsorption |
75 |
100 |
nd |
[25] |
Thermotoga maritima (XynB) |
Nickel-chelate Eupergit C 250L |
40 |
Adsorption / Covalent |
76.3 |
98.7 |
nd |
[26] |
Thermotoga sp. (strain FjSS3-B.1) |
Porous glass beads |
31 |
Covalent |
nd |
85 |
5 (105°C) |
[27] |
Trichoderma reesei |
Polysulfone acrylate membranes |
nd |
Covalent |
nd |
nd |
nd |
[28] |
Trichoderma reesei |
Eudragit L-100 |
nd |
Adsorption / Covalent |
59 |
69 |
nd |
[29] |
Trichoderma viride |
UV-curable polymeric support |
nd |
Covalent |
nd |
nd |
nd |
[30] |
Trichoderma viride |
Chitosan-xanthan hydrogels |
nd |
Covalent |
177 |
92 |
nd |
[31] |
Trichoderma viride |
Polyaniline treated with glutaraldehyde |
nd |
Covalent |
nd |
nd |
nd |
[32] |
nd: not determined
aRecovered activity refers to the activity observed in the derivative compared with the activity bound to the support. b Immobilization yield refers to the percentage of activity bound to the support compared to the total activity offered to it. c Stability factor refers to the ratio between the half-life values of the derivative and the soluble enzyme, at the specific temperatures described in parentheses.
Therefore, in this work, a simple protocol for the purification, immobilization and stabilization of two different xylanases from A. niger is presented. The optimal catalyst was used to study and optimize the reaction of hydrolysis of xylan into different oligosaccharides with industrial interest.
Materials
Endo-1,4-β-xylanase was produced by A. niger. Agarose 10 BCL was purchased from Agarose Bead Technologies (Madrid, Spain). Epichlorhydrine, iminodiacetic acid, p-aminophenyl boronic acid, triethylamine, ethanolamine, polyethyleneimine, beechwood xylan, xylose, glycidol, sodium borohydride, sodium periodate and 3-5´-dinitrosalicylic acid were obtained from Sigma-Aldrich Co (St. Louis, United States). Powdered corncob was kindly donated by Rasul (Andirá, Brazil). Carboxymethyl-sepharose fast flow, Q-sepharose fast flow, CNBr-activated 4B sepharose and SDS-PAGE low molecular weight standards were purchased from GE Healthcare Life Sciences (Uppsala, Sweden), and the xylo-oligosaccharides standards (X2-X6) from Megazyme (Bray, Ireland). All reagents were of analytical grade.
Endo-1,4-β-xylanase production
Aspergillus niger was used for xylanase production. Cultures were grown on 2% (w/v) agar and 4% (w/v) oat flour at 40°C for four days. Enzyme production was performed as described by Benedetti et al. [33], with powdered corncobs as carbon source. The crude extract was filtered through filter paper under vacuum and lyophilized.
Enzyme assay and protein determination
Reducing sugar released after enzymatic hydrolysis of xylan was colorimetrically determined by reaction with 3-5´-dinitrosalicylic acid (DNS), according the described method [34]. Xylose was used as standard reducing sugar for calibrations.
Soluble substrate was prepared mixing 40 mg/mL of beechwood xylan in 100 mM sodium acetate at pH 5. The mixture was stirred for 30 minutes at 25°C and centrifuged for 20 minutes at 5,000 g. The soluble fraction was separated and used as substrate for the reaction. Thus, the final substrate concentration used in this study was 20 mg/mL. The assay was performed at 25°C with mild stirring. This temperature was chosen against the optimal (around 55°C) to facilitate the measures in standard conditions and eliminating the possibility of inactivation at higher temperatures by long time reactions. One enzyme unit (U) was defined as the amount of enzyme capable of producing 1 µmol of reducing sugar per minute in these conditions.
Protein was determined with the Bradford´s method [35] using bovine serum albumin (fraction V) as the protein standard.
Preparation of immobilization supports
Glyoxyl supports were obtained after activation of agarose 10 BCL with aldehyde groups as previously described by Guisan [36]. Polyethylenimine-agarose (PEI-agarose) support was prepared according to Mateo et al. [37] using glyoxyl agarose supports as starting material. Iminodiacetic acid-agarose (IDA-agarose), boronate-agarose and amino-agarose supports were prepared as described by Mateo et al. [38] using epoxy-activated agarose as base support. Cu2+-chelate-agarose was prepared by incubating the support previously activated with iminodiacetic acid in a 20 mM solution of CuSO4 for 30 minutes and then washed with distilled water [39].
Enzyme purification and immobilization
The different preparations were all performed with a low enzymatic load in order to avoid possible mass limitation transfers.
Purification: The preparation of the enzyme using the crude strain as starting material was performed depending on the support used. Thus, 2 mg of xylanase (measured by the Bradford method) containing 2 U/mg was solubilized in sodium phosphate buffer at pH 7 (5 mM phosphate for immobilizing on ion-exchange supports; 50 mM phosphate for immobilization on boronate support and 100 mM for use with Cu2+-chelate agarose). Then, the immobilization on different supports was performed by adding 1 g of support in 10 mL of the enzyme solution at 25°C. The enzyme adsorbed on the Cu2+-chelate agarose was desorbed with 20 mM imidazol.
Immobilization: 2 mg of pure xylanase (containing 16.8 U/mg) was diluted in 10 mL of the buffer solution (100 mM sodium bicarbonate pH 10 or 100 mM sodium phosphate pH 7) and offered to 1 g of glyoxyl agarose or CNBr-activated Sepharose supports. When the immobilization was completed the glyoxyl derivative was reduced by adding 1 mg/mL of solid NaBH4 for 30 minutes and then washed with water. The immobilization on CNBr-support was performed at 4°C for 15 minutes and then blocked with 1 M ethanolamine at pH 8 for 2 hours. Finally derivative was washed with water. The purification-immobilization in one step was also performed using agarose heterofunctional supports activated with Cu2+-chelate groups and glyoxyl groups. For this, the first adsorption step was performed as was described above for monofunctional Copper activated supports and then the adsorbed enzyme was incubated at pH 10 during 2 hours. Finally, the preparation was reduced with 1 mg/mL of solid NaBH4 for 30 minutes and then washed with water.
In order to control the processes of purification and immobilization, periodically, samples of the supernatant (enzyme solution without the support) and suspension (the whole mixture) were withdrawn, and the enzyme activity was measured.
The yield of immobilization (YI) and the expressed activity (EA) were determined as:

The total activity offered to support is the total number of units (U) added to the support for immobilization, and the unbound enzyme activity refers to the number of units (U) found in the supernatant at the end of the immobilization process.
Thermal stability studies
Thermal inactivation was carried out at different temperatures, with 1 g of immobilized derivative suspended in a 10 mL solution of 0.1 M acetate buffer at pH 5. Periodically samples of the suspension were withdrawn and their activities were tested as was described above. The initial activity was considered as 100%.
Inactivation was modeled based on the deactivation theory proposed by Henley and Sadana [40]. Inactivation parameters were determined from the best-fit model of the experimental data which was the one based on two-stage series inactivation mechanism with no residual activity. According to it, biocatalyst inactivation proceeds through two sequential steps of progressively less active enzyme species until a final completely inactive species is obtained, as represented in the following scheme:
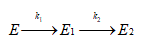
where k1 and k2 are first-order transition rates constants, E, E1 and E2 are the corresponding enzyme species. The mathematical model representing this mechanism is:
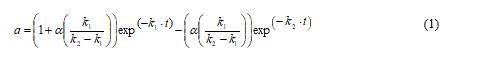
where ɑ represents the residual activity at time t and α is the ratio of specific activity of enzyme species E1 with respect of that of the native enzyme species E.
Considering only one step mechanism of enzyme inactivation (k2=0), and residual activity (α≠0), the model is first order inactivation with residual activity, represented by:
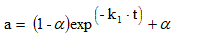 (2)
(2)
Inactivation parameters were determined from the best-fit model of the experimental data. Half-life (time at which the residual enzyme activity is half of its initial value; t1/2) was used to compare the stability of the different biocatalysts, being determined by interpolation from the respective models described by Eq. (1) or Eq. (2).
Influence of temperature on the activity of immobilized xylanase
The activity of glyoxyl-agarose preparation or the soluble enzyme was assayed at different temperatures by diluting 2 mg of soluble or 1 g of the derivative in 10 mL of 100 mM sodium acetate buffer at pH 5. The activity was measured as previously described.
SDS-PAGE
Samples were submitted to denaturing electrophoresis, based on the method described by Laemmli [41], using 12% polyacrylamide gel. Molecular mass standard consisted on phosphorylase b (97 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa) and α-lactalbumin (14.4 kDa). Gels were stained with Coomassie brilliant blue or with silver nitrate, according to Heukeshoven and Dernick [42].
High-Performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) analysis
Xylose and xylo-oligosaccharides (XOS) were analyzed by HPAEC-PAD using an ICS2500 Dionex system consisting of GP50 gradient pump, and ED50 electrochemical detector with a gold working electrode and Ag/AgCl reference electrode. Analyses were carried out at 25°C on a CarboPac PA-1 column (250×4 mm) in combination with a CarboPac PA-1 (50×4 mm) guard column. Separations were performed at a flow rate of 1 mL/min as previously described [21]. Quantification of xylose and XOS was performed by external calibration using standard solutions (XOS1 to XOS6).
Preparation of xylanase catalyst
Purification and immobilization: The strain from A. niger was grown with powdered corncobs as carbon source. The obtained culture was composed by different proteins (Figure 2, lane 2). High enzymatic activity was detected when xylose was used as substrate confirming the production of endo-xylanases; on the opposite, activity was not detected when avicel, carboxymethylcellulose and p-nitrophenyl-β-D-xylopyranoside were used as substrates suggesting that these activities were not significantly produced in the conditions of the strain growing. Due that several xylanases were produced, a purification step is necessary. When the catalytic properties were evaluated, the obtained values represented an average of the individual properties of each enzyme, making difficult to find stability in production during the process. Thus, purification of the target enzymes was performed using different supports activated with groups capable of physically adsorb proteins, as cationic or anionic, metal chelate and phenyl boronate groups (Table 2). The amount of xylanase adsorbed varied depending on the used support. The maximal adsorption (50%) was produced when Cu2+-chelate support was used for the purification. The adsorbed fraction was desorbed with 20 mM imidazole and analyzed by SDS-PAGE, showing a single 34 kDa-protein with xylanolytic activity (Figure 2, lane 4). This enzyme, here named xylanase I (xyl I), corresponds with the 50% of the total endo-xylanase activity. The purification factor was 4.14 (Table 3).
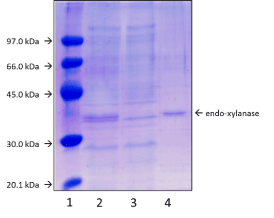
Figure 2. SDS-PAGE gel of the endo-1,4-β-xylanase purification. Lanes: (1) molecular weight markers; (2) crude protein extract from Aspergillus niger; (3) proteins non-adsorbed on Cu-chelate agarose support; (4) endo-1,4-β-xylanase after desorption from Cu-chelate agarose with 20mM imidazole. Experiment was performed as described in Methods.
Table 2. Adsorption of xylanase from A. niger on different supports
Activated supports |
Yield of immobilization (%) |
Carboxymethyl-sepharose |
11 |
IDA-agarose |
17 |
Q-sepharose |
13 |
PEI-agarose |
8 |
Amino-agarose |
3 |
Cu2+-chelate agarose |
50 |
Boronate-agarose |
5 |
Table 3. Protein and activity values after purification of the endo-1,4-β-xylanase from Aspergillus niger on Cu2+-chelate agarose
|
Total protein (mg) |
Total activity (U) |
Specific activity (U.mg-1) |
Yield (%) |
Purification factor |
Crude extract |
26 |
52,8 |
2,03 |
100 |
1,00 |
Endo-xylanase I |
2,7 |
22,7 |
8,41 |
43 |
4,14 |
The use of enzymes in industrial processes makes necessary its immobilization to be used as heterogeneous catalysts. Considering that the enzyme was selectively adsorbed and purified by adsorption on metal-chelate supports, the first tried strategy consisted in using a heterofunctional metal-aldehyde activated support. This support is able to covalently immobilize the selectively adsorbed enzyme through an increase of the incubation pH that increase the nucleophilicity of the amine moieties of the enzyme improving the covalent immobilization to the aldehyde groups on the support surface [38]. The use of this support may permit the purification and covalent immobilization of the enzyme in only one step. The enzyme was immobilized at neutral pH and then the pH was increased to 10 to favor the covalent immobilization. Finally, a reduction process to convert the process into irreversible was performed. This process allowed the complete immobilization of xylanase I (50% of the total xylanase activity) and no leakages were detected after measuring the amount of proteins by the Bradford´s method, confirming that the enzyme was covalently immobilized. However, this protocol (incubation at alkaline pH plus reduction step) yielded inactive derivatives. Considering that a blank of soluble enzyme was active in alkaline conditions as well as in the presence of reductor, the inactivation has to come from the rigidification produced by the covalent multi-interaction with aldehyde groups of the support.
Thus, a protocol in two steps was tried. In a first step the enzyme was purified using Cu2+-activated supports as was described above and then the enzyme was immobilized. Considering that supports activated with different ionic exchangers or others as boronate activated supports were not able to immobilize this protein during the purification, the immobilization was tried using as aldehyde activated support. The immobilization on this support has to be performed at pH 10. This permits the immobilization through the richest region in deprotonated amine groups that corresponds with the richest place in lysines [43]. The purified Xyl I was rapidly immobilized, in fact in 15 minutes more than 80% of this enzyme was bound to the support resulting completely immobilized after 2 hours (Figure 3). Finally the preparation was reduced to stabilize the linkages. The final expressed activity was around 80% compared with that of the soluble enzyme. The observed activity was 13 U/g of derivative.
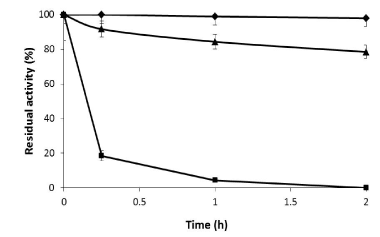
Figure 3. Immobilization of Xylanase I from A. niger on glyoxyl-agarose. Soluble enzyme (♦) supernatant (■) and suspension (▲). The reaction was performed in 100 mM sodium bicarbonate buffer pH 10 at 25°C.
The fraction of enzymes with xylanase activity not adsorbed to Cu2+-support was also offered to glyoxyl support. The immobilization resulted very slow, in fact, after 2 h it was not detectable and only after 48 hours 70% of this fraction was covalently attached to support. This result confirms that the xyl II is an enzyme with different characteristics. Xyl I and xyl II were the two main xylanases in the extract and were the candidates to be studied in later studies. Both proteins were also immobilized using a highly reactive support as CNBr-activated Sepharose. This support is able to immobilize through few linkages having similar properties to that of the soluble enzyme [43]. Using short immobilization times (around 15 minutes) only about 30% of each enzyme (xyl I and xyl II) were separately immobilized keeping unaltered the initial activity.
The thermal stability of the different preparations was evaluated. After incubation at 60° C the half-life of the soluble enzymes xyl I and xyl II was around 18 and 7 minutes respectively. The stabilization factor of the CNBr derivatives was 6 and 5.5 fold compared with the respective soluble enzymes. The activity of the preparations immobilized on glyoxyl-agarose supports remained almost unaltered (Figure 4A). The incubation at higher temperature (75° C) promoted the inactivation of the glyoxyl derivatives with a half-life of 3.5 and 0.8 hours for xyl I and II respectively (Figure 4B). The thermal stabilities of the different preparations of both enzymes suggested again that they were different enzymes.
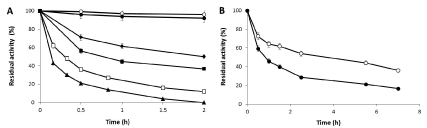
Figure 4. Thermal inactivation courses of different preparations of xylanase I and xylanase II. Soluble Xyl I (□), Soluble xyl II (▲), CNBr-xyl I (♦), CNBr-xyl II (■), Glyoxyl-xyl I (○) and Glyoxyl-xyl II (●). Experiments were carried out at 60°C (A) and 75°C (B) and pH 5.
Optimization of the stability of xyl I-glyoxyl-agarose derivative: Xyl I-glyoxyl derivative immobilized for 2 hours at alkaline pH resulted the most stable derivative. However, the increase of the incubation time at alkaline pH of different enzymes with glyoxyl supports produce derivatives with a higher number of covalent linkages resulting in preparations with different stabilities [36]. Considering this, the just immobilized enzyme was incubated with the support during different times before reduction and then the thermal stability was evaluated. The stability of the different derivatives increased with the incubation time. The maximal stability was obtained after incubation for 20 hours (Figure 5A). Longer incubation time did not yield more stable derivatives (data not shown).
The optimal preparation was compared with the soluble xyl I at 60° C (Figure 5B). While the half-life of the soluble enzyme was around 18 minutes, the optimal preparation had a half-life of around 13.8 days. This resulted in a preparation with a stabilization factor of around 1100 fold compared with the soluble enzyme.
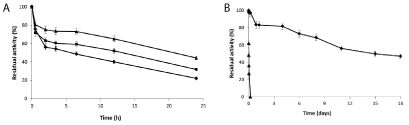
Figure 5. A) Thermal inactivation of Xyl I glyoxyl agarose preparations immobilized incubated for 2 hours and then incubated for different times at pH 10. Derivatives obtained with no extra incubation (♦) and after 5h (■) and 20h (▲) of incubation. Experiments were carried out at 70°C and pH 5. B) Thermal inactivation of xyl I immobilized on glyoxyl-agarose and incubated at pH 10 for 20 h (♦) and the soluble enzyme (▲). Experiments were carried out at pH 5, at 60°C.
Activity of the optimal catalyst in different temperatures: The activity of the optimal immobilized preparation compared with the soluble enzyme (xyl I) was measured at different temperatures. The maximal activity of the soluble enzyme was shown at 55° C and then a decay of the activity was produced until less than 20% at 82° C. The maximal activity of the optimal derivative was obtained at 65° C. The activity was around 50% when it was measured at 82° C (Figure 6). This fact was related with the higher thermal stability of the immobilized preparation compared with the soluble enzyme.
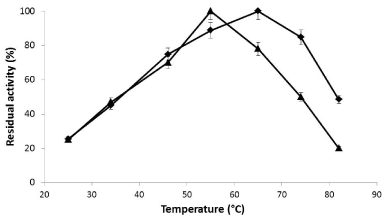
Figure 6. Influence of temperature on the enzymatic activity of xyl I soluble (▲) and immobilized on glyoxyl-agarose (♦).
Hydrolysis of xylan by the optimal glyoxyl derivative
Hydrolysis of beechwood xylan catalyzed by the optimal preparation of endo-1,4-β-xylanase (xyl I) immobilized-stabilized on glyoxyl-agarose was performed at different temperatures (4, 25 and 55°C) and measured using the detection of reducing sugars (Figure 7). As expected the reaction rate strongly depended on the temperature. However the reaction rate is strongly decreased, the maximal conversion was the same independent of the conditions. This conversion value was the maximal using this preparation due that the derivative was performed with a pure endo-xylanase and it is not able to hydrolyze other esterifications with arabinose, glucuronic or acetylations in different positions. In order to compare the reaction curses at different temperatures this value was considered as 100% (maximal conversion measured by DNS method).
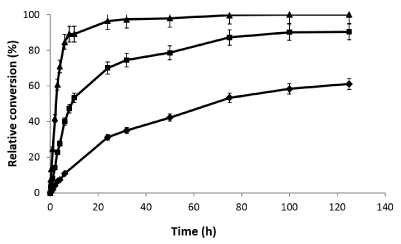
Figure 7. Hydrolysis course of beechwood xylan (20 mg/mL) by the xylanase I immobilized on glyoxyl-agarose at 4°C (♦), 25°C (■) and 55°C (▲) followed by measurement of reducing power (DNS method). Experiments were carried out at pH 5.
The different compounds produced at different times and temperatures were also studied (Table 4). Interestingly, the composition of the different reactions was different depending on the temperature which it was performed. Lower temperatures favored the appearance of compounds with 1-3 units of xylose. The formation of XOS with higher degree of polymerization (DP> 4 units of xylose) was favored by the increase of temperature. For instance, when the conversions were around 42-45% of the maximal obtained with this catalyst, the total amount of XOS 1-XOS 3 was 3.02, 1.64 and 1.18 mg/mL for 4, 25 and 55 °C, respectively and at 90% conversion, the amount of xylose was higher at 25 than at 55°C (1.17 and 0.3 mg/mL respectively); contrarily, the production of XOS 4-XOS 6 was 0.78, 0.91 and 1.06 mg/mL when the reactions were performed at 4, 25 and 55° C respectively. When 90% conversion was obtained, the amount of xylose was higher at 25 than at 55°C (1.17 and 0.3 mg/mL respectively). Contrarily, the production of X4-X6 was 0.78, 0.91 and 1.06 mg/mL when the reactions were performed at 4, 25 and 55° C respectively (Table 4).
Table 4. Xylose and xylo-oligosaccharides (XOS) production after hydrolysis of beechwood xylan (20 mg/mL) by the xylanase immobilized on glyoxyl-agarose, at different temperatures.
Temperature (°C) |
Relative
Conversion |
Time |
Xylose and XOS (mg/mL) |
(%) |
(h) |
X1 |
XOS2 |
XOS3 |
X4 |
X5 |
X6 |
Total |
Control (xylan) |
4.5 |
- |
0.00 |
0.00 |
0.00 |
0.09 |
0.03 |
0.04 |
0.16 |
4 |
5.3 |
0.5 |
0.00 |
0.00 |
0.00 |
0.10 |
0.03 |
0.04 |
0.17 |
6.1 |
1 |
0.00 |
0.00 |
0.00 |
0.11 |
0.04 |
0.05 |
0.20 |
34.3 |
24 |
0.04 |
0.90 |
0.58 |
0.44 |
0.17 |
0.10 |
2.23 |
44.9 |
50 |
0.10 |
1.77 |
1.15 |
0.53 |
0.16 |
0.09 |
3.80 |
55.5 |
75 |
0.20 |
2.61 |
1.46 |
0.50 |
0.13 |
0.08 |
4.98 |
61.5 |
125 |
0.37 |
3.65 |
1.64 |
0.36 |
0.08 |
0.06 |
6.16 |
25 |
8.6 |
0.5 |
0.00 |
0.00 |
0.00 |
0.12 |
0.05 |
0.05 |
0.22 |
12.1 |
1 |
0.00 |
0.05 |
0.04 |
0.16 |
0.07 |
0.06 |
0.38 |
42.7 |
24 |
0.01 |
0.89 |
0.74 |
0.53 |
0.24 |
0.14 |
2.55 |
75.7 |
32 |
0.43 |
4.65 |
1.88 |
0.32 |
0.06 |
0.06 |
7.40 |
90.9 |
125 |
1.17 |
6.69 |
1.26 |
0.15 |
0.04 |
0.05 |
9.36 |
55 |
17.3 |
0.5 |
0.00 |
0.08 |
0.08 |
0.20 |
0.11 |
0.09 |
0.56 |
28.0 |
1 |
0.00 |
0.24 |
0.27 |
0.31 |
0.18 |
0.13 |
1.13 |
44.4 |
2 |
0.00 |
0.56 |
0.62 |
0.53 |
0.32 |
0.21 |
2.24 |
89.6 |
10 |
0.30 |
5.36 |
2.99 |
0.91 |
0.13 |
0.07 |
9.76 |
96.8 |
24 |
0.98 |
8.50 |
3.10 |
0.22 |
0.05 |
0.06 |
12.91 |
100.0 |
100 |
2.20 |
2021 Copyright OAT. All rights reserv
11.17 |
1.05 |
0.14 |
0.05 |
0.07 |
14.68 |
X1) xylose, XOS: 2 xylobiose, 3 xylotriose, 4 xylotetrose, 5) xylopentose, 6) xylohexose. Experiments were performed as described in the Methods section.
Considering that the initial concentration of xylan solution was 20 mg/mL, the highest yield of production of xylose (X1) and XOS (XOS 2-XOS 6) was obtained when the reaction was performed at 55° C and at 100% of xylan conversion, achieving values of 73.4% (14.68 mg/mL), 11% (2.20 mg/mL)corresponding to xylose (Figure 8). However, when the conversion was 96.8%, the production of xylose decreased to 5% (0.98 mg/mL), and 62% (11.93 mg/mL) of prebiotic XOS (X2-X6) was obtained This is a very high conversion considering that in the extract apparently there are not another enzymes such as esterases and others and the rest of linkages with other sugars as arabinose or acetylations cannot be hydrolyzed.
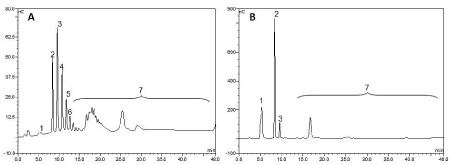
Figure 8. HPAEC-PAD chromatograms of products of hydrolysis of the soluble beechwood xylan (20 mg/mL) by the derivative xyl I-glyoxyl-agarose, after [A] 2 h (44% conversion) and [B] 100 h (100% conversion) of hydrolysis, both at 55°C. 1) xylose, 2) xylobiose, 3) xylotriose, 4) xylotetrose, 5) xylopentose, 6) xylohexose, 7) other XOS of DP>6. Experiments were performed as described in the Methods section.
The extracellular xylanase from A. niger produced with corncob as carbon source was easily purified, immobilized and stabilized in only two steps. The glyoxyl-derivative was around 1100 times more stable than soluble enzyme at 60°C.
The optimal biocatalyst herein described presented qualities that allow the production of functional XOS with high added value from lignocellulosic materials. The high stability of the optimal catalyst allowed performing the hydrolysis at higher temperatures, increasing the rate of reaction and avoiding possible microbial contaminations. The final profile of XOS could be controlled by varying the temperature and time used in the process. The use of the developed process allowed producing 62% of oligo-saccharides of alimentary interest (X2-X6) from soluble xylan.
Ramón Areces foundation financial support is highly recognized. C.C. Aragon thanks Brazilian agencies FAPESP (2008/09332-8) and CAPES (3756-10-6) for financial support. This work was supported by the Spanish Government (AGL2017-84614-C2-1-R).
- Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29: 3-23. [Crossref]
- Azevedo AFC, de Oliva Neto P, Silva DF, Pastore GM (2013) Xylo-oligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res Int 51: 75–85.
- Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, et al. (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67: 577-591. [Crossref]
- Lv Z, Yang J, Yuan H (2008) Production, purification and characterization of an alkaliphilic endo-β-14-xylanase. Enzyme Microb Technol 43: 343–348.
- Biely P, Markovic O, Mislovicová D (1985) Sensitive detection of endo-1,4-beta-glucanases and endo-1,4-beta-xylanases in gels. Anal Biochem 144: 147-151. [Crossref]
- Dhiman SS, Kalyani D, Jagtap SS, Haw JR, Kang YC, et al. (2013) Characterization of a novel xylanase from Armillaria gemina and its immobilization onto SiO2 nanoparticles. Appl Microbiol Biotechnol 97: 1081-1091. [Crossref]
- Sardar M, Roy I, Gupta MN (2000) Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer Eudragit (TM) L-100. Enzyme Microb Technol 27: 672–679.
- Chen H, Liu L, Lv S, Liu X, Wang M, et al. (2010) Immobilization of Aspergillus niger xylanase on chitosan using dialdehyde starch as a coupling agent. Appl Biochem Biotechnol 162: 24–32. [Crossref]
- Pal A, Khanum F (2011) Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of immobilized enzyme. Process Biochem 46: 1315–1322.
- Damásio ARDL, Silva TM, Almeida FBDR, Squina FM, Ribeiro DA, et al. (2011) Heterologous expression of an Aspergillus niveus xylanase GH11 in Aspergillus nidulans and its characterization and application. Process Biochem 46: 1236–1242.
- Gawande PV, Kamat MY (1998) Preparation, characterization and application of Aspergillus sp. xylanase immobilized on Eudragit S-100. J Biotechnol 66: 165-175. [Crossref]
- Gouda MK, Abdel-Naby MA (2002) Catalytic properties of the immobilized Aspergillus tamarii xylanase. Microbiol Res 157: 275–81.
- Rogalski J, Szczodrak J, Dawidowicz A, Ilczuk Z, Leonowicz A (1985) Immobilization of cellulase and D-xylanase complexes from Aspergillus terreus F-413 on controlled porosity glasses. Enzyme Microb Technol 7: 395–400.
- Aragon CC, Santos AF, Ruiz-Matute AI, Corzo, N, Guisan JM, et al. (2013) Continuous production of xylooligosaccharides in a packed bed reactor with immobilized–stabilized biocatalysts of xylanase from Aspergillus versicolor. J Mol Catal B Enzym 98: 8–14.
- Lin YS, Tseng MJ, Lee WC (2011) Production of xylooligosaccharides using immobilized endo-xylanase of Bacillus halodurans. Process Biochem 46: 2117–2121.
- Kapoor M, Kuhad RC (2007) Immobilization of xylanase from Bacillus pumilus strain MK001 and its application in production of xylo-oligosaccharides. Appl Biochem Biotechnol 142: 125-138. [Crossref]
- Nagar S, Mittal A, Kumar D, Kumar L, Gupta VK (2012) Immobilization of xylanase on glutaraldehyde activated aluminum oxide pellets for increasing digestibility of poultry feed. Process Biochem 47: 1402–1410.
- Manrich A, Komesu A, Adriano WS, Tardioli PW, Giordano RLC (2010) Immobilization and stabilization of xylanase by multipoint covalent attachment on agarose and on chitosan supports. Appl Biochem Biotechnol 161: 455–467.
- Dhiman SS, Jagtap SS, Jeya M, Haw JR, Kang YC, et al. (2012) Immobilization of Pholiota adiposa xylanase onto SiO₂ nanoparticles and its application for production of xylooligosaccharides. Biotechnol Lett 34: 1307-1313. [Crossref]
- Sarbu A, de Pinho MN, Freixo MDR, Goncalves F, Udrea I (2006) New method for the covalent immobilization of a xylanase by radical grafting of acrylamide on cellulose acetate membranes. Enzyme Microb Technol 39: 125–130.
- Aragon CC, Mateo C, Ruiz-Matute AI, Corzo N, Fernandez-Lorente G, et al. (2013) Production of xylo-oligosaccharides by immobilized-stabilized derivatives of endo-xylanase from Streptomyces halstedii. Process Biochem 48: 478–483.
- Ai Z, Jiang Z, Li L, Deng W, Kusakabe I, et al. (2005) Immobilization of Streptomyces olivaceoviridis E-86 xylanase on Eudragit S-100 for xylo-oligosaccharide production. Process Biochem 40: 2707–2714.
- Maalej-Achouri I, Guerfali M, Gargouri A, Belghith H (2009) Production of xylo-oligosaccharides from agro-industrial residues using immobilized Talaromyces thermophilus xylanase. J Mol Catal B Enzym 59: 145–152.
- Yan X, Wang X, Zhao P, Zhang Y, Xu P, et al. (2012) Xylanase immobilized nanoporous gold as a highly active and stable biocatalyst. Microporous Mesoporous Mater 161: 1–6.
- Edward VA, Pillay VL, Swart P, Singh S (2002) Immobilization of xylanase from Thermomyces lanuginosus SSBP using Eudragit S-100. S Afr J Sci 98: 553–554.
- Li L, Zhua Y, Huang Z, Jiang Z, Chena W (2007) Immobilization of the recombinant xylanase B (XynB) from the hyperthermophilic Thermotoga maritima on metal-chelate Eupergit C 250L. Enzyme Microb Technol 41: 278-285.
- Simpson HD, Haufler UR, Daniel RM (1991) An extremely thermostable xylanase from the thermophilic eubacterium Thermotoga. Biochem J 277: 413–417.
- Cano Á, Minguillón C, Palet C (2006) Immobilization of endo-1,4-β-xylanase on polysulfone acrylate membranes. J Membr Sci 280: 383–388.
- Xu Z, Miao Y, Chen JY, Jiang X, Lin L, et al. (2011) Co-immobilization mechanism of cellulase and xylanase on a reversibly soluble polymer. Appl Biochem Biotech 163: 153–161.
- Akdemir ZS, Demir S, Kahraman MV, Apohan NK (2011) Preparation and characterization of UV-curable polymeric support for covalent immobilization of xylanase enzyme. J Mol Catal B Enzym 68: 104–108.
- Dumitriu S, Chornet E (1997) Immobilization of xylanase in chitosan-xanthan hydrogels. Biotechnol Prog 13: 539–545.
- Madakbas S, Demir S, Kahraman MV (2013) Xylanase immobilization on functionalized polyaniline support by covalent attachment. Starch-Stärke 65: 146–150.
- Benedetti ACEP, Costa ED, Aragon CC, Santos AF, Goulart AJ, et al. (2013) Low-cost carbon sources for the production of a thermostable xylanase by Aspergillus niger. Rev Cienc Farm Basica Apl 34: 25–31.
- Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31: 426–428.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254. [Crossref]
- Guisán JM (1988) Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzyme Microb Technol 10: 375–382.
- Mateo C, Abian O, Fernández-Lafuente R, Guisan JM (2000) Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support-polyethylenimine composites. Biotechnol Bioeng 68: 98–105.
- Mateo C, Bolivar JM, Godoy C, Rocha-Martin J, Pessela BC, et al. (2010) Improvement of enzyme properties with a two-step immobilization process on novel heterofunctional supports. Biomacromolecules 11: 3112–3117. [Crossref]
- Armisen P, Mateo C, Cortés E, Barredo JL, Salto F, et al. (1999) Selective adsorption of poly-His tagged glutaryl acylase on tailor-made metal chelate supports. J Chromatogr A 848: 61–70.
- Henley JP, Sadana A (1986) Deactivation theory. Biotechnol Bioeng 28: 1277-1285. [Crossref]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [Crossref]
- Heukeshoven J, Dernick R (1985) Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis 6: 103–112.
- Mateo C, Abian O, Bernedo M, Cuenca E, Fuentes M, et al. (2005) Some special features of glyoxyl supports to immobilize proteins. Enzyme Microb Technol 37: 456–462.



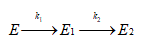
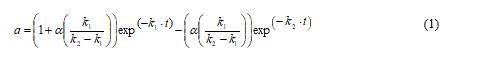
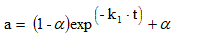 (2)
(2)





