PET Myocardial Perfusion Imaging is one of the most useful tools for clinicians when assessing coronary artery disease, due to its increasing availability, it is of superb importance to recognize the physiological basis and the basic principles for its interpretation. The most common protocols make use of Rubidium or Ammonia as radiotracers to evaluate myocardial perfusion and these agents have different characteristics, one caveat of Ammonia is that an on-site cyclotron is needed due to its short half-life. PET-MPI exhibits abnormalities not only on epicardial arteries but also in the microcirculation, as well as other parameters such as left ventricle ejection fraction and coronary flow reserve. Hybrid studies combine a functional with an anatomical study (i.e. PET-MPI and coronary computed tomography, respectively) and allows for a more complete evaluation of patients and to predict outcomes with great quality.
nuclear cardiology, PET-MPI, physiology, coronary artery disease, radiotracers, myocardial infarction
Cardiovascular Disease (CVD) is common around the world, affecting most adults over the age of 60 [1]. In 2012 and 2013, CVD was estimated to have caused around 17 million deaths worldwide [2]. The 2019 Heart Disease and Stroke Statistics published by the American Heart Association (AHA) states that 48% of the population over the age of 20 have some type of cardiovascular disease, with prevalence increasing with age for both men and women [3]. According to the World Health Organization (WHO), in the year 2030, CVD will account for the death of nearly 23.6 million people [4].
CAD is responsible for around one-third to one-half of overall CVD cases, with ischemic heart disease as the first cause of death in adults from both developing and developed countries [3,5]. According to a study that evaluated patients from the Framingham study, the risk of developing some type of CAD at some point in life was 40 to 49% for males and 32% for females [6].
The Mexican National Health System singles out Ischemic Heart Disease (IHD), along with type 2 diabetes, as the two leading health problems nationally. IHD has been identified as the leading cause of death in both males and females older than 65 [4]. In 2020, IHD was documented as the leading cause of cardiovascular morbidity (30%) and the second cause of cardiovascular mortality (18%) [7]. The estimated spendings by the Mexican National Health System on MI alone amount to approximately 39 billion pesos every year.
In the last decades, Positron-Emission Tomography Myocardial Perfusion Imaging (PET-MPI) has increased its clinical utility in detecting CAD with relevant hemodynamical obstructions. Additionally, its usefulness has extended to cardiovascular risk stratification [8]. PET-MPI evaluates myocardial perfusion with certainty. It is considered the gold standard for measuring absolute myocardial flow and myocardial contractile function during rest and stress in one single session [9,10].
PET-MPI uses radiotracers to produce images that reflect the metabolic activity of body tissues. More than a graphic illustration of heart segments, it is a functional and physiological representation of the patient’s heart [11]. PET-MPI has multiple applications, besides the study of myocardial perfusion. It is also helpful for evaluating ventricular function and volumes by applying the GATED-PET technique; it calculates the left ventricular ejection fraction (LVEF) by combining the imaging acquisition with the electrocardiogram to identify the cardiac cycle phases. Furthermore, PET-MPI is able to quantify the myocardium's coronary flow in milliliters per minute per gram of tissue (mL/min/g) units. It assesses endothelial function integrity with a cold pressor test (CPT) by immersing a thoracic or pelvic limb into a solution at 3°C [12].
The obtained data helps to discriminate between different cardiovascular risk strata in diverse patient populations, such as mortality rates, which are higher in women than in men, even though cardiovascular diseases are less frequent in women [13,14].
Alternative imaging modalities, such as Single-Positron Emission Computed Tomography (SPECT), have failed to show an equivalent diagnostic capacity due to PET-MPI's advantage of quantifying flow rates, allowing to diagnose pathologies such as microvascular disease or multiple vessel disease. PET- MPI scanners’ distribution and accessibility are rising; they are usually combined with a Computed Tomography (CT) system and an on-site cyclotron, but new radiotracers will suppress the need for the latter, allowing a greater clinical use [15].
In Mexico, the use of PET-MPI started in 1999 with the founding of the first PET/CT at the “Facultad de Medicina” of the National Autonomous University of Mexico (UNAM). Furthermore, the first PET-MPI study was performed in August 2000, using 18-Fluorodeoxyglucose on myocardial viability assessment [15]. In 2002, the implementation of 13N-ammonia radiotracer started, beginning a new cardiac imaging era in Mexico [16]. In 2007, a 64-slice PET/CT hybrid system was added to the unit, making it the first of its class in Latin America, and since then, it has evaluated more than 100,000 patients in the unit [12].
The existence of multiple and diverse radiotracers reflects the need for an extensive array of applications, such as perfusion, metabolic, or even inflammation assessment [17]. Some have similar properties and differ in their advantages and disadvantages. These determine the quality, time, and accessibility of the study. The most used radiotracers in PET-MPI and quantification of myocardial flow are Rubidium-82 (82Rb) and 13N-ammonia [18]. Other radiotracers such as 18-F-Flurpiridaz, and [O- 15] -H2O are used in a lower proportion [19].
The function of a radiotracer is to emit positrons that interact with the free electrons of the surrounding tissues, generating a positron-electron annihilation reaction. This reaction emits a pair of photons (gamma rays) at an angle of 180 degrees between them. These photons must be detected in a narrow window of time by the sensor ring of the PET scanner, which determines the origin of the annihilation reaction and generates data that reflects the physiological activity of the tissue of interest (Figure 1) [18].
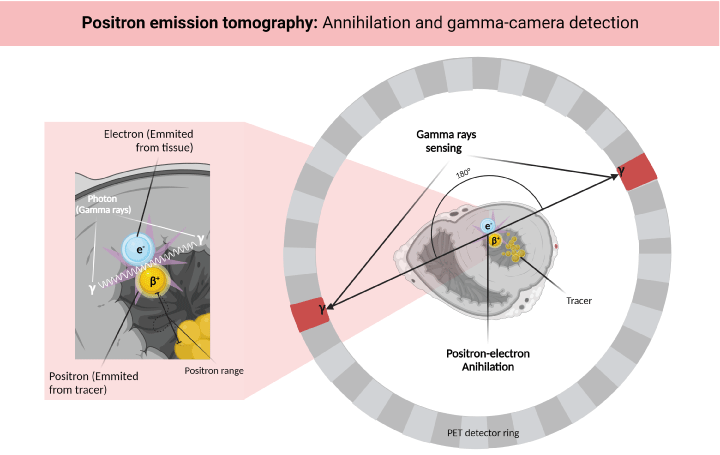
Figure 1. Physical principles of PET-MPI. The radiotracer emits positrons that interact with the free electrons of the surrounding tissues, generating an annihilation reaction that emits a pair of photons, and each photon is detected by the PET scanner’s sensor ring. Created with BioRender.com
An ideal myocardial perfusion radiotracer would have a 100% extraction fraction (from blood to tissue) and a 100% retention in the tissue. These properties would reflect the actual physiological behavior of the tissue [19].
13N-ammonia
It is an inorganic compound that enters the cardiomyocyte by passively diffusing through its cell membrane, transforming into 13N-Glutamine and thus being trapped. The tissues that absorb 13N- ammonia are the myocardium, brain, liver, kidneys, and skeletal muscle [16]. The tracer has a half-life of 9.96 minutes, allowing its production and transport from a regional cyclotron located a few kilometers away from the PET/CT center [20].
13N-ammonia has a low positron range (2.53mm) and an extraction fraction of 80% [18]. Although, in some states of myocardial flow, it is close to 100%, hence reflecting the actual value of Myocardial Blood Flow (MBF) [19]. Likewise, its high extraction fraction and its prolonged half-life generate high-contrast and high-resolution images of intermediate to high quality [16].
82Rb
82Rb remains the most famous PET-MPI radiotracer for its versatility, and most perfusion studies still use it [18]. 82Rb is a metal that acts as a potassium analog that moves through the cardiomyocyte membrane via active transport. Its half-life is 76 seconds, and it has a positron range of 8.6 mm, which generates images of limited resolution. It has a low extraction fraction (65%) that limits the study from reflecting minor differences between consumption of the radiotracer in low-perfusion and normal- perfusion regions [18,20]. The half-life of 82Rb is also very short, but its main advantage is that it can be produced with commercial generators from 82Sr, enabling its storage from 4 to 8 weeks [19].
Comparison
13N-ammonia has the lowest positron range among popular radiotracers (2.53 mm, versus 8.1 mm of 82Rb), and generates excellent spatial image resolution, its prolonged half-life, allows its use in PET centers that transport the tracer over short distances [16].
The main limitations of 13N-ammonia are its general need for an on-site or nearby cyclotron and the need for longer time intervals between acquisitions in stress and rest, making it challenging to apply it in hospital settings [17].
The short half-life of 82Rb allows for a rapid stress-rest protocol, although physicians should pay attention to the decrease in image quality caused by the rapid decay of the isotope. Its long positron range is not a significant limitation in practice; on the other hand, its reduced extraction fraction (35% in peak stress) and retention limit the resolution and image quality to a greater degree [19].
PET-MPI provides a significant amount of advantages, even when accounting for radiation exposure considerations. However, there is a need for developing protocols that balance the low exposure risk with the high quality of images [21].
The acquisition protocol starts with the patient fasting for a minimum of 6 hours. Imaging acquisition has various phases: starting at rest and continuing with pharmacological/physical stress or with a cold pressor test (CPT) [11,18].
PET 3D technology is better for data reconstruction due to the 50% radioactive dose reduction to which the patient is exposed [22]. Likewise, the "list-mode" is preferred for acquiring quantitative and qualitative data [19].
A CT scan is used for proper positioning of the patient, therefore avoiding an extra radiotracer scan [22]. In order to avoid artifacts in the image, the patient should breathe normally, limiting his or her movement within the detector [23].
The dose of 13N-ammonia in adults is 20mCi (equivalent to ≈1.48mSv) and, in patients with high Body Mass Index (BMI), increases to 25-30mCi [11]. Ideally, an automated injection system administers the ammonia, and before the beginning of the image acquisition at rest, we must wait a minimum of 90 seconds after completely infusing the radiotracer. This delay avoids artifacts caused by a fast and variable radiotracer intake. The approximate acquisition time is 10-20 minutes per phase [16]. In the case of a three-phase protocol, a CPT is performed after rest and before stress [18].
A minimum of 5 half-lives (≈50 minutes) must pass between acquisition at rest and stress. When starting a new acquisition phase, the repositioning of the patient is done again with a CT scan; this allows to perform an attenuation correction later [23]. When continuing with the stress image acquisition, adenosine (dipyridamole or regadenoson as an alternative) is administered two to three minutes before injecting the radiotracer in an infusion of 140 μg / Kg/min for four to six minutes [18].
Hybrid cardiac studies combine different imaging modalities in such a way that they contribute with complementary information. The added value of hybrid studies originates from the structural and functional correlation fused in a single image. PET/CT assesses the molecular cardiac function, while CT provides valuable anatomic information of the heart, coronary arteries, and related structures. The PET/MRI protocol achieves better contrast in soft tissues with the great benefit of less radiation exposure. The newest generation of hybrid scanners offers the combination of PET/CT with Magnetic Resonance Imaging (MRI). Currently, most myocardial perfusion and flow measurement studies with PET are performed on hybrid scanners. Those that combine PET with high-resolution CT are becoming the standard for almost all available systems [24].
PET and Coronary Computed Tomographic Angiography (CCTA) are acquired in the same session and equipment. At the same time, coronary calcium quantification can be estimated (CaC) with an increase of only ≈1mSv to the study, achieving lower exposure than CCTA, even the most efficient protocols using an ECG-triggered step-and-shoot acquisition uses doses of 2-5 mSv. [23]. Therefore, in the hybrid protocol, CCTA can be acquired before PET to pre-select patients who can obtain further benefits from a functional study [18].
Adding CCTA to the protocol makes it possible to differentiate patients with extensive obstructive CAD from those with microvascular dysfunction, especially in patients with abnormal myocardial flow values. The CaC allows to establish the risk and prognosis of developing an atherosclerotic plaque. Both CCTA and CaC can be performed immediately after the 13N-ammonia image acquisition and they can be used even as attenuation correctors at rest and under stress, reducing radiation exposure [16].
The use of coronary artery calcium scores (CAC) in conjunction with CCTA allows identifying the etiology of perfusion defects. The CAC has the great advantage of being a reliable noninvasive tool in identifying coronary stenosis in asymptomatic patients. Patients with a CAC of 0 or less than three risk factors (male sex, older age, hypertension and diabetes mellitus) may not require a CCTA [25].
The study carried out by Dedic A, et al. established that the patients with a CAC of 0 have an excellent prognosis, and it is not necessary to carry out further imaging studies such as CCTA. On the other hand, in patients with a CAC of 1 to 100, the CCTA distinguished between patients who experienced adverse events and those who did not. Patients with a high CAC (> 400) experienced the highest incidence of adverse events, and those who also had obstructive CAD identified in the CCTA had the worst prognosis. Therefore, in patients with a positive CAC, CCTA is helpful to identify the patients with a greater risk of experiencing an adverse effect [26].
CAC along with PET-MPI are excellent risk predictors, even in patients without evidence of myocardial ischemia. Coronary calcium determination during a stress study improves the pre-test risk assessment, increases the interpreter's certainty, and leads to more risk-based preventive decision-making than the stress test alone [27].
PET-MPI detects significant coronary stenoses by studying perfusion defects, it can evaluate vasomotor abnormalities, estimate the burden of CAD throughout the coronary system, and determine the risk-benefit assessment of the available interventions, such as coronary angiography [18].
The registered activity of the radiotracer in the myocardium generates qualitative and quantitative data using different techniques. The first is the use of a static acquisition that enables us to evaluate the uptake of the tracer by regions, showing regional abnormalities when comparing it with a standard distribution. The second one is the dynamic acquisition, remarkable for its ability to interpret tracer uptake in specific time frames, generating time-activity curves that represent the quantification of absolute myocardial flow. The third one uses Gated-PET, which syncs the tracer data with the R-R interval produced by the electrocardiogram to evaluate the ventricular function. It is necessary to emphasize that the latter's use should not affect the data we can initially obtain in static and dynamic acquisitions (Figure 2) [11].
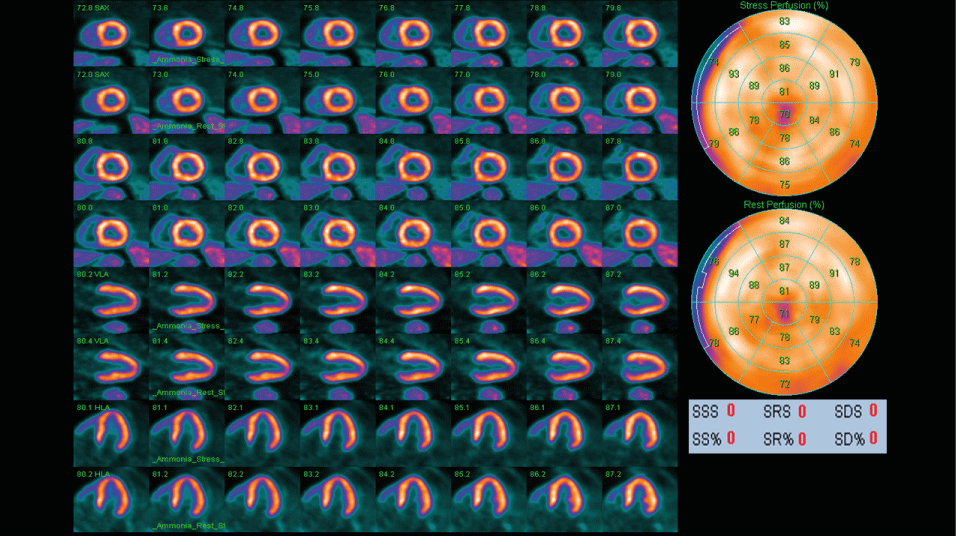
Figure 2. GATED PET-CT scan of normal myocardium. This PET-CT scan used 13N-Ammonia as a radiotracer.
Quantification of myocardial perfusion
In the quantification of myocardial perfusion, dynamic acquisitions are used to regionally identify the radiotracer’s distribution and concentration during stress and rest, being represented in the 17-segment model proposed by the AHA (Figure 3). Kinetic tracing models are used to register the transport rate of the radiotracer from the blood to the tissue (one-compartment model), thus establishing the MBF when tracers like 13N-ammonia metabolize inside the myocardium a two-compartment model is preferred. Subsequently, we can obtain the ratio between MBF in stress and rest to estimate the coronary flow reserve (CFR), which reflects the vasodilator capacity of the coronary system both by region and globally [18].
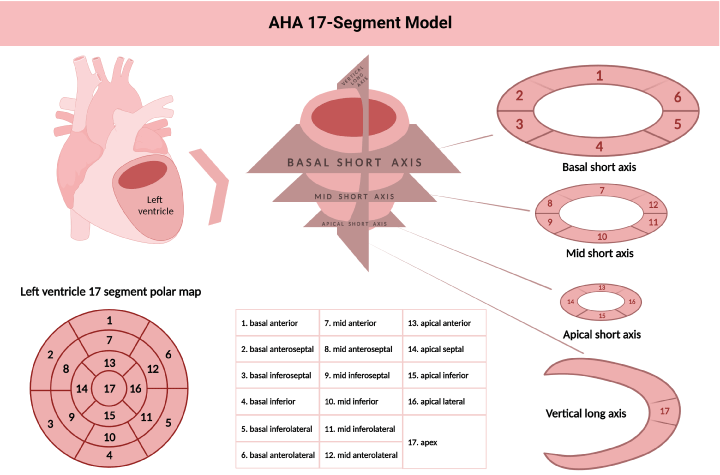
Figure 3. American Heart Association 17 segment model for left ventricle segmentation, nomenclature and 17 segment polar map modified from reference 36. Created with BioRender.com
Qualitative assessment
The extension, severity, and relative location of perfusion defects in the myocardial wall are described in qualitative assessment. According to their extension, defects may be categorized as small (5-10% of the left ventricle), medium (10-20%), or large (> 20%). Severity is similarly expressed as mild, moderate, and severe [11].
Measurements of MBF at stress and rest are compared to determine the extent and severity of perfusion defects, reflecting ischemia or scar regions in the myocardium. Tissue areas that are normal at rest but show perfusion abnormalities under stress are considered reversible lesions and represent ischemia (Figure 4). On the other hand, areas that manifest perfusion abnormalities under rest and stress are considered irreversible lesions and commonly represent areas of previous MI (Figure 5). The difficulty of establishing the reversibility of a myocardial injury increases when ischemic and scarring tissues coexist in the same region; in these situations, expert determination is required [11].
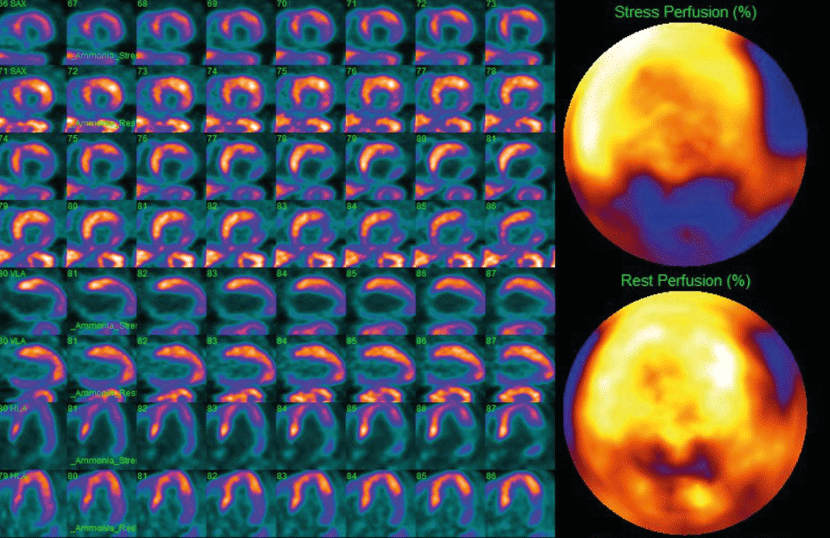
Figure 4. PET-CT scan of an inferolateral transmural MI with slight ischemia of the remaining tissue, along with a moderate anterior wall ischemia that extends anteroseptally, anterolaterally and to the apex
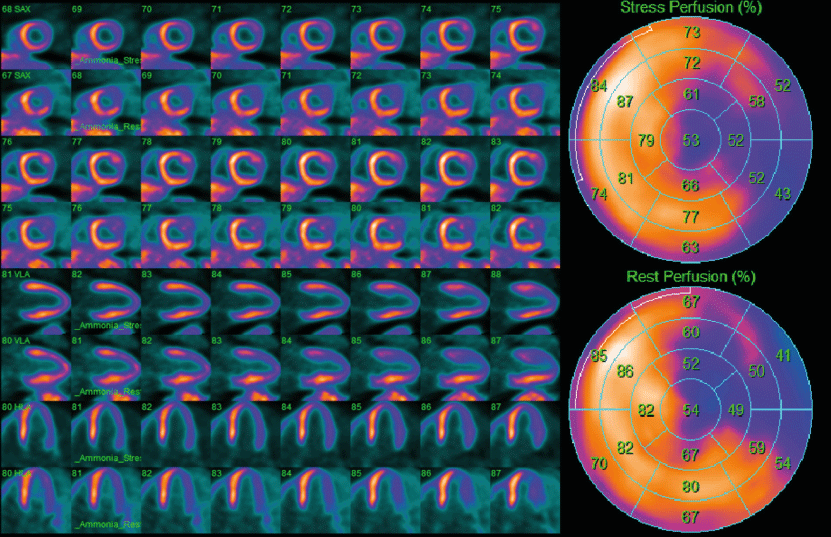
Figure 5. PET-CT scan of a lateral non-transmural MI extended inferiorly, with slight ischemia of the remaining tissue
Semiquantitative assessment
Semiquantitative assessment visually evaluates the myocardium using the 17-segment model established by the AHA. These qualitative data are classified on a 5-point scale that establishes the magnitude of perfusion defects, 0 points mean no defects, 1 point equates to slightly reduced perfusion, 2 points mean moderately reduced perfusion, 3 points indicate severely reduced perfusion, and 4 points translate to no perfusion [23].
The 17-segment model represents the left ventricle in three main short-axis slices (basal, medial, and apical) and a vertical slice (long axis) to show the cardiac apex. The basal and medial slices are divided into six segments and the apical slice into four segments. Each of the 17 segments has a specific name correlated with coronary territories (anatomical variants must be considered). Several scores can be established by counting the number of abnormal segments, such as Summed Stress Score (SSS). The SSS is the sum of the perfusion scores of all the segments during stress and reflects perfusion defects consequent to previous MI or stress-induced ischemia. In contrast, the Summed Score at Rest (SRS) comprises the scores of all the segments during rest and reflects the extent of hibernating or scarring myocardium. The difference between SSS and SRS generates the Summed Differential Score (SDS), a variable that indicates stress-induced ischemia and is used to estimate the reversibility of these lesions [11]. The cut-off values may vary; however, the most widely accepted value for an abnormal SSS is ≥ 4. Some authors propose more restrictive scales, like Hsiao, et al. that classify an SSS > 0 as abnormal [23].
Absolute quantification (quantitative assessment)
Contrary to qualitative assessment, the essential characteristics of a quantitative assessment of myocardial blood flow are reproducibility and objectivity. Absolute quantification of MBF clarifies the diagnosis and prognosis in patients with special results, such as intermediate severity defects or balanced reductions in MBF generated by multivessel disease or balanced multivessel CAD [11].
Some of the patients who benefit the most from absolute MBF quantification are: [11]
1. Those without a previous history of CVD and with symptoms that suggest IHD.
2. Patients with known CAD who require a more specific physiological evaluation.
3. Patients with a high suspicion of multivessel disease.
4. Patients who need to clarify ambiguous results: abnormalities in the qualitative assessment and a normal coronary system (suggesting microvascular disease).
It is accepted that CFR values are generally interpreted as:
1. A CFR > 2.3 mL/min/g establishes a favorable prognosis, excluding the high risk of coronary heart disease (in the absence of lower regional values) [11].
2. A CFR <1.5 mL/min/g suggests an increased risk of developing major adverse cardiovascular events (in the absence of an elevated MBF at rest) [11].
3. The regional decrease in MBF under stress and a CFR <1.5 mL/min/g in avascular territory suggests flow-limiting disease [19].
The images acquired by Gated-PET require regular R-R intervals in an electrocardiogram study; however, some systems already acquire adequate images even with irregular rhythms. This study provides information on ventricular function and volumes during pharmacological stress [11].
The software used to establish ventricular volumes and LVEF in SPECT studies is also used in PET-MPI; therefore, different reference values have not been set. Despite the lack of Gated-PET studies with 13N-ammonia, there are enough similarities between data obtained through PET-MPI and Cardiac MRI (C-MRI) for assessing LVEF and ventricular volumes. For example, in the context of CAD, an LVEF reserve (difference between LVEF in stress and LVEF at rest) less than -5% determines a high Positive Predictive Value (PPV) for severe CAD. Conversely, a value greater than +5% establishes a high Negative Predictive Value (NPV) to rule out severe CAD [23].
Since 1989, Muller, et al. defined plaque vulnerability as the susceptibility to rupture when exposed to stressful events, which increases the risk of thrombosis. (Muller, 1989) The most common mechanism that leads to an acute coronary event is the rupture of an atherosclerotic plaque, followed by vascular thrombosis [28]. PET/CT is able to characterize the plaque’s vulnerability and, hence, patients with high cardiovascular risk. In case of identifying a vulnerable plaque, systemic interventions with statins are the basis for preventing the development of culprit lesions [29].
In the context of vulnerable plaque, 18-FDG, which is a glucose analog, is interiorized by metabolic- accelerated cells; therefore, the intensity of 18-FDG uptake detected by PET reflects the metabolic activity of the cells within the plaque and, therefore, the degree of inflammation [30].
Currently, there is no clinical use for the identification of coronary vulnerable plaques using FDG-PET imaging. In the future, the use of PET/CT in the clinical management of CAD might play an important role by phenotyping the molecular activity of atherosclerotic plaques in order to select patients for targeted treatments, but further studies are required [31].
Coronary Artery Disease Reporting and Data System (CAD-RADS) (Table 1 and 2) is a standardized way of reporting findings of coronary CT angiography. This classification is applied in patients presenting stable chest pain. Any vessel greater than 1.5 millimeters should be evaluated for stenosis using this system. Any patient classified as CAD RADS 3 should be considered for a functional assessment [32].
Table 1. Comparison between 13N-ammonia and 82-rubidium radiotracers





