Abstract
Introduction: Friction blisters, caused by prolonged friction of the skin against a sock or footwear, are one of the most common minor dermatologic lesions, causing discomfort and pain. Prevention strategies seek to reduce friction, pressure and/or shear stress. COMPEED® hydrocolloid plasters for Blisters protect and cushion foot blisters and provide immediate pain and pressure relief.This clinical investigation aimed at evaluating the clinical performance of COMPEED® hydrocolloid plasters in providing adequate protection against rubbing to prevent blisters development.
Method: This was an interventional subject-centered, pre/post, randomized, open labelled superiority investigation performed on paired groups; each subject was her/his own control, by applying one COMPEED®hydrocolloid plaster on one foot and one regular plaster on the other. Included subjects were ≥ 14 years-old, at risk of developing blisters and intending to participate in targeted sports events likely to induce blisters on foot.
Results: From June 27th to July 10th, 2021, 607 subjects were enrolled during 4 sports events of whom 604 (99.5%) were randomized, and 310 (51.1%) completed the post-event questionnaire. Among the later, 45 subjects (14.5%) reported occurrence of hot spots/blisters under the COMPEED® hydrocolloid plaster; 79 subjects (25.5%) under regular plaster; 111 subjects (36.9%) on foot locations outside of the plasters. Occurrence under COMPEED® hydrocolloid plaster was significantly lower compared to regular plaster (p=0.0001). Global impression and satisfaction of subjects on the plaster’s capacity to prevent blister/hot spot development was significantly in favor of COMPEED®hydrocolloid plasters (77.9% vs 20.7%; p<.0001).
Conclusion: We demonstrated that COMPEED® hydrocolloid plasters prevent the occurrence of foot blisters/hot spots under the plaster’s location after an event likely to induce blisters. Overall, our results demonstrate the subjects’ higher perception of satisfaction and efficacy. Excepting the randomization, this investigation emulated real-life use of COMPEED®hydrocolloid plasters.
Keywords
hydrocolloid plasters, foot blister, prevention, skin protection, skin healing
Introduction
Prolonged friction of the skin rubbing against another surface (such as a sock or footwear), causes shear forces within the skin and erythema in and around the rubbing zone. The area encompassed by the erythema is commonly referred to as the “hot spot”, due to the increased burning sensation [1]. Continuous shear further causes epidermal cell necrosis followed by accumulation of serum-like fluid, filling the intra-epidermal split and leading to formation of a blister [2]. Friction blisters are one of the most common minor dermatologic lesions of the human skin causing discomfort and pain, and may constitute entry portals for infections [3], which if aggravated, may trigger cellulitis or sepsis and even toxic shock. Among risk factors, affecting friction forces [1,2], intermediate levels of heat and moisture tend to potentiate the development of blisters [2]. It is, therefore, not surprising that foot blister occurrence is particularly high in certain sportswhichplace considerable performance demands on the feet. Estimates show that up to 39% of marathon runners, over 40% of soldiers in training, and over 50% of hikers are affected by this condition [4]. Blister severity varies from hot spot to intact blister (bubble filled with clear fluid), blood blister (bubble filled with blood), torn blister (blister not sealed by skin), bleeding blister, deroofed blister (blister upper skin, or roof, rubbed off) [5,6].
Prevention strategies seek to reduce friction, pressure and/or shear stress. There is currently no evidence supporting efficacy of preventive measures such as sock fibres, tapes, antiperspirants, lubricants [7]. Moreover, the aim of treating foot blisters is to minimize pain, limit blister size and severity, heal the skin and prevent complications such as skin infections and optimize return to full activities [8]. One of the most frequent treatment strategies consists of covering and protecting the blister with a plaster, in order to keep the blister roof intact [9].
COMPEED® hydrocolloid plasters for Blisters relieve blister pain and discomfort by acting as a second skin thus protecting from further skin shearing/rubbing. They could therefore be indicated for the prevention of blister occurrence. The goal of this clinical investigation was to evaluate the clinical performance/effectiveness of COMPEED® hydrocolloid plasters as compared to regular non hydrocolloid plasters in the prevention of blisters in the population participating in sports events likely to induce blisters, such as running, trail-running, hiking.
Materials and Methods
Study Design and subjects
This was an interventional, subject-centered, pre/post (pre, as subject inclusion and baseline data collection were done before the sport event and post as the evaluation was done after the event with no additional follow-up), randomized, open-labelled superiority investigation performed on paired groups conducted from June 2021 until August 2021 (date of last questionnaire completion of last included subject) and enrolling subjects during 4 sports events in France.
Eligible subjects were aged 14 years old or more at enrolment and participated in an event likely to induce foot hot spots/blisters (running, trail-running, hiking). Subjects understood the full nature and purpose of the study, were willing to sign a written consent (or parent/legal representative consent as applicable) and were willing to complete the French questionnaire booklet. Finally, they had to be covered by a healthcare insurance. Those excluded from the investigation: subjects planning to use other specific plaster or preventive material/treatment during the event; subjects having participated in a consumer testing for blister plasters in the past two weeks; presenting with any uncontrolled systemic disease (e.g. diabetes, cardiovascular disease, etc.) or with a contraindication/hypersensitivity/allergy to any component of any plaster.
The expected subject’s study duration was maximum 3 days (the day when the subject picked-up his/her event materials and performed his/her study enrolment up to 48h post event for self-completion of the electronic Patient Reported Outcome (ePRO) questionnaire. The end of the study was defined as the date of completion of the last expected ePRO questionnaire.
Investigational products
The COMPEED® hydrocolloid plasters for Blisters considered in this clinical investigation are medical devices intended to be used for the protection and cushioning of foot blisters. They are non‐invasive and non‐sterile dressings composed of: a semi-permeable membrane (polyurethane film) allowing the skin/wound to breathe and protecting it from external contaminants such as dirt and bacteria; a hydrocolloid adhesive, adhering to skin and providing wound micro-environment moisturizing capabilities that contributes to the healing process. Five hydrocolloid plasters of the COMPEED® hydrocolloid plasters range were used during the investigation differing in size and shape, according to the expected locations/sizes of blisters as per previous experiences from the participants: COMPEED® Blister Small (herein referred to as Small), COMPEED® Blister Medium (Medium), COMPEED® Blister On Toes (On Toes), COMPEED® Blister Underfoot (Underfoot) and COMPEED® Sports Heel Blister (Medium Extreme). The chosen comparator was HANSAPLAST UNIVERSAL, a CE marked regular non hydrocolloid plaster, available in 4 different sizes (herein, the two smaller sizes grouped in “small” category and the two bigger ones in “big” category). The choice of such regular plasters has been driven by their wide availability and common use in the general European population.
Ethical aspects
The protocol was reviewed and approved by the French Ethics Committee (EC) Nord-Ouest I, on June 17th, 2021, prior to inclusion of subjects. This clinical investigation was conducted in compliance with the latest version of the Declaration of Helsinki (October 2013), the international standard EN ISO 14155:2020 ('Clinical Investigation of medical devices for human subjects – Good Clinical Practice'), Regulation (EU) 2017/745 of 5 April 2017, MEDDEV 2.12/2 Post market clinical follow-up studies, for France, French Public Health Code. Written informed consent was obtained by subjects before start of any study-related procedure.
Study procedures
Subjects were made aware of the clinical investigation through an awareness campaign prior to each selected event. They performed their study enrolment when picking up their event’s materials (i.e. at least their race/event bib number), prior to the event and presenting to the study booth. A dedicated trained study team, including a study nurse, explained the investigation, verified subject’s investigation understanding and eligibility criteria and obtained each subject informed consent to participate in the investigation. The study nurse also collected the randomization variables (as described below).
An inclusion package was provided to each randomized subject, including plasters and identification card to access the dedicated and secured subject website for ePRO questionnaire completion. The procedure to put COMPEED®hydrocolloid plaster on one foot and regular plaster on the other foot prior to the event was explained by the study team to each randomized subject and a written procedure was also given in the inclusion package as a memory tip. Subjects were invited to keep backup plasters in their pockets to change them if needed, during the event.
On the day of the event and prior to the race, each subject placed both plasters (COMPEED®hydrocolloid plaster and regular plaster) according to the procedure given as per the randomization results. Upon event completion, subjects completed the ePRO questionnaires. In this questionnaire, each subject evaluated: hot spot/blister occurrence under COMPEED® hydrocolloid plaster, under regular plaster and outside plaster’s location; blister characteristics (e.g. location, size, severity) upon plaster self-removal. If the subject was not capable of determining hot spot/blister formation at the end of the event (i.e. if the plaster was still in place), s/he had access to the self-completion ePRO questionnaire for up to 2 days after the event execution.
Randomization
Each subject applied a COMPEED®hydrocolloid plaster on one foot and a regular plaster on the other foot (the choice between small or big plasters was left to the user). The foot where COMPEED®hydrocolloid plaster was applied, was randomized. The randomization was done centrally using an interactive web response system (IWRS). For each participating subject, both plasters were applied at the same location on each foot (toe, heel, underfoot or arch/top of foot). The randomization consisted in a permuted block randomization with size block randomly assigned to 4 and 6. To preserve the blinded concealment principle, (a) the randomization list was generated, loaded within the IWRS and only accessible by a study independent statistician and (b) the block’s size remained unknown to any operational stakeholders until the end of the recruitment period.
An additional algorithm (i.e. decision tree) was integrated to the IWRS in order to indicate the location on foot and type of COMPEED® plaster to be used. For a given subject, the algorithm took into account the foot area (i.e. heel, underfoot, arch/top of the foot, toe) where a blister was most likely to occur as well as the size and severity of the potential blisters as per prior subject experience.
1.4.1.6.Sports events characteristics
Subjects included in the study participated in sports events where distances varied between 10km to 175km, according to the event, and could therefore last for several hours.
Primary outcome
The primary outcome was to describe the percentage of subjects presenting at least one blister ± hot spot under COMPEED®hydrocolloidplaster, under regular plaster and outside the plaster’s location at the end of the event. Moreover, percentage of subjects presenting at least one blister ± hot spot under COMPEED®hydrocolloidplaster was compared to regular plaster.
Secondary outcomes
All secondary outcomes were assessed for each type of COMPEED® hydrocolloid plaster compared to regular plaster, unless stated otherwise.
Hot spot and blister occurrence and characteristics: described as the number of subjects presenting at least one blister or hot spot at the plaster location (COMPEED® hydrocolloid plaster compared to regular plaster) at the end of the event; and in case of blister at the plaster location, the overall comparison of the blister severity was described using a 6-point scale (1=Hot spot; 2=Intact blister; 3=Blood blister; 4=Torn blister; 5=Bleeding blister; 6=Deroofed blister).
Plaster staying-in-place: rated using a 7-point Likert scale and overall comparison of the plaster’s staying-in-place profile (1=‘very bad’ and 7=‘excellent’) and rubbing-off (plaster’s edges rolled) (1= ‘Complete rub-off’ and 7=’No rub-off at all’).
Duration: overall comparison of the distance ran with the plaster staying in place.
Global perception/satisfaction: subject’s global impression of COMPEED® hydrocolloid plaster compared to regular plaster was rated using a 7-point Likert scale (1=‘not at all’ and 7=‘excellent prevention’); subject’s overall satisfaction using a 7-point Likert scale (1=‘very unsatisfied and 7=‘very satisfied); subject’s likeliness to recommend COMPEED® hydrocolloid plaster compared to regular plaster to family/friends as evaluated post-event using a 5-point Likert scale (1=’certainly not’ and 5=’absolutely’); subject’s willingness to use each COMPEED® hydrocolloid plaster compared to regular plaster again for blister prevention.
Medical Device Vigilance of COMPEED® hydrocolloid plasters only: all adverse device events related to each COMPEED® hydrocolloid plaster, anticipated or unanticipated.
Statistical analysis
Five analysis populations were defined:
- Enrolled setincluded all subjects who provided their information consent. The study subject’s disposition was described on the enrolled analysis set.
- Randomized set consisted of all subjects randomized in the investigation.
- Reference set consisted of all randomized subjects for whom the variables concerning the primary endpoint (i.e. the type of plaster and the occurrence of hot spot/blister) have been completed and for whom the event was terminated or interrupted prematurely after running/walking a distance considered significant with the regard to the risk of appearance of blisters (at least 1 km) or upon hot spot/blister occurrence preventing the subjects to carry on the event. All efficacy endpoints were analysed on the reference set. The demographics and other baseline characteristics were described on the reference.
- Safety set consisted of all randomized subjects having applied at least one COMPEED® plaster. The safety data, including the analysis of adverse device effects with COMPEED®hydrocolloid plasters were described on the safety set.
- Per Protocol (PP) set included all subjects of the reference set free from major protocol deviation, which can bias the efficacy results.
All analyses were performed according to the plaster brand except for safety data which was described for each COMPEED®hydrocolloid plaster. All statistical analyses were performed using Statistical Analysis Systems (SAS®) release 9.4.Descriptive summary measures were presented for hot spot/blisters occurrence rates, demographic and baseline characteristics. Comparisons between COMPEED®hydrocolloid and regular plaster used tests for paired datawhen applicable: Mc Nemar test was used for binary data and a paired t-test or its non-parametric equivalent for ordinal and continuous data. Two-sided confidence intervals (CI) were given where appropriate and provided 95% confidence. The Agresti-Coull 95% CI was provided for discrete variables and the Wald 95% CI for continuous variables. The Adjusted Wald 95% CI of the inter-group difference was also produced to each test along with the number of subjects with non-missing data for both plasters on which p-value, difference and corresponding 95% CI were calculated. The statistical significance level of the various two-sided tests performed was 5.0%.
Results
Subject’s disposition and baseline characteristics
From June 27th to July 10th, 2021, 607 subjects were enrolled during 4 sports events of whom 604 (99.5%) were randomized (Figure 1). Among these subjects, 293 subjects (48.5%) did not enter information in the post-event ePRO questionnaire. Further, 311 randomised subjects (51.2%) had applied a COMPEED® hydrocolloid plaster and completed the post-event ePRO questionnaire (i.e. safety set). Within the safety set, only one subject did not complete the primary endpoint data in the ePRO questionnaire, therefore the reference set was composed of 310 subjects. These 310 subjects applied one plaster of each brand, of which 72 (23.2%) Small, 72 (23.2%) Medium, 42 (13.5%) On Toes, 35 (11.3%) Underfoot and 89 (28.7%) Sports Heel/Extreme. Finally, 280 out of 310 subjects (90.3%) were free from any major protocol deviations (Figure 1).
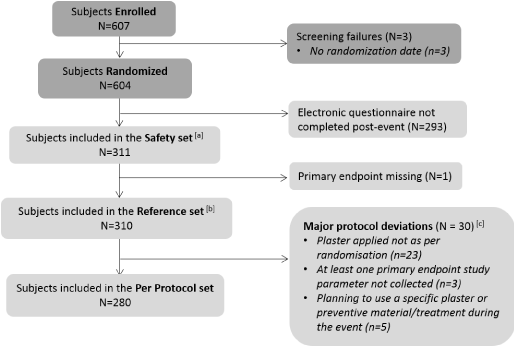
Figure 1. Study Flow chart. Flow chart of participants enrolled, randomized and included in the analysis sets of the study. [a] All randomized subjects having applied at least one COMPEED® plaster. [b] All randomized subjects for whom the variables concerning the primary endpoint (the type of plaster and the occurrence of hot spot/blister) have been completed and the event was terminated or interrupted prematurely after running/walking at least 1 km or upon blister/hot spot occurrence preventing the subjects to carry on the event. [c] A subject may have several protocol deviations
Median age of subjects was 42 years old (ranging from 15 to 71 years old) and 92.9% of subjects were aged between 18-60 years old. Among 310 subjects, 58.7% were male and 75.4% had a normal weight (Table 1); 43.7% performed a physical activity 2-3 times per week or more than 3 times per week (47.6%); 73.6% had normal feet (as opposed to flat or hollow foot) and 68.3% of subjects self-evaluated they had sensitive skin (Table 1); 95.8% of subjects reported they had no disease or chronic controlled condition.
Table 1. Subjects’ baseline characteristics
|
Reference Set (N=310) |
Age (years) |
Mean ± SD |
42.22 ± 11.46 |
|
Min; Max |
15.0 ; 71.0 |
Age categories (years) |
< 18 years |
1 (0.3%) |
|
[18 - 60[years |
288 (92.9%) |
|
≥ 60 years |
21 (6.8%) |
Gender |
Female |
127 (41.0%) |
|
Male |
182 (58.7%) |
|
Does not want to reply |
1 (0.3%) |
Body mass index categories (kg/m²)* |
< 18.5 ‘Underweight’ |
16 (5.2%) |
|
[18.5 ; 25[‘Normal weight’ |
233 (75.4%) |
|
[25 ; 30[‘Overweight’ |
57 (18.4%) |
|
≥ 30 ‘Obese’ |
3 (1.0%) |
Level of physical activity* |
Less than 1 time/month |
0 (0.0%) |
|
2-3 times/month |
9 (2.9%) |
|
1 time/week |
18 (5.8%) |
|
2-3 times/week |
135 (43.7%) |
|
Superior to 3 times/week |
147 (47.6%) |
Sensitive foot skin* |
No |
98 (31.7%) |
|
Yes |
211 (68.3%) |
Type of foot** |
Flat foot (Fallen arch) |
38 (12.8%) |
|
Normal foot |
218 (73.6%) |
|
Hollow foot (High arch) |
40 (13.5%) |
Values are presented as mean ± SD or numbers (%).* 1 missing value, ** 14 missing values.
1.5.1.2.Primary outcome
Hot spot/blister occurrence rate on the foot: After the event, subjects reported presence of hot spot or blister (whichever one occurred) under the applied plaster and on foot locations outside the plaster (Table 2). Forty-five subjects (14.5%) reported hot spot or blister occurrence under COMPEED® hydrocolloid plaster; 79 subjects (25.5%) reported hot spot or blister occurrence under the regular plaster; 111 subjects (36.9%) reported hot spot or blister occurrence on foot locations outside plasters.
Table 2. Hot spot or blister occurrence rate on the foot
|
COMPEED® plasters
(N = 310) |
Regular plasters
(N = 310) |
Occurrence of hot spot/blister under plaster |
No |
265 (85.5%) |
231 (74.5%) |
Yes |
45 (14.5%) |
79 (25.5%) |
Presence of at least one hot spot or blister outside any of the plaster location* |
No |
190 (63.1%) |
190 (63.1%) |
Yes |
111 (36.9%) |
111 (36.9%) |
Data are presented as n (%);*9 values missing.
Hot spot/blister occurrence rate under COMPEED® hydrocolloid plaster was statistically significantly smaller compared to regular plaster (25.5%; p=0.0001) (Table 3). The sensitivity analysis performed on the 280 subjects free from major deviations (PP set) also showed a statistically significant hot spot/blister occurrence rate difference between the 2 plaster brands.
Table 3. Comparison Blister occurrence rate according to plaster brand
|
COMPEED®plasters
(N = 310) |
Regular plasters
(N = 310) |
P-value
Difference (n) [95% CI][a] |
Occurrence of hot spot/blister under plaster |
No [95% CI] |
265 (85.5%)
[81.09%;88.98%] |
231 (74.5%)
[69.36%;79.04%] |
0.0001
11% (310) [5.4%;16.4%] |
Yes [95% CI] |
45 (14.5%)
[11.02%;18.91%] |
79 (25.5%)
[20.96%;30.64%] |
Data are presented as n (%); 95% CI of the difference, n is the number of subjects with no missing data for both plasters on which p-value and the difference and corresponding 95% CI are calculated.
Secondary outcomes
Hot spot and blister occurrence under plasters: The number of individual hot spots and blisters present distinctly under applied plasters was further described in Table 4. Among the 310 subjects, 10.0% presented at least one blister and 4.2% presented at least one hot spot under their COMPEED® hydrocolloid plaster vs 14.8% and 10.6% under regular plaster (Table 4). Mean (± SD) number of blisters was 1.13 (± 0.43) vs 1.09 (± 0.28), under COMPEED® hydrocolloid plaster and regular plaster respectively. Mean (± SD) number of hot spots was 1.08 (± 0.28) vs 1.00 (± 0.00), under COMPEED® hydrocolloid plaster and regular plaster respectively. In terms of number of reported blisters, over 90.0% of subjects reported just 1 blister and 1 hot spot under COMPEED® hydrocolloid plaster or regular plaster.
Table 4. Presence of blister(s) and hot spot(s) under the plaster according to plaster brand
|
COMPEED® plasters
(N = 310) |
Regular plasters
(N = 310) |
At least one blister under plaster |
No |
279 (90.0%) |
264 (85.2%) |
Yes |
31* (10.0%) |
46 (14.8%) |
Number of blister under plaster |
Mean ± SD |
1.13 ± 0.43 |
1.09 ± 0.28 |
Number of blister under plaster (categories) |
1 |
28 (90.3%) |
42 (91.3%) |
2 |
2 (6.5%) |
4 (8.7%) |
3 |
1 (3.2%) |
0 (0.0%) |
Presence of at least one blister of size |
< 1 cm |
10 (32.3%) |
18 (39.1%) |
[1 ; 2] cm |
15 (48.4%) |
21 (45.7%) |
> 2 cm |
7 (22.6%) |
7 (15.2%) |
At least one hot spot under plaster |
No |
297 (95.8%) |
277 (89.4%) |
Yes |
13 (4.2%) |
33 (10.6%) |
Number of hot spot under plaster |
Mean ± SD |
1.08 ± 0.28 |
1.00 ± 0.00 |
Number of hot spot under plaster (categories) |
1 |
12 (92.3%) |
33 (100.0%) |
2 |
1 (7.7%) |
0 (0.0%) |
Presence of at least one hot spot of size |
< 1 cm |
8 (61.5%) |
19 (57.6%) |
[1 ; 2] cm |
3 (23.1%) |
11 (33.3%) |
> 2 cm |
2 (15.4%) |
2 (6.1%) |
Severity profile of hot spots and blisters under plaster |
Not severe |
31 (70.5%) |
61 (78.2%) |
Severe |
13 (29.5%) |
17 (21.8%) |
Data are presented as n (%) or mean ± SD (standard deviation); Blister includes intact blisters, blood blisters, torn blisters, bleeding blisters and deroofed blisters. Severe = Blood blister + Torn blister + Bleeding blister + Deroofed blister / Not severe = Hot spot + Intact blister. * 1 missing value for severity.
Occurring hot spot/blister size and location
Under both plaster brands, in more than 80.0% of subjects, blisters and hot spots sizes ranged between <1cm and 2 cm. Over 70.0% of subjects reported that their hot spots and blisters were not severe (Table 4). Hot spots/blisters were mainly present on toes or arch/top of foot of 47.0% of the 45 subjects with hot spot/blister under COMPEED® hydrocolloid plaster and 32.0% of the 79 subjects with hot spots/blisters under regular plaster. It is noteworthy, that subjects had positioned plasters before the event, according to their previous experience, meaning 55.0% of subjects had positioned plasters on toes or arch/top of foot. Even though presence of hot spots and blisters was quite low under COMPEED® hydrocolloid plaster, occurrences according to COMPEED® hydrocolloid plaster type were quite comparable.
Subject’s global Impression and satisfaction
Statistically significantly more subjects (77.9% vs 20.7%, p<.0001) reported good ability of COMPEED® hydrocolloid plaster to prevent blister/hot spot development vs the regular plaster (Figure 2a). Likewise, 83.8% of subjects reported good satisfaction with COMPEED® hydrocolloid plaster to prevent hot spot/blister development during the event compared to only 31.9% of subjects for regular plasters (Figure 2b). Finally, subjects were significantly more likely to recommend COMPEED® hydrocolloid plaster to family or friends as compared to regular plaster (90.6% vs. 23.2% respectively; p<.0001). Likewise, 285 subjects (92.5%) were willing to use COMPEED® hydrocolloid plaster again while only 76 subjects (25.4%) were willing to use regular plaster again to prevent blister formation (p<.0001).
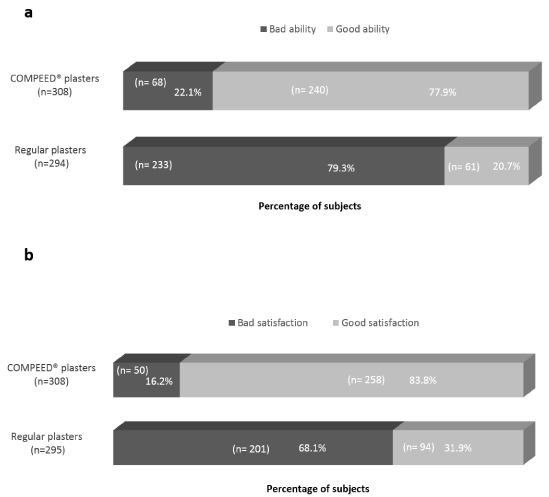
Figure 2. Global impression and satisfaction on plaster’s ability to prevent hot spot/blister development during the event. (a) Global impression; Bad ability = Not at all + Little + Moderate + Fairly good; Good ability = Good + Very good + Excellent. COMPEED® plasters: 2 values missing; regular plasters: 16 values missing. P-value <.0001 and difference (n) [95% CI]: -56.8% (294) [-62.7%;-50.1%]. (b) Global satisfaction; Bad satisfaction = Very unsatisfied + moderately unsatisfied + A little unsatisfied + Neither satisfied nor unsatisfied; Good satisfaction = A little satisfied + Moderately satisfied + Very satisfied. COMPEED®plasters: 2 values missing; regular plasters: 15 values missing. P-value <.0001 and difference (n) [95% CI]: 52.5% (295) [-58.7%;-45.7%]
Plaster duration on skin
COMPEED®hydrocolloid plasterswere reported to stay in place after the event by 273 subjects (88.6%) while this was true for 143 subjects (49.0%) for regular plasters (p <.0001) (Table 5). COMPEED®hydrocolloid plaster “staying-in-place” profile was assessed as “good” by 82.5% of subjects as compared to only 20.5% of subjects for regular plaster. Conversely, 79.5% of subjects reported that the plaster did not stay in place (staying-in-place profile) for regular plasters as compared to 17.5% of subjects for COMPEED®hydrocolloid plaster. Likewise, mean (± SD) “staying-in-place” score was significantly higher for COMPEED® (5.68 ± 1.36) than regular plasters (3.15 ± 1.58) (p<.0001) (Table 5). Moreover, 84.4% of subjects found COMPEED® plaster’s rubbing-off profile (absence of plaster’s edges rolled) as “good” as compared to 53.4% of subjects for regular plasters (p <.0001) (Table 5). Plaster “staying-in-place” was comparable among all COMPEED®hydrocolloid plaster types, with “good staying-in-place”” profile ranging from 71.4% to 91.2% of subjects. Likewise, 79.4% to 86.1% of subjects reported plasters among the different plaster types did not rub off (had “good rubbing off” profile). Interestingly, COMPEED®hydrocolloid plasters stayed in place in 87.0% of subjects vs. 47.0% for regular plasters after ≥ 20 km. Similarly, COMPEED® plasters stayed in place for 96.6% and 77.4% of subjects who ran between 1-2 hours to over 16 hours, respectively, as opposed to regular plasters which stayed in place in 39.3% and 32.1% subjects (Figure 3).
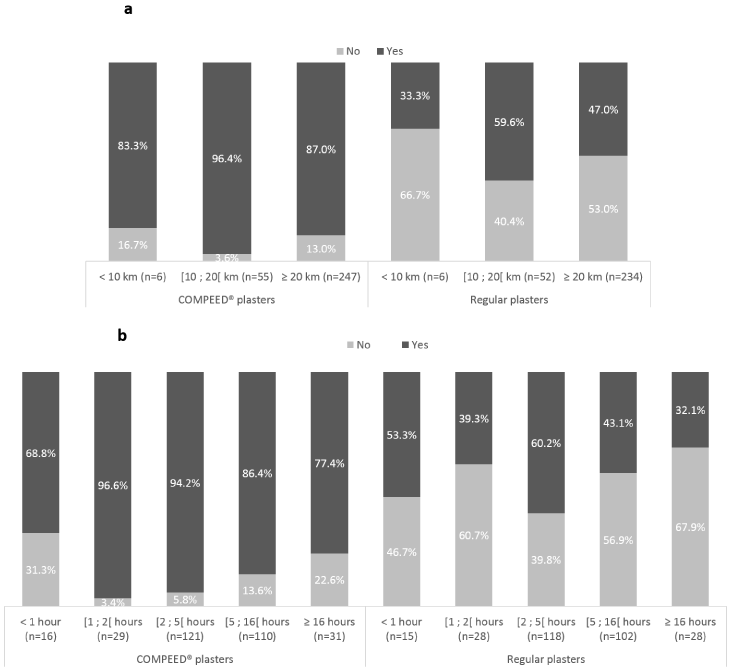
Figure 3. Plaster duration according to plaster brand. (a) Percentage of subjects reporting plaster still on skin according to distance ran (km); COMPEED® plasters: 2 values missing (≥20km); Regular plasters: 3 and 15 values were missing, respectively in 10-20km and ≥20km (b)Percentage of subjects reporting plaster still on skin according to duration ran (hours). COMPEED® plasters: 2 values missing (2h-5h); regular plasters: 1, 1, 5, 8 and 3 values missing (each duration respectively)
Table 5. Plaster on skin according to plaster brand
|
COMPEED® plasters
(N = 310) |
Regular plasters
(N = 310) |
P-value
Difference (n) [95% CI] [a] |
Plaster still on skin after event |
N |
308* |
292** |
<.0001
-39.4% (292)
[-45.2%;-33.0%] |
No |
35 (11.4%) |
149 (51.0%) |
Yes |
273 (88.6%) |
143 (49.0%) |
“Staying-in-place” profile[b] |
N |
308* |
302**** |
<.0001
-62.3% (302)
[-67.9%;-55.7%] |
Bad |
54 (17.5%) |
240 (79.5%) |
Good |
254 (82.5%) |
62 (20.5%) |
“Staying-in-place” score |
N (mv) |
308* |
302*** |
<.0001
2.54 (302) [2.312;2.767] |
Mean ± SD |
5.68 ± 1.36 |
3.15 ± 1.58 |
Rubbing-off profile[c] |
N (mv) |
308* |
305**** |
<.0001
-30.8% (305)
[-37.0%;-24.2%] |
Bad |
48 (15.6%) |
142 (46.6%) |
Good |
260 (84.4%) |
163 (53.4%) |
Rubbing-off score |
N (mv) |
308 (2) |
305 (5) |
<.0001
1.321 (305) [1.083;1.56] |
Mean ± SD |
5.74 ± 1.48 |
4.42 ± 1.98 |
Data are presented as n (%) or mean ± SD (standard deviation); * 2 missing values, ** 18 missing values, *** 8 missing values, ****5 missing values. 95% CI of the difference, n is the number of subjects with no missing data for both plasters on which p-value and the difference and corresponding 95% CI are calculated. Bad “Staying-in-place”= scores <5 / Good “Staying-in-place” = scores ≥5; Bad rubbing off profile = scores <5 / Good rubbing off profile = scores ≥5.
Adverse events and Adverse Device effects
Overall, during the clinical investigation, only 4 adverse device effects (ADEs) (1.3%) were reported by subjects concerning each COMPEED® hydrocolloid plaster type (except COMPEED® Underfoot). None of the reported ADEs were among the most frequently listed ADEs (i.e., application site pain/burning/stinging, irritation/itching/redness/pruritus, swelling, skin torn upon plaster removal, condition worsened, wound appearance at the application site/blister infection); subjects reported blister formation under the plaster.
Discussion
This subject-centred, pre/post, randomized, open-labelled clinical investigation demonstrated the superiority of COMPEED® hydrocolloid plasters as compared to regular plasters in preventing the occurrence of foot hot spots or blisters on plasters’ location after an event likely to induce blisters. Our clinical investigation was conducted in real-life settings (excepted the randomisation procedure) thus presenting several inherent issues, such as: (a) the impossibility in making contact with enrolled subjects post-event, and (b) the impossibility to send reminders through the ePRO without any contact details collected because of regulation restrictions; leading to a high proportion of non-respondents, thus a smaller number of assessable subjects. Nonetheless, the final sample size was large enough to allow statistical comparison between COMPEED® hydrocolloid plasters and regular plasters.
Overall, we described that hot spot/blister occurrence rate under COMPEED® hydrocolloid plaster was 14.5% while it was 25.5% under regular plaster (a statistically significant result (p=0.0001)) and 36.9% outside plaster location. Global impression and satisfaction of subjects in the ability to prevent hot spot/blisters was markedly and statistically significantly in favour of COMPEED®hydrocolloid plaster rather than regular plasters. Results on the plaster staying in place in particularly difficult conditions (subjects ran between 3km-175km and up to 16h), were also superior for COMPEED® hydrocolloid plaster as compared to regular plasters. COMPEED® hydrocolloid plaster “staying-in-place” profile was assessed as “good” by 82.5% of subjects as compared to 20.5% for regular plaster (p<.0001); 84.4% of subjects found the rubbing-off profile of COMPEED® hydrocolloid plaster as “good” as compared to 53.4% of subjects for regular plasters (p <.0001).
To our knowledge, there are no existing data on prevention of hot spot/blister occurrence under hydrocolloid plasters reported in literature [10,11]. We demonstrated that COMPEED®hydrocolloid plasters contributed to skin protection from shearing/rubbing therefore preventing hot spots and blisters development. Moreover, results of our study on plaster staying in place are in line with previous, scarce reports on hydrocolloid dressings [4,12-14]. The main strength of the study was the pragmatic approach reflecting the real-life setting, thus supporting the external validity of our study result. Finally, randomization on paired groups minimized selection bias as well as inter-subject variability further reinforcing a better evaluation of each plaster performances since all subjects had both types of plasters.
Acknowledgments
HRA Pharma funded this study. We are thankful to all ICTA employees and nurses who volunteered to be part of the site staff and recruit participants for their collaboration and fieldwork.
Conflicts of interest
JZB (Medical Operations Lead), PA (Scientific Affairs Assistant & Clinical Operations Assistant), TJ (Global Category Lead Wound Care) and CAA (Global Head of Medical Affairs) are employees at HRA Pharma (manufacturers of the COMPEED® brand of products). MK (Medical writer), VC (Head of Biometrics Department), CP (Chief Operating Officer & Head of Clinical Operations), SW (Head of Medical and Scientific Affairs Department), RG (Head of Business Operations) are employees at ICTA, the organization to which the study conception, operational set-up and conduct, analysis, article writing was subcontracted.
References
- Knapik JJ, Reynolds KL, Duplantis KL, Jones BH (1995) Friction blisters. Pathophysiology, prevention and treatment. Sports Med20:136-147. [Crossref]
- King MJ (1997) Dermatologic problems in podiatric sports medicine. Clin Podiatr Med Surg14:511-524. [Crossref]
- Cortese TAJr, Fukuyama K, Epstein W, Sulzberger MB (1968) Treatment of friction blisters. An experimental study. Arch Dermatol97:717-721. [Crossref]
- Artus-Arduise C, James T, Monteil C, Hammond F, Carr A, et al. (2020) Hydrocolloid blister plasters vs. standard plasters for foot blisters treatment in real life: a comparative, nonrandomised, international, superiority study. Clin Res Trials6:1-7.
- Krabak BJ (2019) Understanding Blisters and How to Prevent Them. International Trail Running Association.
- Rushton R (2015) The Blister Prone Athlete's Guide to Preventing Foot Blisters. In: Lake S [Ed]. The Blister Prone Athlete's Guide to Preventing Foot Blisters.
- Worthing RM, Percy RL, Joslin JD (2017)Prevention of Friction Blisters in Outdoor Pursuits: A Systematic Review. Wilderness Environ Med28:139-149. [Crossref]
- Brennan FHJ (2013) Treatment and Prevention of Foot Friction Blisters. ACSM’s HEALTH & FITNESS JOURNAL17:45-6.
- Lipman GS, Scheer BV (2015) Blisters: the enemy of the feet. Wilderness Environ Med26:275-6. [Crossref]
- Rushton R, Richie D (2023) Friction blisters: A new paradigm to explain causation. J Athl Train.[Crossref]
- Rushton R, Richie D (2023) Friction blisters on the feet: A critical assessment of current prevention strategies. J Athl Train. [Crossref]
- EAME (2009) Johnson & Johnson. Topical Health Care, EU Blister 2nd wave consumer test DEBRIEF March 2009.
- Karlsmark T (2000) Data on File: Protocol 7151071.TK1: An open, randomised,comparative,explorative phase IV clinical investigation of the efficacy and safety of the COMPEED® HydroCure system as hydrocolloid dressing vs. a conventional gauze dressing on experimental blisters and superficial wounds. Sponsored by Coloplast.
- Steinkraus V (2008) Randomized, Evaluator‐Blind, Parallel Group, Clinical Study. Sponsored by Johnson & Johnson.



