Infertility is a global public health issue, attributable to male factors in 30%-50% of cases. By 2025, almost 10 million couples will encounter problems in having a baby. In assisted reproduction, Pentoxifylline improves sperm motility both in vitro and after oral administration in patients with asthenozoospermia. In this phase I dose escalation study, we aimed to assess the pharmacokinetics, safety, and tolerability of three doses of Pentoxifylline formulated as a gel (PKB171 gel) for intravaginal administration in healthy female volunteers and to determine the maximum tolerated dose (MTD) after single doses of 100 mg, 150 mg or 200 mg of Pentoxifylline, following by an extension substudy involving multiple-dose vaginal administration. For both studies, the main variable was the incidence of adverse events (AEs) after each dose. In the single dose study, plasma concentrations of Pentoxifylline and 5-Hydroxy Pentoxifylline were determined at baseline and at +10, +20, +30, +45 minutes, +1, +2, +4, +8 and +12 hours after dose. Vaginally administered PKB171 gel at all study doses was well-tolerated and MTD was 200 mg. The incidence of AEs did not increase as a function of dose. Linearity in Pentoxifylline and 5-Hydroxy Pentoxifylline plasma concentrations was observed for the three doses studied.
intravaginal, Pentoxifylline, asthenozoospermia, male infertility, pharmacokinetics, safety, tolerability
Currently, the world is experiencing a major crisis in birth rates, mainly due to a combination of social and biological factors (e.g., infertility). In developed countries, infertility affects up to 15% of reproductive-aged couples [1]. Infertility (a term used interchangeably with subfertility) is defined as the inability to conceive after 12 months of regular, unprotected sexual intercourse or an impairment in a person’s capacity to reproduce, either as an individual or with his/her partner [1-5]. Subfertility describes any form of reduced fertility that results in an undesired delay in conception.
The cause of the infertility could be attributable to male or female factors, with male factors accounting for 30%-50% of cases [6]. Male infertility is associated with low sperm quality, which may be due to a variety of different causes [7], including asthenozoospermia (reduced sperm motility: < 40% of mobile spermatozoa) or oligozoospermia (low sperm concentration in the ejaculate: < 15×106 spermatozoa/mL) [5,8]. The combination of these two conditions is known as oligoasthenozoospermia [8,9].
The precise cause of male infertility is often unknown (idiopathic infertility) [10]. In cases of idiopathic infertility, empirical treatment (hormonal or non hormonal) can be used but these therapies usually require long-term administration (from 3 to 5 months), the efficacy is highly variable and must be personalized for each patient [11], and there is a risk of side effects [12]. Consequently, many experts believe that the best option for couples facing male infertility is assisted reproductive technology (ART), such as artificial insemination (AI) or in vitro fertilisation (IVF). Although these methods have become common in recent decades, these invasive techniques require a medical intervention, are expensive, and the success rate is only moderate, and often make couples uncomfortable [13,14].
Pentoxifylline is a methylxanthine derivative belonging to a group of vasoactive drugs, wich is a non-specific inhibitor of phosphodiesterase, that improve peripheral blood flow, thus enhancing peripheral tissue oxygenation. In assisted reproduction, in vitro Pentoxifylline has been used to treat sperm samples due to its ability to improve progressive sperm motility. Various studies have demonstrated the effects of Pentoxifylline on sperm motility in vitro [15], before AI [16], in IVF or intracytoplasmic sperm injection (ICSI) [17], and after three [18] and six months [19] of oral administration of 1200 mg/day in patients with asthenozoospermia. The use of Pentoxylline in the laboratory has been associated with improving counts of total motile and total progressively motile spermatozoa, of both fresh and cryopreserved sperm [15,20-22] especially in couples with oligozoospermia/asthenozoospermia and previous IVF failure, encouraging further clinical evaluation.
For more than 30 years, both oral and intravenous Pentoxifylline has been authorised as a treatment for several vascular pathologies [23-26]. Clinical safety data have been reported for systemic exposure to Pentoxifylline, as well as in non-clinical safety studies for marketing authorization [27]. However, to our knowledge, systemic absorption after vaginal administration of Pentoxifylline has not been evaluated to date.
PKB171 gel, developed by Prokrea S.A., is a vaginal gel whose active ingredient is Pentoxifylline. Studies have shown that vaginally-administered PKB171 gel may be a less expensive, less invasive, home-based alternative to current fertility treatments [28.29]. Additionally, this drug could potentially benefit couples with fertility problems attributable to male infertility (asthenozoospermia or low sperm quality).
The available evidence on the different uses of Pentoxifylline in the field of infertility generate the hypothesis that a topical formulation of Pentoxifylline administered intravaginally before or after intercourse lead to an improvement in sperm mobility would result in an increase in the rate pregnancy. In consequence, we conducted this randomised, parallel, double-blind, placebo-controlled, scaled dose clinical trial to determine the maximum tolerated dose (MTD) of PKB171 gel and to evaluate the safety and tolerability of this medication after single-dose vaginal administration in healthy female volunteers. Subsequently, we carried out an extension substudy to investigate the safety, tolerability, and pharmacokinetics (PK) of PKB171 gel after both single and multiple doses. This is the first study designed to evaluate the tolerability, safety and pharmacokinetics of PKB171 gel after vaginal administration.
Ethics approval
The study protocol and the informed consent form were reviewed and approved by the Ethics Committee for Clinical Research of the Hospital de la Santa Creu i Sant Pau. All participants gave written informed consent prior to initial screening. The phase I study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice.
Study design
The study was performed at Drug Research Center (CIM), Sant Pau Institute of Biomedical Research (IIB-Sant Pau), Barcelona, Spain and was registered with EudraCT (2015-004611-21).
This was a phase I, randomised, single-centre, parallel, double-blind, placebo-controlled, dose-escalation study with three consecutive cohorts of healthy female volunteers (n=24) who received a single dose of 5 g of PKB171 gel containing 100 mg (2%), 150 mg (3%), or 200 mg (4%) of Pentoxifylline (n=6 for each dose) or placebo (n=6). The study drug and placebo were administered intravaginally between days 7 and 21 of the participant’s menstrual cycle. The MTD, in terms of local tolerability after single administration, was assessed by means of dose escalation. MTD was defined as the maximum dose at which a no more than one of the six participants reported moderate or severe drug-related adverse events (AE) after administration of the gel.
After completing the dose-escalation study to establish the MTD, we performed an extension substudy to assess the tolerability of PKB171 gel at the MTD dose delivered intravaginally in multiple doses (two consecutive days) in healthy women. The extension substudy, involving six volunteers who had participated in the phase I trial, was also randomized, parallel-group, double-blind, and placebo-controlled. The six healthy women were randomly assigned to receive MTD (n=4) or placebo (n=2) between days 7 and 21 of their menstrual cycle. Table 1 shows the study evaluations for the dose escalation study and the extension substudy.
Table 1. Procedures performed in the dose escalation study and the extension substudy
Dose Escalation Study Extension Substudy |
Procedures |
Screening
Visit |
Visit 1 (V1) |
Visit 2 (V2) |
Final Visit
(FV) |
|
|
|
|
Visit 2 (V2) |
Visit 3 (V3) |
Final Visit
(FV) |
-1h |
0 |
+10’ |
+20’ |
+30’ |
+45’ |
+1h |
+2h |
+4h |
+8h |
+12h |
+24h |
+72h |
-1h |
0 |
+1h |
+4h |
+24h |
+48h |
+96h |
IC / EC |
X |
X |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
CH |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ME |
X |
X |
|
|
|
|
|
|
|
|
|
|
X |
X |
X |
|
|
|
X |
X |
X |
GE |
X |
X |
|
X |
|
|
|
|
|
X |
|
|
X |
X |
X |
|
X |
X |
X |
X |
X |
VS |
X |
X |
|
|
|
X |
|
X |
X |
X |
|
X |
X |
X |
X |
|
|
|
X |
X |
X |
EKG |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
BPT |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DU |
X |
X |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
VAS training |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
UA |
X |
|
|
|
|
|
|
|
|
|
|
|
X |
X |
X |
|
|
|
|
|
X |
BT |
X |
|
|
|
|
|
|
|
|
|
|
|
|
X |
X |
|
|
|
|
|
X |
SERO |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
UPT |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
RANDOM |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
PK |
|
X |
|
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
|
X |
|
|
|
X |
X |
|
DA |
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
X |
X |
|
|
X |
|
|
VAS |
|
X |
|
|
|
|
|
|
|
X |
|
|
X |
X |
X |
|
|
|
X |
X |
|
AE |
|
X ----------------------------------------------------------------------------------------------------------------------------X |
X----------------------------------------------------------------------------X |
The initials corresponds to: IC: Inclusion Criteria; EC: Exclusion Criteria; CH: Clinical History; ME: Medical Examination; GE: Gynaecological Examination; VS: Vital Signs; EKG: Electrocardiogram; BPT: Blood Pregnancy Test; DU: Drugs in Urine; VAS: Visual Analogic Scale; UA: Urinalysis; BT: Blood test; SERO: Serology; UPT: Urinary Pregnancy Test; RANDOM: Randomization; PK: Pharmacokinetics; DA: Drug Administration; AE: Adverse Events. |
Since this was a pilot study, we didn’t perform a formal sample size calculation. However, the number of participants was considered adequate for the study objectives.
Participants
A complete clinical history (CH) including demographic data, weight, height, BMI, electrocardiogram (EKG), vital signs (VS) [systolic and diastolic blood pressure (SBP, DBP), heart rate (HR), and body temperature], blood test (BT) to determine haematological and biochemical parameters and urinalysis (UA) to evaluate density and pH, and to determine the presence of nitrites, leucocytes, proteins, glucose, ketonic bodies, urobilinogen, bilirubin and erythrocytes in urine were performed to premenopausal women in order to verify the inclusion (IC) and exclusion criteria (EC).
All the participants included met the inclusion criteria. They were premenopausal women (age 18-45 years) with a normal body mass index (BMI 18.5- 24.99 kg/m2), non-smokers or smoked ≤ 5 cigarettes/day. None of the participants presented any organic, psychiatric, or gynaecological abnormalities on the medical examination (ME). All participants had a regular menstrual cycle and they agreed to use condom as a contraception method. All participants agreed to avoid sexual intercourse with penetration for at least 24 hours before each study visit.
Exclusion criteria (EC) were as follows: history of serious adverse reactions or hypersensitivity to any drug; any clinically-important abnormal physical findings, particularly gynaecological; any history of drug and alcohol abuse; clinically significant abnormal laboratory and/or EKG values; presence of any significant surgical or medical condition; regular use of medication in the month prior to study initiation; any contraindication for oral Pentoxifylline as specified in the product leaflet.
Gynaecological examination (GE) included the evaluation of signs/symptoms of vaginal inflammation, vaginal infection, and excluding gynaecological diseases: vulvovaginitis; infectious vulvovaginitis, candidal vulvovaginitis or fungal infection; trichomoniasis; bacterial vaginosis; irritative vulvovaginitis; condylomata or genital warts; bartholinitis or cervicitis; increased vaginal discharge and characteristic changes (colour, smell, texture and appearance); redness of the vulvar and vaginal mucosae; warts on the labia and/or anus; and painful lump (with abscess). In addition, the examining gynaecologist asked about the presence of any of the following symptoms: pruritus or itching of the external genitalia and/or vagina, burning or stinging sensation in the genitalia; urinary tract problems such as frequent urination, burning urination, heavy flow and any other symptoms. Participants were instructed in the completion of Visual Analogue Scale (VAS) for evaluate local symptoms. VAS were graded for severity using on the visual analogue scale (0 to 100 mm), being 0 “without intensity” and 100 “maximum intensity””. The following symptoms were assessed: genital itching; irritation; genital burning or stinging; increased vaginal discharge; thicker vaginal discharge; greenish-yellowish vaginal discharge; burning with urination; swelling of the vulva; frequent urination; and “other symptoms”. Moreover, vaginal culture (VC) was performed in the screening (SV) and final visits (FV). Serology (SERO) to check for hepatitis B and C, HIV, and syphilis were performed. A blood pregnancy test (BPT) and a test to check the presence of drugs [opiates, ethanol, cocaine, cannabis, benzodiazepines and amphetamines] in urine (DU) were performed.
Medication
PKB171 gel is an aqueous-based vaginal gel with applicator device (syringe) whose active ingredient is Pentoxifylline. This investigational medicinal product is formulated in a conventional vehicle for vaginal administration in accordance with the guidelines for the Neutral Gel indicated in the Spanish National Formulary [30]. The PKB171 gel formulation has a pH value of 6.3 ± 0.5, suitable for intravaginal pH.
Treatment was provided to the volunteer by a member of the research team and self-administered by the volunteer herself under clinical supervision, provided with the privacy to carry out the self-administration. The volunteers were instructed on how to apply the gel: i) in lying down position, insert the applicator as deeply as possible into the vagina, ii) push the plunger of the syringe all the way, iii) remove the applicator, iv) use toilet paper for the necessary external cleaning and v) remain in lying down position for 1 hour.
The PKB171 gel vaginal was self-administered after randomization, at visit 1 in the dose-escalation study and at visits 1 and 2 in the extension substudy.
We previously confirmed local tolerability of PKB171 gel in two preclinical studies conducted in animal models. The gel, administered at concentration of 8% and 4%, was well-tolerated after repeated vaginal application in rabbits for seven consecutive days [data on file] and showed no evidence of sensitising potential in the Buehler test in guinea pigs [data on file], respectively. Pharmacokinetic studies were also carried out in rabbits after vaginal application.
Randomization and masking
Randomization in both the dose-escalation and extension studies was performed using a randomization table generated by an ad hoc programme based on the pseudo-random routine using SAS (v.9.4) for Windows [31]. For blinding purposes, both the active and placebo gels were identical in appearance and packaging. The investigator received a treatment allocation number for each participant. The investigators and all participants remained blinded to the treatment allocation for the duration of the study.
Pharmacokinetics
Ten venous samples of 10 mL were collected into EDTA K3 plastic tubes at baseline (premedication), and at +10, +20, +30, +45 minutes and at +1, +2, +4, +8 and +12 hours after PKB171 gel vaginal administration through a cannula placed in the arm of the volunteer.
Blood samples were centrifuged within 30 minutes after extraction for 10 minutes at 3000 rpm and at 4˚C and the resulting plasma samples were separated into two aliquots of 1 mL for Pentoxifylline and its metabolite, 5-hydroxi Pentoxifylline respectively that were stored at -20˚C ± 5˚C until assayed.
Bioanalytical determinations for Pentoxifylline and 5-hydroxi Pentoxifylline were performed at the Bioanalysis Unit of Laboratorios Echevarne (Barcelona, Spain) using the previously-validated technique of Liquid chromatography coupled to tandem mass spectrometry (triple quadrupole) with electrospray probe (LC-MS/MS) following a full validated method coded TB/16/002 according to Guideline on bioanalytical method validation (Guideline on bioanalytical method validation [32], with a limit of quantification of 5 ng/mL for both Pentoxifylline and 5-hydroxi Pentoxifylline. Analytical work was performed according to Good Laboratory Practices (GLP).
The calibration curve ranged from 5 to 5000 ng/mL for 5 ng/mL for both Pentoxifylline and 5-hydroxi Pentoxifylline. The internal standard for Pentoxifylline and 5-hydroxi Pentoxifylline was 7-(β-hydroxyethyl) theophylline. Withinrun accuracy (at 5,50,200,600,1000 and 5000 ng/mL) for Pentoxifylline and 5-hydroxi Pentoxifylline ranged from 87.19–108.43% to 85–107.62% for Pentoxifylline and 5-hydroxi Pentoxifylline, respectively. Between-run precision was not higher than 7.71% and 7.84% for Pentoxifylline and 5-hydroxi Pentoxifylline, respectively.
Pharmacokinetic parameters to evaluate absorption and to determine the PK profile of Pentoxifylline and its metabolite, 5-hydroxi Pentoxifylline were calculated by non-compartmental analysis using Profesional WinNonlin-Pro version 2.1 (Pharsight Corporation, Saint Louis, MO). Missing samples were treated as non-reportable concentration. Peak plasma concentration (Cmax) and the time to reach peak concentration (tmax) were obtained directly from the raw data. The terminal plasma elimination half-life (t½), was calculated as t½ = 0.639/ke, where ke represents the first-order elimination rate constant associated with the terminal (log-linear) portion of the curve, estimated via linear regression of time versus log concentration. The area under the plasma concentration-time curve (AUC) from 0 to ∞ (AUC0∞) was calculated as AUC0∞= AUC0tx + C tx /ke, where tx is the time of the last concentration (Ctx), exceeding the limit of quantification. The apparent volume of distribution (V/F) was calculated as V/F=D/(ke*AUC0∞), where D is dose and F is bioavailability. Plasma clearance (Cl/F) was calculated as D/AUC0-t. Dose proportionality was assessed by expressing dose dependent pharmacokinetic parameters (AUC0t and Cmax) for Pentoxifylline and 5-hydroxi Pentoxifylline in the three doses versus the 100 mg dose.
Safety and tolerability
Safety and tolerability were evaluated according to presence of any spontaneously-reported AEs, in addition to the presence of any other clinically-significant abnormalities in the following investigator-determined variables: vital signs, laboratory parameters evaluated in the blood test (BT), and gynaecological evaluation (GE).
The evaluations performed in both studies (dose-escalation and extension substudy) are summarised in Table 1. Inclusion criteria (IC) including vital signs (VS), blood pregnancy test (BPT), and drugs in urine (DU) were verified at visit 1 before PKB171 gel vaginal administration (DA). Spontaneously reported AEs were recorded through the study period.
Study outcomes
The primary endpoint for the dose escalation study was to determine the maximum tolerated dose (MTD) of PKB171 in terms of local tolerability after intravaginal single dose administration to healthy premenopausal women. Secondary endpoints included (i) to evaluate safety and tolerability of PKB171 after intravaginal single dose administration to healthy premenopausal women, (ii) to describe the pharmacokinetic profile of PKB171 after intravaginal single dose administration to healthy premenopausal women. For the extension substudy the endpoint was to evaluate safety and tolerability of PKB171 after intravaginal multiple dose administration to healthy premenopausal women.
Statistical analysis
Descriptive statistics were calculated for all pharmacokinetic parametres for both studies. Dose linearity for Cmax and AUC was evaluated by means of an ANOVA of 1 factor (dose).
For the safety and tolerability analysis, all participants who completed the study were included. The incidence of treatment-emergent AEs (TEAEs) was classified by system organ class and preferred term according to the Medical Dictionary of Regulatory Activities (MedDRA version 21.1), the relationship to the study drug, and the severity for each dose.
Changes from baseline in the VAS scores were summarized by descriptive statistics. The mean change in VAS scores at 4 hours or 24 hours after vaginal administration was compared to the values at the final visit by means of the t-test of paired data or its nonparametric equivalent, as appropriate, for each treatment. All statistical analyses were performed with the SAS, v9.4 for Windows.
Study population
In the dose-escalation study, 37 volunteers were screened and 24 were randomized to receive active treatment or placebo, as follows: PKB171 gel 100 mg (n=6), 150 mg (n=6), 200 mg (n=6) or placebo (n=6). All participants (n=24) completed the study (Figure 1).
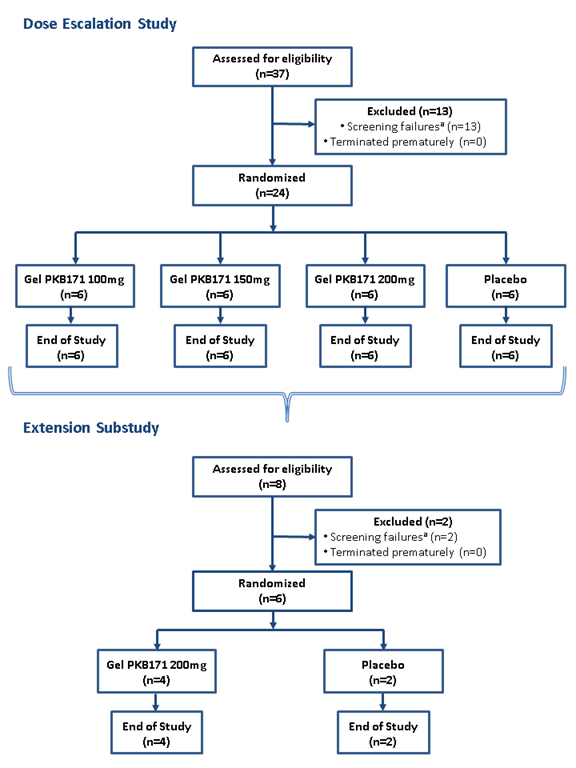
Figure 1. Participant distribution (CONSORT flow-diagram). aDetermined by inclusion/exclusion criteria
In the extension study, 6 volunteers recruited from the dose-escalation study were randomized to receive either the MTD (200 mg) or placebo in a 4:2 ratio (Figure 1).
The study drugs were dosed at the study site under medical supervision, so the treatment compliance was confirmed in all participants in both studies.
Twenty-four females (age, 20 to 31 years) and six females (age, 22 to 31 years) were included in the dose-escalation study and extension substudy, respectively. All treatment groups were comparable with regard to the sociodemographic variables (Table 2). All serology, blood pregnancy (at screening), drugs in urine, and pregnancy test in urine (predose) were negative and all ECG were normal for both the dose-escalation study and the extension substudy.
Table 2. Demographic and baseline characteristics
Dose Escalation Study Extension Substudy |
|
PKB171 Gel 100mg
(N=6) |
PKB171 Gel 150mg
(N=6) |
PKB171 Gel 200mg
(N=6) |
PKB171 Gel All doses
(N=18) |
PKB171 Gel Placebo
(N=6) |
PKB171 Gel 200mg
(N=4) |
PKB171 Gel
Placebo
(N=2) |
Caucasian n (%) |
6 (100.0) |
6 (100.0) |
6 (100.0) |
18 (100.0) |
6 (100.0) |
4 (100.0) |
2 (100.0) |
Age (years) |
23.5 (3.9) |
23.3 (1.8) |
25.3 (4.1) |
24.1 (3.4) |
22.3 (2.9) |
26.3 (4.0) |
23.0 (0.0) |
Weight (Kg) |
61.1 (7.0) |
60.9 (3.0) |
58.9 (7.6) |
60.3 (6.0) |
58.0 (2.3) |
63.6 (5.2) |
59.7 (5.9) |
Height (cm) |
166.0 (5.0) |
170.0 (3.0) |
166.2 (7.7) |
167.4 (5.6) |
164.2 (4.4) |
170.5 (7.2) |
163.0 (0.0) |
BMI (kg/m2) |
22.2 (2.1) |
21.1 (1.0) |
21.3 (2.1) |
21.5 (1.8) |
21.6 (1.2) |
21.9 (2.1) |
22.5 (2.2) |
Duration of menstrual cycle |
|
|
|
28.39 (0.85) |
27.33 (1.75) |
28.00 (0.82) |
29.00 (1.41) |
Data are presented as mean (standard deviation) |
|
|
The demographics and baseline characteristics for each group are summarized in Table 2. In both studies (dose-escalation and extension substudy), the treatment groups were comparable with regard to the duration of the participant’s menstrual cycle.
Pharmacokinetics
The Per-Protocol population, (defined as all randomized subjects who met the entry criteria,
received all study medication, completed the study and did not present protocol violations)
was used for the pharmacokinetic analysis. Plasma concentration over time obtained in dose escalation study for Pentoxifylline and 5-hydroxi Pentoxifylline after vaginal administration of PKB171 gel are displayed for the 3 doses in normal scale (Figure 2) and log scale (Figure 3).
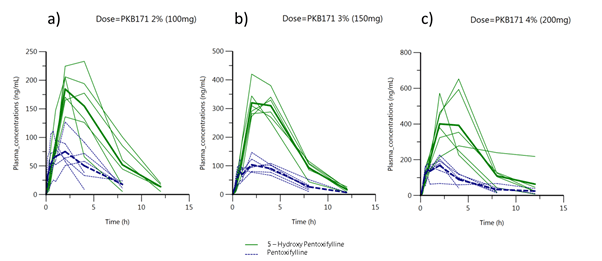
Figure 2. Pentoxifylline and 5-hydroxi Pentoxifylline plasma concentrations obtained in dose escalation study in normal scale versus time profiles for PKB171 gel (2%) 100mg dose (a), PKB171 gel (3%) 150mg dose (b) and PKB171 gel (4%) 200mg dose (c). The green lines represent individual values for 5-hydroxiPentoxifylline and the solid green line depicts the mean values. The blue dotted lines represent individual values for Pentoxifylline and solid blue dotted line represents the mean values
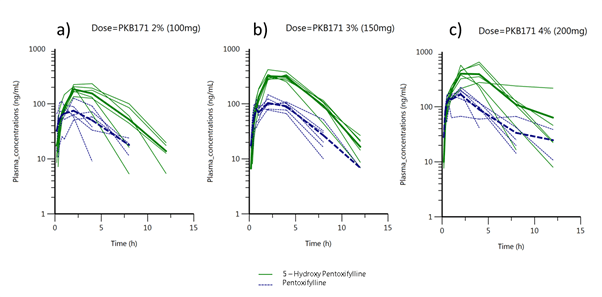
Figure 3. Pentoxifylline and 5-hydroxiPentoxifylline plasma concentrations obtained in dose escalation study in log scale versus time profiles for PKB171 gel (2%) 100mg dose (a), PKB171 gel (3%) 150mg dose (b) and PKB171 gel (4%) 200mg dose (c). Green lines represent individual observations and green solid line the mean of the observations for 5-hydroxiPentoxifylline. Blue dotted lines represent individual observations and blue solid dotted line the mean of the observations for Pentoxifylline
Mean pharmacokinetic parameters for Pentoxifylline and 5-hydroxi Pentoxifylline are displayed for the three doses in Table 3.
Table 3. Mean pharmacokinetic parameters and normalized data for Pentoxifylline and 5-hidroxiPentoxifylline in healthy female volunteers
Parameters |
Pentoxifylline |
5-hidroxiPentoxifylline |
N |
Arithmetic mean (sd)
Median [min-max] |
N |
Arithmetic mean (sd)
Median [range] |
|
100 mg |
AUC0t (ng·h/mL) |
6 |
351.81 |
(115.30) |
6 |
1048.46 |
(319.64) |
Cmax (ng/mL) |
6 |
90.34 |
(25.65) |
6 |
187.96 |
(33.99) |
Tmax (h) |
6 |
1.50 |
[0.75-4.00] |
6 |
2.00 |
[2.00-4.00] |
t1/2 (h) |
4 |
2.20 |
(1.36) |
6 |
1.92 |
(0.56) |
V/F (L) |
4 |
749.01 |
(367.97) |
0 |
- |
- |
Cl/F (L/h) |
4 |
264.16 |
(108.75) |
0 |
- |
- |
|
150 mg |
AUC0t (ng·h/mL) |
6 |
584.19
389* |
(138.77) |
6 |
1934.52 1289* |
(265.15) |
Cmax (ng/mL) |
6 |
114.03
76* |
(22.58) |
6 |
338.35
225* |
(45.04) |
Tmax (h) |
5 |
2.00 |
[0.75-4.00] |
6 |
3.00 |
[2.00-4.00] |
t1/2 (h) |
6 |
2.44 |
(0.36) |
6 |
1.87 |
(0.28) |
V/F (L) |
5 |
841.29 |
(215.75) |
0 |
- |
- |
Cl/F (L/h) |
5 |
240.37 |
(64.36) |
6 |
1.98 |
(0.28) |
|
200 mg |
AUC0t (ng·h/mL) |
6 |
779.57
390* |
(196.05) |
6 |
2514.80 1257* |
(697.96) |
Cmax (ng/mL) |
6 |
178.39
89* |
(42.20) |
6 |
471.55
236* |
(153.21) |
Tmax (h) |
6 |
1.63 |
[0.75-2.00] |
6 |
3.33 |
[2.00-4.00] |
t1/2 (h) |
5 |
4.13 |
(4.83) |
6 |
5.30 |
(8.65) |
V/F (L) |
5 |
1010.32 |
(874.66) |
6 |
286.93 |
(198.33) |
Cl/F (L/h) |
5 |
204.28 |
(45.67) |
6 |
74.15 |
(35.47) |
Data are presented as arithmetic mean (standard deviation), normalized by 100 mg dose* and median [minimum-maximum]. N is number of individuals with data to obtain the parameters |
As Table 3 shows, for all three doses, mean plasma concentrations and total exposure of the active metabolite were higher than for the parent drug. The mean Cmax values for the 100 mg, 150 mg, and 200 mg dose were, respectively, two, three, and 2.5 times higher. The mean AUC0t values were three times higher at all three doses. The median tmax values were similar for Pentoxifylline (1.5-2 h) and its metabolite (2-3.3 h) at all three doses. The mean t1/2 was similar for Pentoxifylline (2.20-2.45 h) and its metabolite (1.85-1.92 h) in 100 and 150 mg doses, but the t1/2 doubled at the 200 mg dose for both the treatment drug and its metabolite.
AUC0t and Cmax obtained after 100 and 200 mg of PKB171 gel maintained the linearity in terms of absorption and disposition for Pentoxifylline and 5-hydroxi Pentoxifylline (Figure 4).
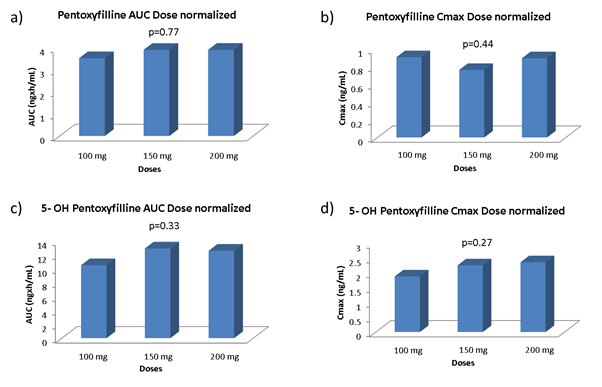
Figure 4. Pentoxifylline dose normalized AUC (a) and Cmax (b) for the three doses. 5- hydroxi Pentoxifylline dose normalized AUC (c) and Cmax (d) for the three doses. p value between the three doses is shown for the 4 comparisons
Safety and tolerability
The safety and tolerability population defined as all randomized subjects who took at least one dose of the study medication was used for safety analyses.
Adverse events / gynecological examination: All AEs reported in this phase I study occurred in the dose-escalation study (no AEs were observed in the extension substudy). A total of 12 participants who received active treatment reported 15 AEs while 5 patients in the placebo group reported 6 AEs. The following AEs were considered to be potentially associated with PKB171 gel medication: vaginal discharge (n=10); vaginal inflammation (1); vaginal pruritus (2); vaginal candida infection (1); and headache (1). In the placebo group, reported AEs included vaginal discharge (n=5) and vaginal candida infection (1). The most common AE in both groups was vaginal discharge (observed in 55.6% and 83.3%) of patients in the active and placebo groups, respectively.
Of the 15 AEs in the active treatment group, 14 were mild while one was moderately severe. All six AEs in the placebo group were considered mild. All 21 AEs (21 events in 17 participants) reported in the study were either mild or moderate, and all but one (headache) was classified as a delayed local reaction. These AEs were considered to be associated with the study treatment. All were resolved with no sequelae. No serious AE was reported during the study in any treatment arm. Table 4 summarizes the reported AEs and other relevant information.
Table 4. Adverse Events (AEs) by System Organ Class (SOC) and Preferred Term (PT) in the dose-escalation study codified with (MedDRA version 21.1)
|
PKB171 Gel 100mg
(N=6) |
PKB171 Gel 150mg
(N=6) |
PKB171 Gel 200mg
(N=6) |
PKB171 Gel All doses
(N=18) |
PKB171 Gel Placebo
(N=6) |
At least one AE, n (%) nAEs |
6 (100.0) 8 |
4 (66.7) 4 |
2 (33.3) 3 |
12 (66.7) 15 |
5 (83.3) 6 |
Infections and Infestations
Candida infection |
1 (16.7) 1 |
0 (0.0) 0 |
0 (0.0) 0 |
1 (5.6) 1 |
1 (16.7) 1 |
Nervous System disorders
Headache |
0 (0.0) 0 |
1 (16.7) 1 |
0 (0.0) 0 |
1 (16.7) 1 |
0 (0.0) 0 |
Reproductive System and breast disorders
Vaginal discharge
Vaginal inflammation |
5 (83.3) 5
1 (16.7) 1 |
3 (50.0) 3
0 (0.0) 0 |
2 (33.3) 2
0 (0.0) 0 |
10 (55.6) 10
1 (5.6) 1 |
5 (83.3) 5
0 (0.0) 0 |
Skin and subcutaneous tissue disorders
Pruritus |
1 (16.7) 1 |
0 (0.0) 0 |
1 (16.7) 1 |
2 (11.1) 2 |
0 (0.0) 0 |
N: number of participants by treatment group; n: number of participants with at least one AE; %: percentages calculated based on number of participants; nAEs: number of Aes |
Biochemistry, haematology and urinalysis: We observed no clinically-significant abnormalities in any laboratory values (biochemistry, haematology, urinalysis) in either the dose-escalation or extension studies in the screening and in the final study.
Vital signs: In the dose-escalation study, all vital signs (blood pressure, heart rate and body temperature) all remained in the normal range at all the time points: Similarly, in the extension substudy, these values were all normal at all assessments: predose, days 2 and 3, and at the final visit.
Visual analogue scale: Nine (37.5%) participants reported a total of 16 symptoms that were graded on the VAS from 0 to 100. Of these, 13 (81.3%) occurred after PKB171 gel administration and 3 (18.8%) after placebo. Twelve symptoms (75%) were reported at the final visit and 4 (25%) at 4 hours post-treatment administration. All symptoms had resolved on the following follow-up visit. In the PKB171 gel group, seven symptoms (43.8%) were reported after the 100 mg dose and 6 (37.5%) after the 200 mg dose.
The most common symptoms were thicker and increased vaginal discharge, each of which occurred in five (20.8%) participants. Genital itching was the second most-commonly reported symptom (n=3, 12.5%). In the active treatment group, thicker vaginal discharge was reported at the final visit by three (12.5%) participants who received the 100 mg dose) and by two (8.3%) participants who received the 200 mg dose. Increased vaginal discharge was reported at the final visit by two (8.3%) participants who received PKB171 gel (200 mg), at +4 hours by two (8.3%) participants in the placebo group, and by one (4.2%) volunteer who received the 200 mg dose of PKB171 gel. The three symptoms with the highest VAS grade were one case of increased vaginal discharge after placebo at 4 h post-administration (VAS=44 mm), one case of genital itching after placebo at the final visit (VAS=36 mm), and one case of “other” (intravaginal itching) after 200 mg dose of PKB171 gel at the final visit (VAS=26 mm).
In the extension substudy, only one VAS-graded symptom was reported: predose genital itching in one volunteer who received the 200 mg dose of PKB171 gel in the dose-escalation study.
This phase I trial was performed to determine the MTD according to local tolerability of intravaginally-administered PKB171 gel in a group of healthy female volunteers. This is the first study to evaluate the pharmacokinetics, safety, and tolerability of PKB171 vaginal gel in this population. Vaginal administration of PKB171 gel presented a good safety and tolerability profile and no serious AEs were observed at any of the study doses. The MTD in the present study was the maximum dose tested, 200 mg (equivalent to 3.3 mg/kg), with a good safety profile. The doses evaluated in this study (100 mg, 150 mg, 200 mg) were based on data obtained in previous studies carried out in rabbits (intravaginal administration [data on file]) and after both oral and intravenous administration in humans. The human equivalent doses in the rabbit study (vaginal administration) and in humans (oral administration) in these studies were 8.4 mg/kg and 6.7 mg/kg, respectively. The maximum dose in the present study (5 g of PKB171 gel at a maximum concentration of 4% of Pentoxifylline once a day for 2 consecutive days), which equates to 200 mg/day, was substantially lower than the maximum recommended dose in patients with intermittent claudication due to chronic occlusive arterial disease, which is 400 mg three times per day (1200 mg/day) [27].
After vaginal administration of PKB171 gel, the peak plasma concentrations of the active metabolite (5-hydroxi Pentoxifylline) were three times greater than the parent drug (Pentoxifylline). This ratio between parent drug and its metabolite is consistent with previous reports after oral [33,34] and intravenous administration of Pentoxifylline [34]. After vaginal administration at doses ranging from 100 to 200 mg, we found that Pentoxifylline was rapidly absorbed, achieving peak plasma concentrations (after dose normalization) of approximately 90 ng/mL at 1.5-2 h for all three doses. For the active metabolite, peak plasma concentrations (after dose normalization) of approximately 200 ng/mL occurred at 2-3.3 h for the three doses studied. These findings are lower than those obtained by Smith, et al. [33], who administered 100 and 200 mg of oral Pentoxifylline, which resulted in a peak plasma concentration of Pentoxifylline (after dose normalization) of approximately 300 ng/mL at 0.4 h and peak plasma concentrations (after dose normalization) of 5-hydroxi Pentoxifylline of approximately 450 ng/mL at 0.7 h. These differences in peak plasma concentrations (for both Pentoxifylline and 5-hydroxi Pentoxifylline) could be attributable to the faster and greater absorption after oral administration compared to vaginal administration, as vaginally-administered drugs must first be dissolved in the vaginal lumen and penetrate the membrane to enter the blood vessels. Consequently, the metabolite generated from the parent drug Pentoxifylline will be lower too.
In terms of dose linearity (at all three dose levels), our findings were consistent with the results reported by Smith, et al. [33] after oral administration of a Pentoxifylline solution, confirming that dose linearity in the absorption and disposition of up to 200 mg is maintained, regardless of the route of administration. The exposure (AUC) of Pentoxifylline and 5-hydroxi Pentoxifylline obtained in our study was lower than that obtained by Beerman, et al. [34], who administered 200 mg of intravenous Pentoxifylline in healthy humans (men and women). Again, these findings were expected, as bioavailability is lower after vaginal administration compared to intravenous administration. However, Beerman and colleagues found that oral administration of Pentoxifylline at a 400 mg dose [34] resulted in less exposure (normalized to the 200 mg dose) than observed in our study after administration of a 200 mg dose. Given the hydrophilic nature of Pentoxifylline, absorption could be expected to be low, but other molecular or pharmacological characteristics of the molecule could influence vaginal absorption. The systemic absorption of PKB171 gel observed in our study after vaginal administration may be due the hydrophilic characteristics of this drug, as the vasoactive effect could facilitate absorption. Moreover, a systemic effect has been observed in several other drugs approved as local treatments after vaginal administration [35]. However, the differences in the findings of Smith, et al. [33] and Beerman, et al. [34] must be interpreted cautiously due to differences in the two study populations (e.g., gender, the methods used to analyse the parent drug and metabolites, the route of administration, and pharmaceutical forms).
In the present trial, we did not observe any clinically relevant changes on the physical examination, nor in vital signs, ECG parameters, or laboratory values in either the dose-escalation study or the extension substudy. The most common AE was vaginal discharge. Notably, we did not observe any episodes of nausea, which is the most commonly-reported AE after oral administration of sustained-release Pentoxifylline [27]. Similarly, we did not observe any of the other AEs—except for headache—commonly observed after Trental administration (dizziness, flushing, malaise, vomiting, abdominal discomfort, bloating, diarrhea or dyspepsia). In general, vaginal administration of PKB171 gel at all three dose levels was well-tolerated. Moreover, the incidence of AEs did not increase as a function of dose, indicating that these effects are not dose dependent. In addition, we observed no differences between the active treatment and placebo in the percentage of volunteers who developed AEs [36-38].
In conclusion, the safety and tolerability profile, and the PK linearity observed at the study doses, suggest that vaginal administration of PKB171 gel 2 days/cycle may represent a novel, safe, and easy to use therapeutically approach for subfertile or infertile couples with low sperm quality. Efficacy trials are currently underway.
The authors thank Mª Angeles Funes for technical editing, Dr. J.M.Palacios, R&D Director of Prokrea BCN SL for making possible the study, Dr, J.L.Fabregas (Laboratorio Sanitatis (Vitoria, Spain)) for coordinating the pharmaceutical development, Innoqua Toxicology SL (Barcelona, Spain) for performing the preclinical studies, TSF SA (Barcelona, Spain) for technical support and Bradley Londres for professional English language editing.
All authors contributed significantly to the study conception and design. JP, JC, MP and MRBV performed research and acquired data, MRBV and IGS analyzed data; MRBV, JC, MP, JP and RMAA interpreted data; MRBV, JC, MP and RMAA wrote the paper. All the authors were involved in revising the manuscript for important intellectual content and approving the final version for publication.
The study was funded by Prokrea BCN, SL.
The authors declare no conflicts of interest.
- Cui W (2010) Mother or nothing: the agony of infertility. Bull World Health Organ 88: 881-882. [Crossref]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J de, et al. (2017) The international glossary on infertility and fertility care. Fertil Steril 8: 393-406. [Crossref]
- Thurston L, Abbara A, Dhillo WS (2019) Investigation and management of subfertility. J Clin Pathol 72: 579-587. [Crossref]
- Agarwal A, Mulgund A, Hamada A, Chyatte MR (2015) A unique view on male infertility around the globe. Reprod Biol Endocrinol 13: 37. [Crossref]
- World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen.
- Brian RW, Walsh TJ (2014) The epidemiology of male infertility. Urol Clin North Am 41: 195-204. [Crossref]
- National Institute for Health and Care Excellence (NICE) (2013) Fertility problems (2013): assessment and treatment. Retrieved from: https://www.nice.org.uk/guidance/cg156/resources/fertility-problems-assessment-and-treatment-35109634660549. Accessed 2020 March 26.
- Cooper TG, Noonan E, Von ES, Auger J, Baker HWG, et al. (2009) World health organization reference values for human semen characteristics. Hum Reprod Update 16: 231-245. [Crossref]
- National collaborating centre for women's and children's health (UK) (2004) Fertility: Assessment and Treatment for People with Fertility Problems. RCOG Press. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK45935/pdf/Bookshelf_NBK45935.pdf. Accessed 2020 February 17.
- Alahmar AT (2018) The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med 45: 57-66. [Crossref]
- EAU Guidelines on Male infertility, (2016) Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Infertility-2016-2.pdf. Accessed 03 June 2020.
- Duca Y, Calogero AE, Cannarella R, Condorelli RA, La Vignera S (2019) Current and emerging medical therapeutic agents for idiopathic male infertility. Expert Opin Pharmacother 20: 55-67. [Crossref]
- Garg H, Kumar R (2015) Empirical drug therapy for idiopathic male infertility: What is the new evidence? Urology 86: 1065-1075. [Crossref]
- Stanhiser J, Steiner AZ (2018) Psychosocial aspects of fertility and assisted reproductive technology. Obstet Gynecol Clin North Am 45: 563-574. [Crossref]
- Chambers GM, Adamson GD, Eijkemans MJ (2013) Acceptable cost for the patient and society. Fertil Steril 100: 319-327. [Crossref]
- Yunes R, Fernández P, Doncel GF (2005) Cyclic nucleotide phosphodiesterase inhibition increases tyrosine phosphorylation and hyper motility in normal and pathological human spermatozoa. Biocell 29: 287-293. [Crossref]
- Mehrannia T (2009) The effect of pentoxifylline in semen preparation for intrauterine insemination. Pak J Med Sci 25: 359-363.
- Kovačič B, Vlaisavljević V, Reljič M (2006) Clinical use of Pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl 27: 45-52. [Crossref]
- Shen MR, Chiang PH, Yang RC, Hong CY, Chen SS (1991) Pentoxifylline stimulates human sperm motility both in vitro and after oral therapy. J Clin Pharmac 31: 711-714. [Crossref]
- Merino G, Martínez Chéquer JC, Barahona E, Bermúdez JA, Morán C, et al. (1997) Effects of Pentoxifylline on sperm motility in normogonadotropic asthenozoospermic men. Arch Androl 39: 65-69. [Crossref]
- Yovich JM, Rohini Edirisinghe W, Cummins JM, Yovich JL (1988) Preliminary results using Pentoxifylline in a pronuclear stage tubal transfer (PROST) program for severe male factor infertility. Fertil Steril 50: 179-181. [Crossref]
- Tesarik J, Thébault A, Testart J (1992) Effect of pentoxifylline on sperm movement characteristics in normozoospermic and asthenozoospermic specimens. Hum Reprod 7: 1257-1263. [Crossref]
- Paul M Sumpter JP, Lindsay KS (1995) Action of pentoxifylline directly on semen. Hum Reprod 10: 354-359. [Crossref]
- Aviado DM, Porter JM (1984) Pentoxifylline: A new drug for the treatment of intermittent claudication. Mechanism of action, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy 4: 297-307. [Crossref]
- Frampton JE, Brogden RN (1995) Pentoxifylline (oxpentifylline) A review of its therapeutic efficacy in the management of peripheral vascular and cerebrovascular disorders. Drugs Aging 7: 480-503. [Crossref]
- Jull AB, Waters J, Arroll B (2000) Oral pentoxifylline for treatment of venous leg ulcers. Cochrane Database Syst Rev 2: CD001733. [Crossref]
- Jacoby D, Mohler ER (2004) Drug treatment of intermittent claudication. Drugs 64: 1657-1670. [Crossref]
- Trental [Pentoxifylline]. Product monograph, [electronic source] [accessed 2020 March 26] Available from: https://www.medicines.org.uk/emc/product/909/smpc.
- Mosher WD BC (1996) Understanding U.S. fertility: continuity and change in the National Survey of Family Growth, 1988-1995. Fam Plann Perspect 28: 4-12. [Crossref]
- Abdelkader AM, Yeh J (2009) The potential use of intrauterine insemination as a basic option for infertility: A review for technology-limited medical settings. Obstet Gynecol Int. [Crossref]
- Formulario Nacional (2015) 2 ª Edición. Ed. Ministerio de Sanidad y Consumo, Madrid.
- SAS [computer program]. Version 9.4. Cary, NC: SAS Institute Inc; 2014. (SAS Institute Inc., Cary, NC, USA).
- Guideline on bioanalytical method validation (EMEA/CHMP/EWP/192217/2009, 21 July 2011) Retrieved from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed 2015 December 03.
- Smith RB, Waller ES, Doluisio JT, Bauza MT, Puri SK, et al. (1986) Pharmacokinetics of Orally Administered Pentoxifylline in Humans. J Pharm Sci 75: 47-52. [Crossref]
- Beermann B, Ings R, Mansby J, Chamberlain J, McDonald A (1985) Kinetics of intravenous and oral Pentoxifylline in healthy participants. Clin Pharmacol Ther 37: 25-28.
- Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, et al. (2004) Why consider vaginal drug administration? Mod Trends Fertil Steril 82: 1-12. [Crossref]
- Matson PL, Yovich JM, Edirisinghe WR, Junk SM, Yovich JL (1995) An argument for the past and continued use of pentoxifylline in assisted reproductive technology. Hum Reprod 1: 67-71. [Crossref]
- Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, et al. (2014) Antioxidants for male subfertility. Cochrane Database Syst Rev 14: CD007411. [Crossref]
Editorial Information
Editor-in-Chief
Ying-Fu Chen
Kaohsiung Medical University, Taiwan
Article Type
Research Article
Publication history
Received date: October 12, 2020
Accepted date: October 29, 2020
Published date: November 02, 2020
Copyright
©2020 Ballester MR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Ballester MR, Puntes M, Coimbra J, Perelló J, Gich I, et al. (2020) Pharmacokinetics, safety, and tolerability of Pentoxifylline intravaginal gel for subfertile/ infertile couples with low sperm quality: phase I randomized controlled clinical trial in healthy women. Trends Med 21: DOI: 10.15761/TiM.1000263




