Abstract
Post-transplant urinary leak remains one of the commonest surgical complications in the early transplant recovery phase causing significant patient morbidity. Timely diagnosis and intervention is needed in minimizing associated morbidity in addition to avoiding unnecessary hospital stay and potential adverse outcomes. Prevention of urinary leak requires meticulous planning and technique in organ harvest as well as implantation. Any suspicious fluid leak from surgical drains or peri-graft collections need to be aggressively investigated in order to differentiate urine leak from benign conditions such as lymphatic leak. A combination of clinical, biochemical and imaging tests assists in clear differentiation and confirmation of diagnosis. While endo-urological management techniques are available especially for small volume leaks and unstable patients, open surgical repair remains the gold standard in treating large volume urinary leak after transplantation.
Keywords
Renal transplant, Urological complications, Urine leak, Ureter dehiscence
Introduction
Compared to the transformations seen in medical management, tissue crossmatch and post-transplant immunosuppression, surgical technique and associated outcomes have shown little change since the inception. While laparoscopic donor nephrectomy has revolutionized the living donor surgery, the recipient operation technique has remained relatively constant, with minimal variations based mostly on individual surgeons’ preferences. Apart from post-operative haemorrhage, the commonest surgical complications following renal transplantation, often termed its ‘Achilles heel’ have been related to the ureteric anastomosis. The reported incidence of major urological complications following transplant vary from 2.4%-9.2% in recent literature [1,2]. Among them, urinary leak has been reported at an incidence of 1.8% - 2.9% and remains the commonest early ureteric complication with potential implications on patient and graft outcome [3].
Surgical drains and excess drainage
Heterotopic renal transplantation in to the iliac fossa, involves preparation of the renal bed with dissection of the iliac vessels in the retroperitoneal space. This dissection leaves behind a potential dead space for fluid collection. Placement of intra-operative drains in this space adjacent to the graft is commonly practiced, although there is no conclusive evidence of its benefit in reducing post-operative complications [4,5]. Nevertheless, when a drain is placed, one advantage is it allows easy bed-side access to any potential peri-graft collection for visual and biochemical assessment.
Excess drainage from surgical drain could be blood, urine, lymph or rarely ascitic fluid. Drainage of blood is obvious due to the colour and appearance and would require prompt intervention, when significant. Leakage of urine, lymph or ascitic fluid may be indistinguishable requiring further investigation.
Urinary leak; the background
Early urinary leakage (day-01-04) is primarily due to technical errors in construction of the neo-uretero-cystostomy. Less frequently, it could be mechanical damage to the graft ureter or renal pelvis during harvesting or stent insertion. It could also occur due to calyceal damage especially after ex-vivo instrumentation such as calculi extraction [2,6].
Late urinary leakage (Day 05-10) is usually due to ischemic necrosis of ureter resulting in anastomotic dehiscence [7]. This is more frequent in deceased donor transplants with prolonged cold ischemia times. Rarely, it is also possible after live donor transplants where peri-ureteric tissue has been damaged, stripping the ureteric blood supply or where excessive length of the ureter renders it ischemic at the distal end [8,9]. Another possibility is thrombosis or ligation of inferior polar arterial branches that supply the ureter [10,11].
Other forms of leakage that could mimic urinary leak include lymphatic leak and ascitic fluid leakage. Lymphatic leak results from damage to recipient pelvic lymphatics during dissection or due to untied donor lymphatics in the allograft hilum [12]. Ascitic fluid drainage is extremely rare and can occur where the peritoneum has been breached in a recipient with pre-existing ascites.
Clinical manifestation
Clinical manifestation of urinary leak depends on timing and degree of leak. High-volume leaks occurring early, would manifest as excess drain output with accompanied reduction in urinary catheter output. Conversely, low-volume leaks may go undetected and manifest later as peri-graft collections with lower abdominal fullness, tenderness and possible graft dysfunction. These can also present late as a urinary fistula with leakage from the wound or complicated with features of sepsis due to infection. Graft dysfunction can occur as a result of ureteric or vascular compression caused by a urinoma [7].
Diagnosis by imaging
Imaging with Ultra-Sound (US) or Computerized Tomography (CT) in a suspected urinoma will reveal a peri-graft collection, often indistinguishable from a lymphocele [13,14]. Urinoma and lymphocele would both appear anechoic on US compared to the hyperechoic appearance of a haematoma or abscess (Figure-2). Uncomplicated urinoma would appear as a clear uniform collection without septae and loculi seen in a lymphocele, although a long-standing urinoma with possible infection can closely mimic a lymphocele [15,16]. CT imaging would show iso-dense collection (Figure-1) in urinoma or lymphocele compared to the hyperdense shadow of a haematoma or abscess [17]. Both US and CT are poor at defining the exact site of leakage and would rather show the mere presence and extent of a peri-graft collection.
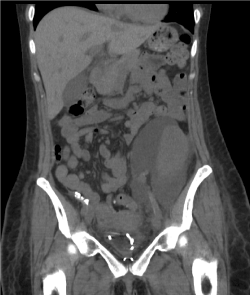
Figure 1: Urinoma (anechoic collection) on ultra-sound scan.
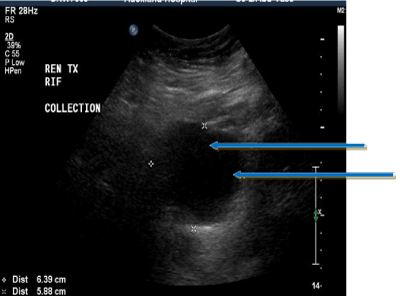
Figure 2: Large peri-graft urinoma on CT.
Although not as readil2021 Copyright OAT. All rights reservgraphy imaging would confirm nuclear contrast extravasation around the graft. Similarly, antegrade contrast uretero-pyelogram can clearly demonstrate the site and degree of leak [18,19]. Hence, this has become the gold-standard investigation of choice for diagnosis of urine leak with the ability for precise localization of leakage [20].
Chemical analysis
Biochemical analysis of drain output or diagnostic needle sampling allows useful information to differentiate urinoma from lymphocele. Chemical analysis for fluid K+, Blood Urea Nitrogen (BUN) and creatinine levels compared with simultaneous samples of serum and urine would allow comparison. All three markers would be markedly higher than serum values and closer to urinary values, confirming urinary leak [11,21]. The presence of a surgical drain allows easy access to such sampling in early leakage whereas late fluid collections need radiological aspiration for sampling.
Routine ureteric stenting
The incidence of post-transplant urinary leak has been shown to have declined significantly with the practice of routine ureteric stenting [22,23]. Majority of such stents are kept in-situ for approximately 6-weeks post-transplant and are expected to avoid minor degree leaks until complete sealing of the neo-uretero-cystostomy. Nevertheless, significant ureteric dehiscence may still cause considerable leakage despite the stent. Some surgeons including the author, also practice attaching the stent to the urinary catheter and early removal of both around day-05-07 [24]. Although recent studies have shown that early stent withdrawal is safe, one potential drawback is that it would offer no protection against minor leaks that could get sealed-off with a stent in-situ.
Recognized causes of post-transplant urinary leak [25] |
- Technical deficiencies in ureteric anastomosis
- Excess length of donor ureter (ischaemia of distal segment)
- Distal ureteric necrosis; excessive dissection and devascularization, donation after cardiac death, extended criteria donors, prolonged cold ischaemia
- Ureteric rotation or excess tension on the anastomosis
- Necrosis of the inferior renal parenchyma (ligation or thrombosis of inferior polar artery)
- Perforation during stent insertion
- Parenchymal leak due to ex-vivo instrumentation
- Premature removal of stent
- Delayed bladder healing (in defunctionalized bladders with no residual native output)
- Distal obstruction and excess bladder pressure (blocked catheter)
|
Management
Once confirmed by clinical, imaging and biochemical analysis, definitive management of high-volume leaks is by surgical repair and restoration of ureteric continuity. Timing of repair and the need for interim measures depends on the degree of leakage and overall status of the patient. If detected late, initial stabilization includes urinary catheterization for distal decompression and commencement of intravenous antibiotics to prevent sepsis. Even during early presentation, the possibility of a blocked catheter causing proximal build-up of pressure and anastomotic disruption should be considered, unless there is free flow in to the urinary catheter.
Endo-urological management
In circumstances where the general condition is unstable with possible graft dysfunction or rejection, radiological placement of a percutaneous nephrostomy would allow temporary urinary diversion until definitive repair is feasible (Figure-3).
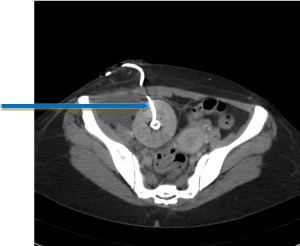
Figure 3: Percutaneous nephrostomy for temporary diversion.
Presence of an established urinoma may warrant percutaneous drainage to prevent sepsis and extrinsic graft compression. Management of low-volume leaks by endourological manoeuvres has been studied in several case series with reported success rates around 60% [26,27]. This would warrant careful patient selection and avoidance of sepsis or graft dysfunction [28-30]. In carefully selected patients with low-volume leak, the combination of percutaneous nephrostomy for proximal decompression with ureteric stenting and urinary catheterization can offer a potential definitive management option with close surveillance of graft function. After a designated period (4-6 weeks), the nephrostomy tube can be used for antegrade contrast study to delineate complete resolution of leakage. While persistent leakage requires open surgical revision, those with no remnant leak can be relieved of stent and catheter while following up for possible secondary ureteric stenosis [31,32]. Hence, the long-term success rate of endo-urological management is well below the above mentioned 60% due to secondary ureteric stenosis [33]. Therefore, in the absence of factors that preclude early surgical revision, open surgical reconstruction remains the best option for long-term success [32,34].
Open surgical revision
Definitive management of complete ureteric dehiscence and those that fail initial endo-urological management, is by open ureteric re-implantation. This requires resection of unhealthy segments of ureter and reconstruction of a new uretero-cystostomy using healthy well-vascularized segment of ureter. Avoiding tension at the new anastomosis may require mobilization of the native bladder, creation of a Boari flap or use of native ipsilateral ureter for a uretero-ureterostomy [34]. Berli and colleagues reported their experience of surgical management in over 2600 transplants and 53 ureteric revisions involving uretero-cystostomy, uretero-ureterostomy and pyelo-ureterostomy with no significant difference in terms or outcome and graft survival [32].
Early versus late surgical revision
Early repair is often technically easier than late repair due to absence of fibrosis and adhesions around the ureter. Dissection along tissue planes becomes easier, avoiding damage to the ureter and graft vasculature. It also minimises the risk of secondary infection in a persistent urinoma.
Late re-implantation either primarily or after failed endo-urological management can be potentially challenging due to presence of fibrosis, possible sepsis and obliteration of tissue planes [11]. Attention should be paid to which donor kidney (left or right) and which anatomical configuration was used in implantation to consider the relation of the ureter and renal pelvis to the hilar vessels. Where the percutaneous nephrostomy tube is in-situ, it can be used for injection of sterile methylene blue that aids in identification of the graft ureter. Antegrade stenting is another technique used by the authors to identify the ureter during the re-operation.
Prevention of urinary leak
Prevention of possible ureteric complications in the recipient involves meticulous planning and technique starting with pre-operative work-up. Patients at high-risk of urological complications post-transplant include those with dysfunctional bladders and previous bladder reconstruction surgery where uretero-cystostomy healing may take longer [8]. Furthermore, the donor operation needs to be well planned with pre-operative imaging and preservation of lower polar arterial supply, especially during laparoscopic live donor retrieval. Avoidance of damaging peri-ureteric tissue during harvest and anastomosis of any inadvertently divided lower polar branches will allow maximal perfusion of the ureter. The ureteric anastomosis needs to be performed with care in avoiding excess length of the ureter and rotation. Routine stenting of the ureter as mentioned above has demonstrated significant benefit in minimising post-transplant urinary leak.
Minimizing Urinary leak in renal transplantation |
- Avoiding ureteric devascularization during organ retrieval
- Avoidance of damaging polar arterial branch
- Keeping the ureter short and well vascularized at anastomotic end
- Avoiding tension at anastomosis
- Routine stenting of ureter
- Delayed stent removal in deceased donor transplants, dysfunctional bladders etc.
|
Conclusion
Urinary leakage is the commonest early ureteric complication following renal transplantation. Although its incidence has reduced with routine ureteric stenting, persistent urinary leak can result in considerable patient morbidity and potential graft loss. Prompt diagnosis requires a combination of clinical, imaging and biochemical findings to differentiate from other peri-graft collections. Minimally invasive endo-urological management can be considered in carefully selected low-volume leaks with overall success rates of approximately 60%. However, early aggressive intervention with surgical repair has shown the highest success rates in terms of minimising long-term complications of sepsis, ureteric stenosis as well as maximising outcome in terms of graft survival.
References
- Lempinen M, Stenman J, Kyllönen L, Salmela K (2015) Surgical complications following 1670 consecutive adult renal transplantations: a single center study. Scand J Surg 104: 254-259.
- Streeter EH, Little DM, Cranston DW, Morris PJ (2002) The urological complications of renal transplantation: a series of 1535 patients. BJU Int 90: 627-634. [Crossref]
- Hamouda M, Sharma A, Halawa A (2018) Urine Leak After Kidney Transplant: A Review of the Literature. Exp Clin Transplant 16: 90-95. [Crossref]
- Derweesh IH, Ismail HR, Goldfarb DA, Araki M, Zhou L, et al. (2008) Intraoperative placing of drains decreases the incidence of lymphocele and deep vein thrombosis after renal transplantation. BJU Int 101: 1415-1419. [Crossref]
- Sidebottom RC, Parsikia A, Chang PN, Berhane Z, Campos S, et al. (2014) No benefit when placing drains after kidney transplant: a complex statistical analysis. Exp Clin Transplant 12: 106-112.
- Shoskes D, Jiménez JA (2014) Chapter 29 – Urological Complications After Kidney Transplantation. Kidney Transplantation–Principles Pract pp: 464-471.
- Sui W, Lipsky MJ, Matulay JT, Robins DJ, Onyeji IC, et al. (2017) Timing and Predictors of Early Urologic and Infectious Complications After Renal Transplant: An Analysis of a New York Statewide Database. Exp Clin Transplant.
- Englesbe MJ, Dubay DA, Gillespie BW, Moyer AS, Pelletier SJ, et al. (2007) Risk factors for urinary complications after renal transplantation. Am J Transplant 7: 1536-1541. [Crossref]
- Di Carlo HN, Darras FS (2015) Urologic Considerations and Complications in Kidney Transplant Recipients. Adv Chronic Kidney Dis 22: 306-311.
- Karam G, Maillet F, Parant S, Soulillou JP, Giral-Classe M (2004) Ureteral necrosis after kidney transplantation: risk factors and impact on graft and patient survival. Transplantation 78: 725-729. [Crossref]
- Branchereau J, Karam G (2016) Management of Urologic Complications of Renal Transplantation. Eur Urol Suppl 15: 408-414.
- Richard HM III (2004) Perirenal transplant fluid collections. Semin Intervent Radiol 21: 235-237.
- Humar A, Matas AJ (2005) Surgical complications after kidney transplantation. Seminars in Dialysis.
- Buresley S, Samhan M, Moniri S, Codaj J, Al-Mousawi M (2008) Postrenal Transplantation Urologic Complications. Transplant Proc 40: 2345-2346.
- Park SB, Kim JK, Cho KS (2007) Complications of renal transplantation: ultrasonographic evaluation. J Ultrasound Med 26: 615-633.
- Brown ED, Chen MY, Wolfman NT, Ott DJ, Watson NE (2000) Complications of renal transplantation: evaluation with US and radionuclide imaging. Radiographics 20: 607-622
- Dirlik A, Erinç R, Özcan Z, Duman S, Özkahya M, et al. (2001) Diagnosis of urinary leakage in renal transplant patients: Ultrasonographic, clinical and scintigfraphic findings. Türk Nefroloji Diyal ve Transplant 10: 239-243.
- Smith TP, Hunter DW, Letourneau JG, Cragg AH, Darcy MD, et al. (1988) Urine leaks after renal transplantation: Value of percutaneous pyelography and drainage for diagnosis and treatment. Am J Roentgenol 151: 511-513.
- Inci MF, Ozkan F, See TC, Tatli S (2014) Renal Transplant Complications: Diagnostic and Therapeutic Role of Radiology. Can Assoc Radiol J 65: 242-252.
- Kumar S, Ameli-Renani S, Hakim A, Jeon JH, Shrivastava S, et al. (2014) Ureteral obstruction following renal transplantation: causes, diagnosis and management. Br J Radiol 87: 20140169.
- Lucewicz A, Wong G, Lam VWT, Hawthorne WJ, Allen R, et al. (2011) Management of primary symptomatic lymphocele after kidney transplantation: a systematic review. Transplantation 92: 663-673.
- Wilson CH, Rix DA, Manas DM (2013) Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev 17: CD004925.
- Dominguez J1, Clase CM, Mahalati K, MacDonald AS, McAlister VC, et al. (2000) Is routine ureteric stenting needed in kidney transplantation? A randomized trial. Transplantation 70: 597-601. [Crossref]
- Wilson CH, Hosgood SA, Nicholson ML (2015) Early versus late ureteric stent removal after kidney transplantation. Cochrane Database Syst Rev 1: CD011455.
- Rahnemai-Azar AA, Gilchrist BF, Kayler LK (2015) Independent risk factors for early urologic complications after kidney transplantation. Clin Transplant 29: 403-408.
- Duty BD, Barry JM (2015) Diagnosis and management of ureteral complications following renal transplantation. Asian J Urol 2: 202-207.
- Duty BD, Conlin MJ, Fuchs EF, Barry JM (2013) The current role of endourologic management of renal transplantation complications. Adv Urol 2013: 246520.
- Campbell SC, Streem SB, Zelch M, Hodge E, Novick AC (1993) Percutaneous management of transplant ureteral fistulas: Patient selection and long-term results. J Urol 150: 1115-1117.
- Aytekin C, Boyvat F, Harman A, Özyer U, Colak T, et al. (2007) Percutaneous therapy of ureteral obstructions and leak after renal transplantation: Long-term results. Cardiovasc Intervent Radiol 30: 1178-1184
- Alcaraz A, Bujons A, Pascual X, Juaneda B, Martí J, et al. (2005) Percutaneous management of transplant ureteral fistulae is feasible in selected cases. Transplant Proc 37: 2111-2114. [Crossref]
- Dinckan A, Tekin A, Turkyilmaz S, Kocak H, Gurkan A, et al. (2007) Early and late urological complications corrected surgically following renal transplantation. Transpl Int 20: 702-707.
- Berli JU, Montgomery JR, Segev DL, Ratner LE, Maley WR, et al. (2015) Surgical management of early and late ureteral complications after renal transplantation: techniques and outcomes. Clin Transplant 29: 26-33.
- Fonio P, Appendino E, Calandri M, Faletti R, Righi D, et al. (2015) Treatment of urological complications in more than 1,000 kidney transplantations: the role of interventional radiology. Radiol Med 120: 206-212.
- Nie ZL, Zhang KQ, Li QS, Jin FS, Zhu FQ, et al. (2009) Treatment of Urinary Fistula After Kidney Transplantation. Transplant Proc 41: 1624-1626.



