Background: Post transplant diabetes mellitus (PTDM) is a common sequela of lung transplant (LTx) that has previously been shown to decrease survival. This study evaluates the incidence and outcomes of PTDM following LTx at a single center.
Methods: LTx recipients from 2004 through 2013 were retrospectively divided into three groups: (1) No diabetes (DM), (2) Pre- and post-transplant diabetes (PDM), and (3) PTDM. Univariate and multivariate analyses were performed to evaluate risk factors for death following LTx, including pre-transplant diagnosis, body mass index (BMI), Hemoglobin A1C, insulin use, oral anti-diabetes agents (OAD) use, lung allocation score, tacrolimus levels, and serum creatinine (Scr).
Results: 293 patients qualified for analysis; 31% had no DM, 17% had PDM and 47% developed PTDM. Median follow-up was 11 years. Survival was not significantly different between groups, although it was shortest in those with PDM. Mean survival was 8.7 years in the no DM group, 7.2 years in PDM, and 8.1 years in PTDM (p=0.09). Scr>2 mg/dL at any time after transplant was the sole predictor of mortality (HR 1.99, CI 1.34-2.96, P<0.01). In PTDM, there was no significant difference in mortality in those with later resolution of DM (29%) versus those with persistent DM (39%) (P=0.22).
Conclusion: Nearly half of LTx recipients developed PTDM. However, development of PTDM did not significantly impact mortality. Later resolution of PTDM similarly did not impact mortality. Renal injury with a Scr>2 mg/dL is associated with increased mortality after LTx.
post-transplant diabetes, diabetes, lung transplant outcomes, lung transplant complications, renal failure/ AKI
Abbreviations
BMI: Body Mass Index; CMV: Cytomegalovirus; COPD: Chronic obstructive pulmonary disease; Hgb A1c: Hemoglobin A1c; LAS: Lung Allocation Score; LTx: Lung Transplant; OAD: Oral Anti-diabetes agent; PDM: Pre- and Post-transplant Diabetes Mellitus; PTDM: Post-transplant Diabetes Mellitus; Scr: Serum Creatinine; UNOS: United Network for Organ Sharing
Post-transplant diabetes mellitus (PTDM) is a common sequela of solid organ and bone marrow transplant. At 12 months post-transplant, the incidence of PTDM in lung recipients has varied from 5-45%, similar to that seen in kidney (20-50%), liver (9-30%) and heart transplantation (28-30%) [1,2]. Prior lung transplant (LTx)-specific work has illustrated that the incidence of PTDM was estimated to plateau around 32% at 3 months, with the diagnosis based on oral glucose tolerance testing [3]. However, the impact of PTDM on morbidity and mortality in the post-transplant population appears variable amongst different solid organ recipient groups. Data from renal transplantation has long illustrated increased mortality as well as diminished graft survival in the PTDM population [4]. It is believed that the primary driver of this mortality difference is attributable to the increased cardiovascular risk associated with PTDM [5]. Following liver transplant, patients with PTDM have been shown to have reduced survival as well as higher incidences of sepsis and chronic kidney disease [6].
Less is known concerning the role of PTDM on outcomes following LTx. Hackman, et al. have previously shown that the presence of diabetes, either pre- and post-transplant (PDM) or PTDM, incurs a higher risk of mortality [3,7]. Recent work has further shown that glycemic control strongly correlates with survival [8]. However, in these studies, PTDM was not delineated from PDM. Further, the pathophysiology behind the increased mortality following LTx remains unknown, particularly considering the increasing incidence of cardiovascular risk factors following LTx [9]. Whether hyperglycemia in patients with PTDM or the mere presence of PTDM is a driving factor for poor post-transplant outcomes remains controversial.
In this study, we retrospectively reviewed the incidence and outcomes of PTDM in our single center LTx cohort. Particularly, we aimed to evaluate the association between PTDM and post-LTx survival. Further, we sought to evaluate whether later resolution of PTDM impacted outcomes.
A retrospective, Institutional Review Board-approved chart review of adult lung transplant recipients was performed at the University of Michigan Health System. Patients were included if they received a single or bilateral lung transplant between January 2004 to July 2013.
Data collection
Data was retrieved from the electronic medical record. Variables collected included patient demographics, body mass index (BMI), pre-transplant diagnosis, lung allocation score (LAS), pre-transplant glucose levels, pre and post-transplant hemoglobin A1C (Hgb A1C), the presence of DM by medical history, insulin or oral antidiabetes agent (OAD) utilization post-transplant, tacrolimus levels and prednisone doses at discharge, 3, 6, and 12 months after transplant, serum creatinine (Scr), patient survival, and cause of death. LTx recipients who died within 90 days of transplant were separated from the remaining dataset and were excluded from further analysis.
Diabetes definition
Patients were classified into three groups according to diabetes status: no diabetes, pre-and post-transplant diabetes (PDM), and post-transplant diabetes (PTDM). Patients were classified as PDM if: (1) a confirmed diagnosis of diabetes pre-transplant was noted or (2) a Hgb A1C of ³ 7 mg/dL was noted during the transplant admission. PTDM was diagnosed based on: (1) a continued need of insulin or OAD post-transplant or (2) Hgb A1C was ³ 7 mg/dL at any time point after transplant.
Immunosuppression/ Prophylaxis
According to our center-specific protocol, all patients received triple immunosuppressive therapy after transplant consisting of tacrolimus, azathioprine or mycophenolate mofetil, and corticosteroids. Induction therapy is utilized in the setting of renal insufficiency in the immediate post-transplant setting, commonly when Scr has doubled from baseline pre-transplant values. Antiviral prophylaxis was assigned according to risk status. Cytomegalovirus (CMV) seronegative recipients of seropositive donor lungs received intravenous ganciclovir for two weeks then converted to valganciclovir for a minimum 1 year. In addition, this subset of patients received six infusions of CMV intravenous immunoglobulin over 6 months. Seropositive recipients of seropositive donor lungs also received intravenous ganciclovir for two weeks then converted to valganciclovir for a minimum of 6 months. Seronegative and seropositive recipients receiving seronegative donor lungs received oral acyclovir. All patients receive pneumocystis jiroveci pneumonia prophylaxis.
Statistical analysis
All statistical computations were performed using PASW (IBM, Armonk, NY) and SAS Version 9.4 (SAS institute, Cary, NC). Categorical variables were compared using Chi-square or Fisher’s exact test and shown as Number (%). Normally and non-normally distributed continuous variables were analyzed using ANOVA or Kruskal-Wallis tests, respectively, and shown as mean (95% CI). Number of days to death within each of the diabetes group were compared using Kaplan-Meier curves. Univariate and multivariate Cox regression models were used to determine the risk factors for mortality. Variables for Cox regression included pre-transplant diagnosis, BMI, Hgb A1C, insulin use, OAD use, LAS, tacrolimus levels, and Scr. A p-value of <0.05 was considered statistically significant.
Two hundred ninety-three patients received a LTx during the study period. Patient demographics are reported in Table 1. Median follow-up was 11 years. The mean age at transplant was similar between groups at 51 years (SD 12.9 years). Males accounted for 64% of LTx recipients. Mean BMI at transplant was 25.5 Kg/m2 (SD 5.07 kg/m2). Mean pre-LTx Scr was 0.78 mg/dL (SD 0.22 mg/dL). PDM was present in 49 patients (17%), while 139 patients (47%) developed PTDM, of which 87 patients (63%) had later resolution. Fourteen patients (5%) died within 90 days of transplant, of which 3 had PDM and 2 developed PTDM. In total, 194 patients (66%) had some form of diabetes. When evaluating patients in different diabetes groups, the only significant baseline differences were pre-transplant glucose levels. Notable post-transplant differences included BMI three months post-transplant, with the PDM patients having the highest BMI (P=0.01), and post-transplant mean Hgb A1c (<0.001), notable for being below 7%.
Table 1. Patient Demographics by Diabetes Mellitus Status
|
No Diabetes (N=91) |
PTDM (N=139) |
Pre & Post Diabetes (N=49) |
Early Death (N=14) |
p-value |
Age at Transplant |
52 ± 10 |
50 ± 14 |
47 ± 15 |
55 ± 9.5 |
0.6 |
Males |
59% |
55% |
68% |
86% |
0.1 |
White |
87% |
84% |
94% |
86% |
0.05 |
Baseline BMI |
25 ± 4.2 |
25 ± 5.2 |
26.2 ± 6.1 |
25.9 ± 5.4 |
0.83 |
BMI 3mon |
24 ± 3.7 |
25 ± 4.6 |
28.2 ± 4.2 |
- |
0.01 |
BMI 6mon |
26 ± 4.5 |
26.4 ± 5.4 |
27.4 ± 5.0 |
- |
0.61 |
BMI1 2mon |
27 ± 4.7 |
27.4 ± 6.2 |
28.8 ± 6.6 |
- |
0.53 |
Baseline Scr |
0.81 ± 0.23 |
0.76 ± 0.23 |
0.74 ± 0.17 |
0.84 ± 0.22 |
0.15 |
Days listed on the lung transplant database |
310 ± 534 |
318 ± 489 |
415 ± 747 |
118 ± 148 |
0.33 |
Pre-transplant Blood Glucose (mg/dL) |
100 ± 21 |
105 ± 27 |
139 ± 56 |
99 ± 26 |
<0.001 |
Post-transplant Hgb A1C (mg/dL) |
5.4 ± 0.9 |
6.0 ± 1.2 |
6.8 ± 1.4 |
5.9 ± 0.5 |
<0.001 |
Tacrolimus Level 3 mon post-LTx |
10.2 ± 4.2 |
10.5 ± 4.4 |
11.3 ± 3.6 |
11.5 ± 10 |
0.57 |
Tacrolimus Level 6 mon post-LTx |
9.8 ± 4.9 |
9.5 ± 4.5 |
10.7 ± 4.4 |
11.5 ± 10 |
0.34 |
Tacrolimus Level 12 mon post-LTx |
10.2 ± 4.2 |
10.5 ± 4.4 |
11.3 ± 3.6 |
11.5 ± 10 |
0.57 |
LAS at listing |
42 ± 14 |
40 ± 12 |
41 ± 11 |
40 ± 5 |
0.8 |
LAS at transplant |
46 ± 19 |
44 ± 16 |
44 ± 14 |
46 ± 15 |
0.9 |
After separating the groups by pre-transplant pathology, the cystic fibrosis group had the highest rate of diabetes overall at 91%, with 38% having PDM (Table 2). The chronic obstructive pulmonary disease (COPD)/bronchiectasis group had the highest rate of PTDM at 52%. Early death rates were highest in the pulmonary fibrosis group.
Table 2. Pre-transplant pathology and Diabetes mellitus status
|
No Diabetes (N%) |
PTDM (N%) |
Pre and post diabetes (N%) |
Early death (N%) |
Total |
Cystic fibrosis |
4 (8) |
25 (53) |
18 (38) |
0 |
47 |
COPD/bronchiectasis/Emphysema |
33 (38) |
45 (52) |
8 (0.09) |
1 (0.01) |
87 |
Pulmonary HT |
6 (43) |
6 (43) |
1 (.07) |
1 (0.07) |
14 |
Pulmonary Fibrosis |
32 (30) |
47 (44) |
20 (19) |
9 (0.08) |
108 |
Note: 2 pts with emphysema also have Pulmonary fibrosis.1 patient has both emphysema and bronchiectasis.1 COPD also has Pulmonary HT.1 Pulmonary HT patient has also Pulmonary fibrosis.1 Pulmonary fibrosis also has sarcoidosis.
Mortality at one year was higher in patients with any form of diabetes (3.3% No diabetes, 7.2% PTDM, 8.2% PDM), but did not reach statistical significance (p=0.38) (Table 3). Overall mean survival was 7.8 years (7.3–8.3 years); 8.7 years (7.9–9.4 years) in the no diabetes group, 8.2 years (7.5–8.8 years) in the PTDM group, and 7.2 years (6.0–8.3 years) in the PDM group. By the end of the study, 65% of the PTDM group survived versus 49% of the PDM group. (Figure 1). However, there was no significant difference in survival time amongst groups (p=0.09). Although patients with PDM were noted to trend towards an increased risk of death, this did not reach statistical significance (HR 1.68, CI 0.99-2.83, P=0.052). PTDM had no significant association with mortality (HR 1.04, CI 0.66-1.66, p=0.85). Re-inclusion of early death group did not yield any significant differences in mortality between diabetes groups.
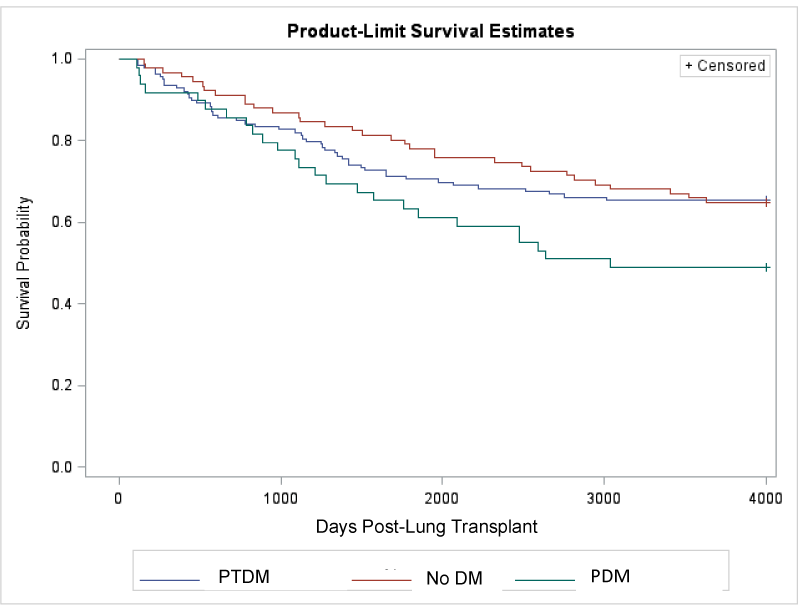
Figure 1. Post-LTx survival by Diabetes group (Early death group has been excluded to allow for scale. Data was censored at 4000 days post-LTx).
Table 3. Mortality at 1 year and 5 years after transplant
Diabetes status |
N |
Overall Mortality
(%) |
Mortality within 1 year |
Mortality within 5 years |
No Diabetes |
91 |
35% |
3.3% |
22% |
PTDM |
139 |
35% |
7.2% |
30% |
Pre and post diabetes |
49 |
51% |
8.2% |
37% |
Overall |
293 |
119 |
31 |
93 |
p-value |
- |
0.10 |
0.38 |
0.16 |
In multivariate analysis, PDM (HR 1.56, CI 0.91–2.66, P=0.09) and PTDM (HR 1.05, CI 0.66-1.64, p=0.83) again did not show any impact on risk of death. When mortality was compared between those with resolution of PTDM (28.7%) versus those with persistent PTDM (39.1%), no statistically significant difference was noted (P=0.22). Mortality was not affected by type of medical therapy received for DM (insulin, OADs or both). The main cause of death in the no diabetes and the diabetes groups were chronic rejection and infection without significant differences in the rates of occurrence, respectively.
Univariate analysis of factors impacting mortality showed that a Scr>2 mg/dL at any point post-transplant was the only predictor of mortality (HR 2.00, CI 1.33-2.78, P<0.001), which was also noted in multivariate analysis (HR 1.99, CI 1.34-2.96, p<0.01)
In this review of 293 LTx recipients, 17% of patients had diabetes prior to transplant and 47% developed PTDM. Although there was a noted trend towards increased mortality in patients with PDM, this did not reach statistical significance. PTDM did not impact mortality.
There were no significant demographic differences between our comparison groups. Previous work has identified similar risk factors for development of PTDM to those noted for development of DM in the general population [9,10]. In a large study utilizing United Network for Organ Sharing (UNOS) data with 3540 lung transplant recipients, the highest risk factors for PTDM included BMI>30, male gender, age>50, and African American race [11]. Notably, BMI in this study (mean 25.5 Kg/m2) was higher than in previous studies, consistent with the present obesity trends in the United States [9,12]. Pre-transplant lung pathology was similar to those previously described, with COPD and pulmonary fibrosis as the most common indications for LTX [8,13]. Additionally, 60% of our study population was male, modestly higher than previous studies [11].
Our incidence of PTDM was 47%, higher than previously reported rates [1,11,13]. We suspect the higher incidence is attributable to consistent use of tacrolimus over cyclosporine in this study, as our institution transitioned to tacrolimus immunosuppression protocol in 2005, making the number of patients on cyclosporine in this study minimal. In comparison, prior studies included data from patients on cyclosporine-based immunosuppression regimens for 8 of 10 years of data collection [7]. Understanding the natural course of PTDM is limited by the variability of immunosuppression, particularly tacrolimus and steroid dosing post-transplant [14]. Tacrolimus has been shown to decrease the rate of serious post-LTx complications, including bronchiolitis obliterans [15]. However, this is done at the expense of elevated rates of diabetes mellitus when compared to the previously used cyclosporine-based regimens, with an estimated relative risk of 4.24 [15]. Resolution of PTDM and improvement in Hgb A1c levels has been noted with when tacrolimus is transitioned to cyclosporine in renal transplant recipients, but this would result in an increased risk of bronchiolitis obliterans in our population [15,16].
In our study, PTDM did not increase the risk of mortality in our evaluation timeframe [3,17]. As has been previously hypothesized, the short duration of DM in these patients may not necessarily impact LTx mortality, given the time course to develop macrovascular and microvascular complications [13]. The primary causes of death in this study were bronchiolitis obliterans and infection, the leading causes of late mortality following LTx nationally, notably with little contribution from diabetes pathology [18]. Previously, PTDM has been shown to increase major cardiac incidents and cardiovascular complications in renal transplant recipients, with mixed data regarding whether this impacts overall post-transplant mortality [5,19]. In this study, cardiovascular etiologies were noted to be much less common causes of mortality in comparison to bronchiolitis obliterans and infection. Perhaps, given the overall shorter median survival of LTx recipients in comparison to renal transplant recipients, macrovascular complications may not be fully appreciated in LTx patients. Of note, our study had a longer median follow-up of 11 years than prior work [7].
PTDM resolved in the majority (63%) of patients in this study. Little work has been done regarding PTDM resolution, but initial work noted resolution to be much less common, occurring in 14% of patients by oral glucose tolerance test [3]. Importantly, our work did not include oral glucose tolerance testing in all patients, but resolution was defined by normalization of the Hgb A1c to <6.5% and/or the lack of further treatment required with insulin or OADs. Mortality was not significantly different between LTx recipients with PTDM resolution and those without resolution, consistent with our findings that PTDM had no significant impact on overall mortality. In addition, the choice of insulin or OADs did not impact mortality. Further research regarding the role of OADs in the care of patients with PTDM is needed.
Scr>2 mg/dL at any point post-LTx was the only variable shown in our analysis to have a significant impact on morality. The impact of renal insufficiency on survival has been noted previously, with EGFR<45 strongly associated with increased post-LTx mortality [20,21]. Prolonged acute kidney injury post-LTx also has been associated with increased mortality [22]. However, impaired renal function may not impact mortality in patients with cystic fibrosis receiving LTx [23].
The strengths of this study include the long follow up of a single cohort for over seven years with the consistent anti-rejection regimen of steroids and tacrolimus throughout this time course. Additionally, immediate post Tx glucose management guidelines were consistent during this period with blood glucose controlled to goal of <150 mg/dl in perioperative period with follow up of PDM or PTDM. Patient follow up was longitudinal, including both inpatient and outpatient data. Limitations include the limited number of preoperative values of Hgb A1c and the absence of oral glucose tolerance tests to diagnose diabetes pre-LTx and normalization of glucose post-LTx. This was a single center retrospective study. Although DM management by a hyperglycemia team headed by an endocrinologist was tightest and most algorithm-based in the perioperative period, there was no specific protocol for management of the patients with DM in the outpatient follow up, as evidenced by the plurality of insulin and OAD regimens. DM was however appropriately managed as evidenced by mean post-Tx Hemoglobin A1c concentrations of <7%. Importantly, our definition of PTDM and A1c was pragmatic but more inclusive than those utilized in some prior works [3,13]. A more rigorous and universally accepted definition of PTDM, as offered by the International consensus meeting on PTDM, is needed for continued investigation and collaboration in the field [24].
In conclusion, this study shows that the incidence of post-LTx PTDM is higher than previously described, impacting nearly half of all LTx recipients. The presence of PTDM, when appropriately managed, had no significant impact on post-transplant mortality when compared with patients with pre- and post-transplant DM and patients without diabetes. Scr>2 mg/dL appears to be a strong marker for mortality following LTx.
- Lane JT, Dagogo-Jack S (2011) Approach to the patient with new-onset diabetes after transplant (NODAT). J Clin Endocrinol Metab 96: 3289-3297.
- Sharif A, Cohney S (2016) Post-transplantation diabetes-state of the art. Lancet Diabetes Endocrinol 4: 337-349.
- Hackman KL, Snell GI, Bach LA (2014) Prevalence and predictors of diabetes after lung transplantation: a prospective, longitudinal study. Diabetes Care 37: 2919-2925.
- Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, et al. (2002) Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 62: 1440-1446.
- Wauters RP, Cosio FG, Suarez Fernandez ML, Kudva Y, Shah P, et al. (2012) Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation 94: 377-382.
- Lv C, Zhang Y, Chen X, Huang X, Xue M, et al. (2015) New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes 7: 881-890.
- Hackman KL, Bailey MJ, Snell GI, Bach LA (2014) Diabetes is a major risk factor for mortality after lung transplantation. Am J Transplant 14: 438-445.
- Hackman KL, Snell GI, Bach LA (2017) Poor Glycemic Control is Associated with Decreased Survival in Lung Transplant Recipients. Transplantation 101: 2200-2206.
- Savioli G, Surbone S, Giovi I, Salinaro F, Preti P, et al. (2013) Early development of metabolic syndrome in patients subjected to lung transplantation. Clin Transplant 27: E237-243.
- Han E, Kim MS, Kim YS, Kang ES (2016) Risk assessment and management of post-transplant diabetes mellitus. Metabolism 65: 1559-1569.
- Ye X, Kuo HT, Sampaio MS, Jiang Y, Bunnapradist S (2011) Risk factors for development of new-onset diabetes mellitus after transplant in adult lung transplant recipients. Clin Transplant 25: 885-891.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL (2016) Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284-2291.
- Ollech JE, Kramer MR, Peled N, Ollech A, Amital A, et al. (2008) Post-transplant diabetes mellitus in lung transplant recipients: incidence and risk factors. Eur J Cardiothorac Surg 33: 844-848.
- Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DER, et al. (1987) The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 44: 376-381.
- Penninga L, Penninga EI, Møller CH, Iversen M, Steinbrüchel DA, et al. (2013) Tacrolimus versus cyclosporin as primary immunosuppression for lung transplant recipients. Cochrane Database Syst Rev 2013: CD008817.
- Wissing KM, Abramowicz D, Weekers L, Budde K, Rath T, et al. (2018) Prospective randomized study of conversion from tacrolimus to cyclosporine A to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. Am J Transplant 18: 1726-1734.
- Klomjit N, Mehrnia A, Sampaio M, Bunnapradist S (2015) Impact of diabetes mellitus on survival outcome of lung transplant recipients: An analysis of OPTN/UNOS data. Clin Transpl 31: 43-55.
- Costa J, Benvenuto LJ, Sonett JR (2017) Long-term outcomes and management of lung transplant recipients. Best Pract Res Clin Anaesthesiol 31: 285-297.
- Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, et al. (2006) The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 69: 588-595.
- Grimm JC, Valero V, Magruder JT, Kilic A, Dungan SP, et al. (2015) A novel risk score that incorporates recipient and donor variables to predict 1-year mortality in the current era of lung transplantation. J Heart Lung Transplant 34: 1449-1454.
- Grimm JC, Lui C, Kilic A, Valero V, Sciortino CM, et al. (2015) A risk score to predict acute renal failure in adult patients after lung transplantation. Ann Thorac Surg 99: 251-257.
- Fidalgo P, Ahmed M, Meyer SR, Lien D, Weinkauf J, et al. (2014) Association between transient acute kidney injury and morbidity and mortality after lung transplantation: a retrospective cohort study. J Crit Care 29: 1028-1034.
- Crawford TC, Magruder JT, Grimm JC, Suarez-Pierre A, Zhou X, et al. (2017) Impaired renal function should not be a barrier to transplantation in patients with cystic fibrosis. Ann Thorac Surg 104: 1231-1236.
- Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, et al. (2014) Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant 14: 1992-2000.

