Background: Peripherally inserted central catheters (PICC) are increasingly used in the treatment of several conditions, including cancer. Use of PICCs may lead to complications, and various potential factors have been associated to their occurrence. Still, quantitative data on the issue are limited.
Objective: Main aims of this study are to provide information on the durability of PICC in oncological patients and to identify which factors are associated to complications leading to PICC removal.
Interventions/Methods: This is an observational, retrospective study of adult patients with onco-haematological diseases. An expert venous access team managed the full pathway of PICC use. Complications were continuously recorded according to hospital protocol. PICC survival was analysed using Kaplan-Meier curves and through multivariate hazard ratios (HR) and corresponding 95% confidence intervals (CI).
Results: A total of 3700 patients were included during 2010-2018, for over 450,000 PICC-days. The HRs of PICC removal were 1.006 (95%CI, 1.001-1.011) for each 1-year increase in patient age, 1.35 (95%CI, 1.08-1.70) for referral to the oncology vs. surgery ward, 1.62 (95%CI, 1.32-1.99) for use of PICC for parenteral nutrition vs. chemotherapy administration, and 3.01 (95%CI, 2.58-3.50) for use of open-tip vs. closed-tip PICC.
Conclusions: This Real-World analysis provided new quantitative evidence showing overall long survival times of PICCs in oncological patients. Both patient-related and treatment-related features were associated to PICC complications.
Implications for Practice: PICCs were confirmed as a secure and long-lasting venous access device for cancer patients undergoing chemotherapy. In this oncological population, closed-tip PICCs showed overall better performances than open-tip PICCs.
complications, hematologic neoplasms, medical oncology, peripheral catheterization, vascular access devices
The selection of the appropriate vascular access device (VAD) is of utmost importance to provide proper intravenous therapy in oncologic patients. As a matter of fact, VAD has a central role with many aspects of managing a patient with cancer: from the initial stages of chemotherapy and surgery to the latest steps of palliative care [1].
Peripherally inserted central catheters (PICC) are increasingly used in the treatment of several acute and chronic conditions as they represent a less invasive and more cost-effective option than other central venous catheters (CVC) [2]. PICCs are used for prolonged continuous or intermittent infusions both in hospitalized patients and outpatients with cancer. As PICCs could stay in place for months, their actual duration depends on several different factors. Use of PICCs may, in fact, lead to complications, particularly thrombosis, catheter-associated infection, catheter occlusion and breakage [1-4].
A number of factors have been related to a different extent to the occurrence of PICC complications. With reference to the characteristics of the PICC itself, some studies reported, in turn, that the material, size, presence/absence of valve, type of valve, and presence of an open- or closed-tip may play a role on the occurrence of different complications, but specific quantitative data on the issue are still relatively limited [4-9].
Aims of this study are thus to provide information on the durability of PICC in oncological patients and to identify which factors are associated to complications leading to PICC removal. In particular, our purpose is to assess the role of using an open- or closed-tip PICC (Groshong®) on subsequent removal of the catheter. Similarly, an additional objective of the investigation is to identify which factors are related to PICC removal after persistent withdrawal obstruction (PWO) of the PICC.
The population in study, enrolled by a venous access team operating for over ten years at a hospital structure in Northern Italy, were oncologic patients, both hospitalized and outpatients. The traceability of all the activities related to PICC use was made possible through the definition of an ad hoc database.
This is an observational, retrospective study conducted at ASST Melegnano della Martesana (Lombardy, Italy), based on data of PICC insertion in patients with onco-haematological diseases. An expert venous access team managed the full pathway of use of venous catheters in oncological patients, from their insertion to removal.
The methods of the study have been described in details in an earlier publication [10]. Here, updated information on data collection in this observational study and an integration of statistical methods are reported. Briefly, a total of 3700 adult oncological patients receiving PICC during the period 2010-2018 were included, for a total of 453,442 PICC-days and 64,777 vascular access management procedures performed by the venous access team.
Various different types of PICC were used: 1) 4F single-lumen silicone, valved-tip PICC (Groshong PICC; Bard Access Systems, Salt Lake City, UT); 2) 4F single-lumen polyurethane power injectable PICC (Turbo-Ject; Cook Medical, Bloomington, IN); 3) 4F single-lumen polyurethane power injectable PICC (Synergy CT PICC; Health Line International Corp, Centerville, UT); 4) 4F single-lumen polyurethane power injectable PICC (Teleflex Medical, Wayne, PA); 5) 4F single-lumen polyurethane power injectable PICC (Pro-PICC; MedComp and Health Line, San Francisco, CA); 6) 4F single-lumen polyurethane power injectable PICC (Bard Access Systems, Salt Lake City, UT).
Indications for PICC use in our hospital were: 1) Need to preserve patient’s vasculature during an infusion with substances harmful to the endothelium either due to chemical or physical characteristics (e.g., pH <5 and >9, or osmolarity >600 mOsm/L) or to drug-related features (e.g., neutral drugs); 2) Patients with life expectancy >30 days requiring administration of continuous or intermittent central intravenous therapies; 3) Patients with ago-phobia requiring administration of continuous or intermittent central intravenous therapies
All PICCs were implanted using sterile technique, inclusive of maximum barrier precautions, and skin antisepsis with 2% chlorhexidine skin preparation. Tools such as ultrasound guidance [11] and micro-introduction were used. The position of the PICC tip was regularly checked by chest radiograph after the procedure. All implanted PICCs had confirmation of correct positioning at atrio-caval junction.
Before the implant, a nurse checked that each patient had been provided with information on the procedure, and collected informed consent that had been given to the patient by a medical doctor. At the end of the placement, the same nurse documented the procedure in the patient’s medical record. The medical doctor authorized the use of catheter after validating the catheter tip location via the chest radiograph. After completion, an operator imputed all information of the procedure in a structured database.
Vascular access management procedures were carried out weekly, and consisted of site inspection, disinfection, evaluation of catheter functionalities, stop and go flushing methodology, dressing with transparent film and stabilization of the catheter (ESD). Complications such as catheter-related bloodstream infection (CRBSI), deep vein thrombosis, mechanical complications and specifically PWO were continuously recorded according to hospital protocol. All these activities and complications were also systematically recorded in a separate section of the structured database.
The diagnosis of CRBSI was based on comparative blood cultures performed on 2-3 samples (each sample was composed of 2 vials, 1 for aerobic and 1 for anaerobic) for a total of 4-6 vials to ensure more sensibility.
Deep vein thrombosis symptoms are a function of thrombus size. The nurse informed a medical doctor if any of the following occurred: 1) pain or arm heaviness (where the catheter was placed); 2) redness and hyperaemia at the exit site; 3) superficial vein dilation; 4) functionally challenged catheters that required validation. The physician then ordered an ultrasound with eco-color Doppler based on the patient exam.
In case of complications due to PWO, a radiological evaluation was performed to rule out that the catheter’s tip had migrated. If the device was in situ and not mispositioned, it was tried to clear the lumen by adopting the negative pressure (two syringes) technique [12], using saline solution. If the complication was resolved, the device could be used. In cases where the complication persisted, the attempt was repeated for a total of 6 times and, if PWO persisted, the catheter was assessed for need of replacement. Clinical decision related to maintenance in situ of catheters with PWO foresees a residual treatment less than 30 days.
The study was conducted in accordance with applicable laws, regulations and guidelines for protection of human subjects. Identifying information of patients was removed from the database to guarantee their privacy.
Comparison between groups were performed by using the contingency table analysis with the Chi-square or Fisher’s exact test, as appropriate, for categorical variables and a Student’s T test or the corresponding non-parametric Wilcoxon rank-sum test (according to the normality of the distribution, based on the Shapiro-Wilk statistic) for continuous data. When comparisons involved more than two groups, analysis of variance models or the non-parametric Kruskal-Wallis test was used. Overall PICC survival was analysed using Kaplan-Meier product-limit survival curve estimates and log-rank tests for comparison between groups [13].
Overall PICC survival was defined as the time from date of PICC insertion to date of removal due to end of therapy, death of the patient (censored observations), or removal due to complications (events). We tested the proportional hazards assumption by including time-dependent effects in the model (i.e., a covariate for interaction of the predictor and the logarithm of survival time), and no violation was found. Hazard ratios (HR) of PICC removal due to complications and the corresponding 95% confidence intervals (CI) were estimated using Cox proportional hazards models including terms for age and sex (model 1), as well as for age, sex, hospitalization type, hospital ward, PICC indication, type of oncological disease, PICC insertion arm and PICC type (model 2) [14].
Odds ratios (OR) of PICC removal due to occlusion in patients with PWO, and the corresponding 95% CI, were calculated using unconditional multiple logistic regression, including terms for age and sex (model 1), as well as for age, sex, hospitalization type, hospital ward, PICC indication, type of oncological disease, PICC insertion arm and PICC type (model 2). All tests were two-sided and a p-value of less than 0.05 was considered as statistically significant. Data analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA), and the figures on overall PICC survival were obtained using STATA 15 (StataCorp LP, College Station, Tex, USA) statistical software (Figure 1 and 2).
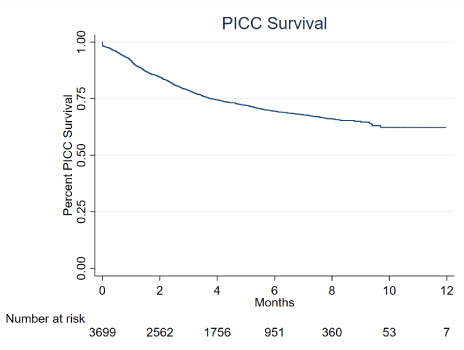
Figure 1. Overall PICC survival in oncological patients
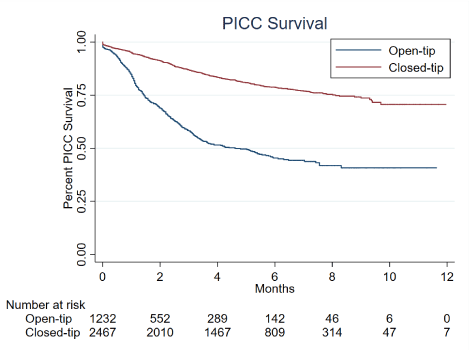
Figure 2. PICC survival in oncological patients, according to type of PICC used
Table 1 shows the main characteristics of oncological patients at baseline (PICC insertion), overall and according to PICC type. The mean age of enrolled subjects was 73.6 years (SD: 13.0) and 55.8% of them were females. Most patients were referred to the oncology ward (85.8%) for a solid tumour (93.2%). PICC was used for chemotherapy administration in 80.9% of patients, being placed in the right arm in 75.7% of cases. With reference to comparison of patients’ characteristics according to PICC type, those treated with a closed-tip PICC (as compared to an open-tip PICC) were younger (73.1 vs 74.7 years, p-value<0.001), were more frequently females (57.4% vs. 52.6%, p-value<0.01), admitted for a day-hospital (65.9% vs. 36.3%, p-value<0.001), referred to the oncology ward (99.1% vs. 59.1%), had more frequently a PICC inserted for chemotherapy administration (95.3% vs. 52.0%, p-value<0.001), a lympho-haematological cancer (8.0% vs. 4.5%, p-value<0.001) and insertion in the left arm (25.9% vs. 21.1%, p-value=0.001). Further, insertion of both micro-introducer and PICC were easier in patients treated with closed-tip
Table 1. Characteristics of 3700 oncological patients at PICC insertion
|
All patients
(n=3700) |
Open-tip PICC
(n=1233) |
Closed-tip PICC
(n=2467) |
p-valuea |
|
n (%) |
n (%) |
n (%) |
|
Age |
Mean ± SD |
73.6 ± 13.0 |
74.7 ± 12.9 |
73.1 ± 12.9 |
<0.001 |
Sex |
Male |
1635 (44.2) |
584 (47.4) |
1051 (42.6) |
|
Female |
2065 (55.8) |
649 (52.6) |
1416 (57.4) |
0.006 |
Hospitalization type |
Hospitalized |
1628 (44.0) |
786 (63.7) |
842 (34.1) |
|
Day hospital |
2072 (56.0) |
447 (36.3) |
1625 (65.9) |
<0.001 |
Referral hospital ward |
Surgery |
527 (14.2) |
504 (40.9) |
23 (0.9) |
|
Oncology |
3173 (85.8) |
729 (59.1) |
2444 (99.1) |
<0.001 |
PICC indication |
Chemotherapy |
2993 (80.9) |
641 (52.0) |
2352 (95.3) |
|
Parenteral nutrition |
707 (19.1) |
592 (48.0) |
115 (4.7) |
<0.001 |
Oncological disease |
Big killersb |
2763 (74.7) |
986 (80.0) |
1777 (72.0) |
|
Other solid tumours |
685 (18.5) |
192 (15.6) |
493 (20.0) |
|
Lympho-hematological cancers |
252 (6.8) |
55 (4.5) |
197 (8.0) |
<0.001 |
PICC insertion arm |
Right |
2800 (75.7) |
973 (78.9) |
1827 (74.1) |
|
Left |
900 (24.3) |
260 (21.1) |
640 (25.9) |
0.001 |
No. of venipuncture at insertion |
1 |
3266 (88.3) |
1092 (88.6) |
2174 (88.1) |
|
≥ 1 |
434 (11.7) |
141 (11.4) |
293 (11.9) |
0.69 |
Ease of insertion of micro-introducer |
Easy |
3281 (88.7) |
1032 (83.7) |
2249 (91.2) |
|
Hard |
419 (11.3) |
201 (16.3) |
218 (8.8) |
<0.001 |
Ease of PICC insertion |
Easy |
3376 (91.2) |
1103 (89.5) |
2273 (92.1) |
|
Hard |
324 (8.8) |
130 (10.5) |
194 (7.9) |
0.007 |
Malposition of PICC |
No |
3489 (94.3) |
1156 (93.8) |
2333 (94.6) |
|
Yes |
211 (5.7) |
77 (6.2) |
134 (5.4) |
0.31 |
Right arm PICC insertion (cm) |
Mean ± SD |
37.8 ± 2.8 |
38.0 ± 2.9 |
37.7 ± 2.7 |
0.03 |
Left arm PICC insertion (cm) |
Mean ± SD |
38.5 ± 3.4 |
38.4 ± 3.2 |
38.5 ± 3.5 |
0.78 |
ap-value for comparison between open-tip and closed-tip PICCs.
bIncluding gastric, colorectal, lung and breast cancers.
HRs of PICC removal due to complications, and the corresponding 95% CI, according to main characteristics at insertion are presented in Table 2. After adjustment for age, sex and other selected baseline characteristics, the HRs of PICC removal were 1.006 (95% CI, 1.001-1.011) for an increase of 1 year of age, 1.35 (95% CI, 1.08-1.70) for referral to the oncology as compared to the surgery ward, 1.62 (95% CI, 1.32-1.99) for use of PICC for parenteral nutrition as compared to chemotherapy administration, and 3.01 (95% CI, 2.58-3.50) for use of open-tip as compared to closed-tip PICC. On the other hand, sex, type of hospitalization, type of oncological disease and insertion arm were not associated with PICC removal due to complications.
Table 2. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) of PICC removal due to complications according to various characteristics, among 3700 oncological patients
|
HR (95% CI),
Model 1a |
HR (95% CI),
Model 2b |
|
n (%) |
n (%) |
Age |
One-year age increase (continuous term) |
1.009 (1.004-1.014) |
1.006 (1.001-1.011) |
Sex |
Male |
1 (reference) |
1 (reference) |
Female |
0.83 (0.73-0.95) |
0.88 (0.77-1.00) |
Hospitalization type |
Hospitalized |
1 (reference) |
1 (reference) |
Day hospital |
0.63 (0.55-0.71) |
0.88 (0.76-1.01) |
Referral hospital ward |
Surgery |
1 (reference) |
1 (reference) |
Oncology |
0.42 (0.35-0.49) |
1.35 (1.08-1.70) |
PICC indication |
Chemotherapy |
1 (reference) |
1 (reference) |
Parenteral nutrition |
2.61 (2.25-3.02) |
1.62 (1.32-1.99) |
Oncological disease |
Big killersb |
1 (reference) |
1 (reference) |
Other solid tumours |
0.87 (0.73-1.02) |
0.94 (0.79-1.11) |
Lympho-hematological cancers |
0.67 (0.50-0.89) |
0.76 (0.57-1.01) |
PICC insertion arm |
Right |
1 (reference) |
1 (reference) |
Left |
1.05 (0.91-1.22) |
1.10 (0.95-1.28) |
PICC type |
Closed-tip |
1 (reference) |
1 (reference) |
Open-tip |
3.34 (2.93-3.80) |
3.01 (2.58-3.50) |
aHR from multivariate Cox regression, including terms for age and sex.
bHR from multivariate Cox regression, including terms for age, sex, hospitalization type, hospital ward, PICC indication, type of oncological disease, PICC insertion arm and PICC type.
Table 3 reports information from 356 oncological patients with PWO of PICC, by relating the characteristic at PICC insertion to the outcome of PWO. Patients with a favourable outcome (end of therapy) had more frequently a closed-tip PICC (80.9%) than those with removal due to clinical decision (57.3%) and removal due to occlusion (40.4%) outcomes (p-value<0.001). Also, patients reaching end of therapy had a day-hospital access (65.9%), were referred to the oncology ward (90.7%) and used PICC for chemotherapy administration (89.6%) more frequently than those in the groups of PICC removal outcome (all p-values<0.01). No other differences between outcome groups emerged in the univariate analyses.
Table 3. Relation between baseline characteristics and outcome in 356 oncological patients after persistent withdrawal occlusion (PWO) of PICC
|
End of therapy
(n=173) |
Removal due to clinical decision (PWO)
(n=89) |
Removal due to occlusion
(n=94) |
p-valuea |
|
n (%) |
n (%) |
n (%) |
|
Age |
Mean ± SD |
73.6 ± 12.5 |
75.0 ± 13.1 |
74.2 ± 13.1 |
0.59 |
Sex |
Male |
76 (43.9) |
45 (50.6) |
38 (40.4) |
|
Female |
97 (56.1) |
44 (49.4) |
56 (59.6) |
0.37 |
PICC type |
Open-tip |
33 (19.1) |
38 (42.7) |
56 (59.6) |
|
Closed-tip |
140 (80.9) |
51 (57.3) |
38 (40.4) |
<0.001 |
Hospitalization type |
Hospitalized |
59 (34.1) |
38 (42.7) |
52 (55.3) |
|
Day hospital |
114 (65.9) |
51 (57.3) |
42 (44.7) |
0.004 |
Referral hospital ward |
Surgery |
16 (9.2) |
23 (25.8) |
25 (26.6) |
|
Oncology |
157 (90.7) |
66 (74.2) |
69 (73.4) |
<0.001 |
PICC indication |
Chemotherapy |
155 (89.6) |
62 (69.7) |
64 (68.1) |
|
Parenteral nutrition |
18 (10.4) |
27 (30.3) |
30 (31.9) |
<0.001 |
Oncological disease |
Big killersb |
125 (72.2) |
68 (76.4) |
76 (80.8) |
|
Other solid tumours |
36 (20.8) |
16 (18.0) |
10 (10.6) |
|
Lympho-hematological cancers |
12 (6.9) |
5 (5.6) |
8 (8.5) |
0.31 |
PICC insertion arm |
Right |
131 (75.7) |
68 (76.4) |
68 (72.3) |
|
Left |
42 (24.3) |
21 (23.6) |
26 (27.7) |
0.78 |
No. of venipuncture at insertion |
1 |
159 (91.9) |
80 (89.9) |
82 (87.02) |
|
≥ 1 |
14 (8.1) |
9 (10.1) |
12 (12.8) |
0.47 |
Ease of insertion of micro-introducer |
Easy |
162 (93.6) |
83 (93.3) |
82 (87.2) |
|
Hard |
11 (6.4) |
6 (6.7) |
12 (12.8) |
0.16 |
Ease of PICC insertion |
Easy |
167 (96.5) |
82 (92.1) |
84 (89.4) |
|
Hard |
6 (3.5) |
7 (7.9) |
10 (10.6) |
0.06 |
Malposition of PICC |
No |
165 (95.4) |
84 (94.4) |
88 (93.6) |
|
Yes |
8 (4.6) |
5 (5.6) |
6 (6.4) |
0.82 |
Right arm PICC insertion (cm) |
Mean ± SD |
37.4 ± 2.9 |
38.0 ± 3.0 |
37.9 ± 3.2 |
0.27 |
Left arm PICC insertion (cm) |
Mean ± SD |
38.7 ± 3.4 |
39.6 ± 3.1 |
39.4 ± 3.4 |
0.41 |
ap-value for comparison between three outcome groups.
bIncluding gastric, colorectal, lung and breast cancers.
Table 4 gives the ORs of PICC removal due to occlusion, and the corresponding 95% CIs, in 356 oncological patients with PWO of PICC, according to main characteristics. In the multivariate model including terms for age, sex and other selected baseline factors, the OR of PICC removal due to occlusion after PWO was 4.83 (95% CI, 2.52-9.26) for the use of open-tip as compared to closed-tip PICC. No other factor considered, including age, sex, type of hospitalization, referral hospital ward, PICC indication of use, type of oncological disease and insertion arm, was found to be associated with PICC removal due to occlusion after PWO.
Table 4. Predictive factors of PICC removal due to occlusiona in 356 oncological patients with persistent withdrawal occlusion (PWO) of PICC, according to various characteristics
|
OR (95% CI),
Model 1b |
OR (95% CI),
Model 2c |
|
n (%) |
n (%) |
Age |
One-year age increase (continuous term) |
1.001 (0.983-1.020) |
1.000 (0.980-1.019) |
Sex |
Male |
1 (reference) |
1 (reference) |
Female |
1.26 (0.78-2.04) |
1.46 (0.87-2.45) |
Hospitalization type |
Hospitalized |
1 (reference) |
1 (reference) |
Day hospital |
0.48 (0.29-0.77) |
0.67 (0.38-1.15) |
Referral hospital ward |
Surgery |
1 (reference) |
1 (reference) |
Oncology |
0.48 (0.27-0.84) |
1.75 (0.72-4.22) |
PICC indication |
Chemotherapy |
1 (reference) |
1 (reference) |
Parenteral nutrition |
2.30 (1.34-3.96) |
1.09 (0.48-2.49) |
Oncological disease |
Big killerb |
1 (reference) |
1 (reference) |
Other solid tumours |
0.49 (0.24-1.02) |
0.61 (0.28-1.32) |
Lympho-hematological cancers |
1.19 (0.49-2.89) |
1.43 (0.54-3.75) |
PICC insertion arm |
Right |
1 (reference) |
1 (reference) |
Left |
1.18 (0.69-2.01) |
1.59 (0.88-2.85) |
PICC type |
Closed-tip |
1 (reference) |
1 (reference) |
Open-tip |
4.15 (2.51-6.85) |
4.83 (2.52-9.26) |
aComparison group included patients whose PICC was removed because of either end of therapy (n=173) or clinical decision (PWO, n=89).
bOdds ratios (OR) and corresponding 95% confidence intervals (CI) computed from multivariate logistic regression, including terms for age and sex.
cOR and corresponding 95% CI computed from multivariate logistic regression, including terms for age, sex, hospitalization type, hospital ward, PICC indication, type of oncological disease, PICC insertion arm and PICC type.
Our study, a retrospective Real-World analysis of 8 years of activity for a total of 3700 PICCs implanted, provided new quantitative evidence showing overall long survival times of peripherally implanted central catheters in oncological patients. It also allowed to investigate factors associated to complications requiring PICC removal, highlighting a number of potential patient-related (e.g., age) and treatment-related (e.g., indication of PICC use, PICC type) features to be considered in the cancer setting. In particular, closed-tip catheters showed better performances than open-tip PICCs. Even after adjusting for several covariates, the risk of PICC removal due to complications was 3-fold-increased in patients treated with open-tip as compared to closed-tip PICCs (Multivariate HR=3.01) and, when PWO occurred, it was almost 5-times more likely that a PICC had to be removed if it was open-tipped (Multivariate OR=4.83).
Ensuring stable and long-lasting access for the administration of chemotherapies to cancer patients has always been one of the main challenges for cancer patients and nurses. In this population of oncological patients, the use of PICC guaranteed an appropriate therapeutic path of patients and a correct administration of medium- and long-term therapies, eliminating any damage caused by extravasation of blistering, stinging, hypo- or hyper-osmotic solutions. The adoption of a pre-defined protocol to standardize both PICC insertion and nursing allowed us to minimize the occurrence of complications.
Despite several studies were conducted, a number of relevant points with the use of PICC in cancer patients remain unsettled. In particular, the choice of the best PICC type (e.g., with or without distal valve, open- or closed-tip, in polyurethane or silicone, etc) for chemotherapy administration has been examined, but is still open to discussion [3,4,15-17]. In this population of both in-patients and out-patients with cancer, we were able to compare the performances of closed-tip and open-tip PICCs over time. Survival time of the closed-tip PICCs at one month was around 95% as compared to 84% of open-tip PICCs, and the difference between types was even larger at 3 months, i.e. 87% vs 58%, respectively. Closed-tip PICCs were used almost exclusively to administer chemotherapy, whereas open-tip PICCs were used for both chemotherapy and parenteral nutrition as, for the latter indication, their use decreases the risk of occlusions related to lipids. We kept into account this and other baseline differences between groups in multivariate analyses, that nevertheless confirmed and further strengthened the reliability of univariate findings.
While various RCT and observational studies provided data on the performances and safety of different types of catheters in various settings [2,6,18,19], only a few earlier studies compared open-tip to closed-tip PICCs in oncological patients. A US retrospective study reviewed the role of PICC on the risk of thrombosis during a 1-year period, reporting an overall low-incidence of symptomatic upper extremity deep venous thrombosis, with no difference between open-tip and closed-tip PICCs [20].
As in our study, about two-thirds of patients of that US investigation were treated with a Groshong PICC. On the other hand, a Chinese prospective study conducted between 2010 and 2013, including 311 cancer patients, found a relatively high incidence of (symptomatic and asymptomatic) PICC-related thrombosis [21].
When the type of PICC was examined, no difference between Groshong and open-ended PICCs was found in univariate analysis (OR=1.06, 95% CI: 0.53-2.08). An Italian study of hematological patients treated with PICC considered both risks of thrombosis and CRBSI in a total of 483 patients enrolled between 2009 and 2012 [8].
Given the low incidence of PICC-related thrombotic complications (0.20 per 1000 PICC-days) and CRBSI (0.59 per 1000 PICC-days), the overall findings of this investigation supported the use of PICCs as an alternative to other central venous access devices. Univariate analyses showed lower risks of both CRBSI (HR=0.71) and thrombotic complications (HR=0.43) for closed-tip as compared to open-tip PICCs, in the absence, however, of statistically significant differences. In two other earlier Italian studies, with partially overlapping clinical records to this analysis, lower complications of closed-tip than open-tip PICCs were found [4,10].
In the first analysis, no formal statistical testing for difference between groups was, however, available 10. In the second one, consistently with our findings, an increased risk of PICC-related adverse events emerged for open-tip vs. valved PICC system (multivariate HR=1.89, 95% CI: 1.24-2.88) [4].
Our study also revealed different outcomes of closed-tip and open-tip PICCs after a PWO. In fact, complications could be solved in 140 out of 229 PWO (61%) when a closed-tip PICC was used, as compared to 33 out of 127 PWO (26%) for open-tip PICCs. Furthermore, catheters removed due to total occlusion were 38 (17%) for closed-tip vs. 56 (44%) for open-tip PICCs.
In multivariate analyses, PICC type emerged as the only factor associated to PICC removal due to occlusion after a PWO in our dataset of oncological patients. This finding may likely be explained by the fact that the distal valve is easily cleared than an open-tip PICC. In fact, the presence of the valve prevents infiltrations of the sheath into the catheter lumen, and it is thus easy to restore its functionality through appropriate operations.
No significant relation with PICC removal due to occlusion after a PWO emerged for any other investigated factor, including age, gender, type of hospitalization, referral hospital ward, indication of PICC use, type of oncological disease and insertion arm.
A limitation of this investigation is its observational, retrospective study design, with corresponding potential presence of bias. In particular, patients treated with closed-tip and open-tip PICCs differed widely in their baseline characteristics. Although we were able to adjust for these factors through multivariate analyses, the risk of residual confounding still remains – particularly for referral ward and PICC indication, that were strongly unbalanced between groups (i.e., very few patients in the surgery ward and indicated for parenteral nutrition were treated with closed-tip PICCs).
Also, a potential role of other yet unidentified covariates cannot be excluded. Among other limits of this study, asymptomatic thrombosis was not examined. In fact, diagnosis of PICC-related thrombosis was suspected on the basis of clinical symptoms (e.g., arm heaviness, pain, redness, hyperaemia, superficial vein dilation); when such symptoms were present, an ultrasound testing with color Doppler was performed to confirm the diagnosis. The role of asymptomatic PICC-related thrombosis is, in any case, still debated and screening with objective tests is generally not recommended [22,23].
Strengths of this study are the large number of patients enrolled and the long period of observation that, to our knowledge, is rare for PICC analyses in a cancer setting, the availability of detailed information on patient- and treatment-related factors that allowed to fit meaningful multivariate models and the very low frequency of missing data (<1% for most variables). Thus, we emphasize the importance of systematically collecting the main information on PICC implant and management, together with any subsequent complication, in a structured database.
In conclusion, in this Real-World observational study, PICCs were confirmed as a secure and long-lasting venous access device in both hospital and non-hospital settings for cancer patients undergoing chemotherapy. In such challenging conditions (i.e., long-term use in cancer patients), closed-tip PICCs (Groshong) showed overall better performances than open-tip PICCs in terms of easiness of insertion (related to microintroducer insertion and catheter progression), longer indwelling over time, lower occurrence of complications and increased likelihood of resolution of PWO. Our investigation suggests, therefore, an important role of the choice of the appropriate device in the initial management phase of patients with cancer, in order to provide patients a proper path in support of humanization of care [24]. Still, in view of the limitations of our observational study, further investigations are needed to confirm these findings.
None.
The authors have no funding or conflicts of interest to disclose.
- Gallieni M, Pittiruti M, Biffi R (2008) Vascular access in oncology patients. CA Cancer J Clin 58: 323-346. [Crossref]
- Chopra V, Anand S, Hickner A (2013) Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 382: 311-325. [Crossref]
- Kang J, Chen W, Sun W, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc 18: 153-157. [Crossref]
- Campagna S, Gonella S, Berchialla P, et al. Can Peripherally Inserted Central Catheters Be Safely Placed in Patients with Cancer Receiving Chemotherapy? A Retrospective Study of Almost 400,000 Catheter-Days. Oncologist 24: e953-e959. [Crossref]
- Ong CK, Venkatesh SK, Lau GB, Wang SC (2010) Prospective randomized comparative evaluation of proximal valve polyurethane and distal valve silicone peripherally inserted central catheters. J Vasc Interv Radiol 21: 1191-1196. [Crossref]
- Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, et al. (2012) The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates--the 'ELeCTRiC' study. J Vasc 13: 421-425. [Crossref]
- Chopra V, Kaatz S, Conlon A (2017) The Michigan Risk Score to predict peripherally inserted central catheter-associated thrombosis. J Thromb Haemost 15: 1951-1962. [Crossref]
- Morano SG, Latagliata R, Girmenia C (2015) Catheter-associated bloodstream infections and thrombotic risk in hematologic patients with peripherally inserted central catheters (PICC). Support Care Cancer 23: 3289-3295. [Crossref]
- Seckold T, Walker S, Dwyer T (2015) A comparison of silicone and polyurethane PICC lines and postinsertion complication rates: a systematic review. J Vasc16: 167-177. [Crossref]
- Zerla PA, Canelli A, Caravella G (2015) Open-vs closed-tip valved peripherally inserted central catheters and midlines: Findings from a vascular access database. Journal of the Association for Vascular 20: 169-176.
- Maecken T, Grau T (2007) Ultrasound imaging in vascular access. Crit Care Med 35(5 Suppl): S178-185. [Crossref]
- Cummings-Winfield C, Mushani-Kanji T (2008) Restoring patency to central venous access devices. Clin J Oncol Nurs 12: 925-934. [Crossref]
- Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457-481.
- Cox DR (1972) Regression models and life‐tables. JR Stat Soc 34: 187-202.
- Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, et al. (2014) A prospective, randomized comparison of three different types of valved and non-valved peripherally inserted central catheters. J Vasc 15: 519-523. [Crossref]
- Xu B, Zhang J, Tang S, Hou J, Ma M (2018) Comparison of two types of catheters through femoral vein catheterization in patients with lung cancer undergoing chemotherapy: A retrospective study. J Vasc 19: 651-657. [Crossref]
- Miyagaki H, Nakajima K, Hara J (2012) Performance comparison of peripherally inserted central venous catheters in gastrointestinal surgery: a randomized controlled trial. Clin Nutr 31: 48-52. [Crossref]
- Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB (1999) Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol 173: 1393-1398. [Crossref]
- Smith SN, Moureau N, Vaughn VM (2017) Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol 28: 749-756.e742. [Crossref]
- Liem TK, Yanit KE, Moseley SE (2012) Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg 55: 761-767. [Crossref]
- Liu Y, Gao Y, Wei L, Chen W, Ma X, et al. (2015) Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: a double-center prospective investigation. Ther Clin Risk Manag 11: 153-160. [Crossref]
- Cortelezzi A, Moia M, Falanga A (2005) Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: a prospective multicentre study. Br J Haematol 129: 811-817. [Crossref]
- Fallouh N, McGuirk HM, Flanders SA, Chopra V (2015) Peripherally Inserted Central Catheter-associated Deep Vein Thrombosis: A Narrative Review. Am J Med 128: 722-738. [Crossref]
- Passalacqua R, Caminiti C, Annunziata M (2012) Final results of a large collaborative, hospital-based quality improvement study aimed at the implementation of interventions for the psychosocial care of adult cancer patients (HUCARE project): American Society of Clinical Oncology.
Editorial Information
Editor-in-Chief
Akira Sugawara
Tohoku University Graduate School of Medicine
Article Type
Research Article
Publication history
Received date: February 23, 2021
Accepted date: March 08, 2021
Published date: March 11, 2021
Copyright
©2021 Zerla PA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Zerla PA, Galeone C, Pelucchi C, Caravella G, Gilardini A, et al. (2021) Peripherally inserted central catheters in the oncological setting: An Italian experience of 3700 patients. Clin Res Trials 7: doi: 10.15761/CRT.1000337

Figure 1. Overall PICC survival in oncological patients

Figure 2. PICC survival in oncological patients, according to type of PICC used
Table 1. Characteristics of 3700 oncological patients at PICC insertion
|
All patients
(n=3700) |
Open-tip PICC
(n=1233) |
Closed-tip PICC
(n=2467) |
p-valuea |
|
n (%) |
n (%) |
n (%) |
|
Age |
Mean ± SD |
73.6 ± 13.0 |
74.7 ± 12.9 |
73.1 ± 12.9 |
<0.001 |
Sex |
Male |
1635 (44.2) |
584 (47.4) |
1051 (42.6) |
|
Female |
2065 (55.8) |
649 (52.6) |
1416 (57.4) |
0.006 |
Hospitalization type |
Hospitalized |
1628 (44.0) |
786 (63.7) |
842 (34.1) |
|
Day hospital |
2072 (56.0) |
447 (36.3) |
1625 (65.9) |
<0.001 |
Referral hospital ward |
Surgery |
527 (14.2) |
504 (40.9) |
23 (0.9) |
|
Oncology |
3173 (85.8) |
729 (59.1) |
2444 (99.1) |
<0.001 |
PICC indication |
Chemotherapy |
2993 (80.9) |
641 (52.0) |
2352 (95.3) |
|
Parenteral nutrition |
707 (19.1) |
592 (48.0) |
115 (4.7) |
<0.001 |
Oncological disease |
Big killersb |
2763 (74.7) |
986 (80.0) |
1777 (72.0) |
|
Other solid tumours |
685 (18.5) |
192 (15.6) |
493 (20.0) |
|
Lympho-hematological cancers |
252 (6.8) |
55 (4.5) |
197 (8.0) |
<0.001 |
PICC insertion arm |
Right |
2800 (75.7) |
973 (78.9) |
1827 (74.1) |
|
Left |
900 (24.3) |
260 (21.1) |
640 (25.9) |
0.001 |
No. of venipuncture at insertion |
1 |
3266 (88.3) |
1092 (88.6) |
2174 (88.1) |
|
≥ 1 |
434 (11.7) |
141 (11.4) |
293 (11.9) |
0.69 |
Ease of insertion of micro-introducer |
Easy |
3281 (88.7) |
1032 (83.7) |
2249 (91.2) |
|
Hard |
419 (11.3) |
201 (16.3) |
218 (8.8) |
<0.001 |
Ease of PICC insertion |
Easy |
3376 (91.2) |
1103 (89.5) |
2273 (92.1) |
|
Hard |
324 (8.8) |
130 (10.5) |
194 (7.9) |
0.007 |
Malposition of PICC |
No |
3489 (94.3) |
1156 (93.8) |
2333 (94.6) |
|
Yes |
211 (5.7) |
77 (6.2) |
134 (5.4) |
0.31 |
Right arm PICC insertion (cm) |
Mean ± SD |
37.8 ± 2.8 |
38.0 ± 2.9 |
37.7 ± 2.7 |
0.03 |
Left arm PICC insertion (cm) |
Mean ± SD |
38.5 ± 3.4 |
38.4 ± 3.2 |
38.5 ± 3.5 |
0.78 |
ap-value for comparison between open-tip and closed-tip PICCs.
bIncluding gastric, colorectal, lung and breast cancers.
Table 2. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) of PICC removal due to complications according to various characteristics, among 3700 oncological patients
|
HR (95% CI),
Model 1a |
HR (95% CI),
Model 2b |
|
n (%) |
n (%) |
Age |
One-year age increase (continuous term) |
1.009 (1.004-1.014) |
1.006 (1.001-1.011) |
Sex |
Male |
1 (reference) |
1 (reference) |
Female |
0.83 (0.73-0.95) |
0.88 (0.77-1.00) |
Hospitalization type |
Hospitalized |
1 (reference) |
1 (reference) |
Day hospital |
0.63 (0.55-0.71) |
0.88 (0.76-1.01) |
Referral hospital ward |
Surgery |
1 (reference) |
1 (reference) |
Oncology |
0.42 (0.35-0.49) |
1.35 (1.08-1.70) |
PICC indication |
Chemotherapy |
1 (reference) |
1 (reference) |
Parenteral nutrition |
2.61 (2.25-3.02) |
1.62 (1.32-1.99) |
Oncological disease |
Big killersb |
1 (reference) |
1 (reference) |
Other solid tumours |
0.87 (0.73-1.02) |
0.94 (0.79-1.11) |
Lympho-hematological cancers |
0.67 (0.50-0.89) |
0.76 (0.57-1.01) |
PICC insertion arm |
Right |
1 (reference) |
1 (reference) |
Left |
1.05 (0.91-1.22) |
1.10 (0.95-1.28) |
PICC type |
Closed-tip |
1 (reference) |
1 (reference) |
Open-tip |
3.34 (2.93-3.80) |
3.01 (2.58-3.50) |
aHR from multivariate Cox regression, including terms for age and sex.
bHR from multivariate Cox regression, including terms for age, sex, hospitalization type, hospital ward, PICC indication, type of oncological disease, PICC insertion arm and PICC type.
Table 3. Relation between baseline characteristics and outcome in 356 oncological patients after persistent withdrawal occlusion (PWO) of PICC
|
End of therapy
(n=173) |
Removal due to clinical decision (PWO)
(n=89) |
Removal due to occlusion
(n=94) |
p-valuea |
|
n (%) |
n (%) |
n (%) |
|
Age |
Mean ± SD |
73.6 ± 12.5 |
75.0 ± 13.1 |
74.2 ± 13.1 |
0.59 |
Sex |
Male |
76 (43.9) |
45 (50.6) |
38 (40.4) |
|
Female |
97 (56.1) |
44 (49.4) |
56 (59.6) |
0.37 |
PICC type |
Open-tip |
33 (19.1) |
38 (42.7) |
56 (59.6) |
|
Closed-tip |
140 (80.9) |
51 (57.3) |
38 (40.4) |
<0.001 |
Hospitalization type |
Hospitalized |
59 (34.1) |
38 (42.7) |
52 (55.3) |
|
Day hospital |
114 (65.9) |
51 (57.3) |
42 (44.7) |
0.004 |
Referral hospital ward |
Surgery |
16 (9.2) |
23 (25.8) |
25 (26.6) |
|
Oncology |
157 (90.7) |
66 (74.2) |
69 (73.4) |
<0.001 |
PICC indication |
Chemotherapy |
155 (89.6) |
62 (69.7) |
64 (68.1) |
|
Parenteral nutrition |
18 (10.4) |
27 (30.3) |
30 (31.9) |
<0.001 |
Oncological disease |
Big killersb |
125 (72.2) |
68 (76.4) |
76 (80.8) |
|
Other solid tumours |
36 (20.8) |
16 (18.0) |
10 (10.6) |
|
Lympho-hematological cancers |
12 (6.9) |
5 (5.6) |
8 (8.5) |
0.31 |
PICC insertion arm |
Right |
131 (75.7) |
68 (76.4) |
68 (72.3) |
|
Left |
42 (24.3) |
21 (23.6) |
26 (27.7) |
0.78 |
No. of venipuncture at insertion |
1 |
159 (91.9) |
80 (89.9) |
82 (87.02) |
|
≥ 1 |
14 (8.1) |
9 (10.1) |
12 (12.8) |
0.47 |
Ease of insertion of micro-introducer |
Easy |
162 (93.6) |
83 (93.3) |
82 (87.2) |
|
Hard |
11 (6.4) |
6 (6.7) |
12 (12.8) |
0.16 |
Ease of PICC insertion |
Easy |
167 (96.5) |
82 (92.1) |
84 (89.4) |
|
Hard |
6 (3.5) |
7 (7.9) |
10 (10.6) |
0.06 |
Malposition of PICC |
No |
165 (95.4) |
84 (94.4) |
88 (93.6) |
|
Yes |
8 (4.6) |
5 (5.6) |
6 (6.4) |
0.82 |
Right arm PICC insertion (cm) |
Mean ± SD |
37.4 ± 2.9 |
38.0 ± 3.0 |
37.9 ± 3.2 |
0.27 |
Left arm PICC insertion (cm) |
Mean ± SD |
38.7 ± 3.4 |
39.6 ± 3.1 |
39.4 ± 3.4 |
0.41 |
ap-value for comparison between three outcome groups.
bIncluding gastric, colorectal, lung and breast cancers.
Table 4. Predictive factors of PICC removal due to occlusiona in 356 oncological patients with persistent withdrawal occlusion (PWO) of PICC, according to various characteristics
|
OR (95% CI),
Model 1b |
OR (95% CI),
Model 2c |
|
n (%) |
n (%) |
Age |
One-year age increase (continuous term) |
1.001 (0.983-1.020) |
1.000 (0.980-1.019) |
Sex |
Male |
1 (reference) |
1 (reference) |
Female |
1.26 (0.78-2.04) |
1.46 (0.87-2.45) |
Hospitalization type |
Hospitalized |
1 (reference) |
1 (reference) |
Day hospital |
0.48 (0.29-0.77) |
0.67 (0.38-1.15) |
Referral hospital ward |
Surgery |
1 (reference) |
1 (reference) |
Oncology |
0.48 (0.27-0.84) |
1.75 (0.72-4.22) |
PICC indication |
Chemotherapy |
1 (reference) |
1 (reference) |
Parenteral nutrition |
2.30 (1.34-3.96) |
1.09 (0.48-2.49) |
Oncological disease |
Big killerb |
1 (reference) |
1 (reference) |
Other solid tumours |
0.49 (0.24-1.02) |
0.61 (0.28-1.32) |
Lympho-hematological cancers |
1.19 (0.49-2.89) |
1.43 (0.54-3.75) |
PICC insertion arm |
Right |
1 (reference) |
1 (reference) |
Left |
1.18 (0.69-2.01) |
1.59 (0.88-2.85) |
PICC type |
Closed-tip |
1 (reference) |
1 (reference) |
Open-tip |
4.15 (2.51-6.85) |
4.83 (2.52-9.26) |
aComparison group included patients whose PICC was removed because of either end of therapy (n=173) or clinical decision (PWO, n=89).
bOdds ratios (OR) and corresponding 95% confidence intervals (CI) computed from multivariate logistic regression, including terms for age and sex.
cOR and corresponding 95% CI computed from multivariate logistic regression, including terms for age, sex, hospitalization type, hospital ward, PICC indication, type of oncological disease, PICC insertion arm and PICC type.


