Spondyloarthropathies (SpA) include a group of inflammatory arthritides encompassing ankylosing spondylitis (AS), reactive arthritis, arthritis/spondylitis associated with psoriasis (PsA) and arthritis/spondylitis associated with inflammatory bowel diseases (IBD). In Spa, inflammation can act either by promoting atherosclerosis or by increasing the effect of conventional CV risk factors. The incidence of cardiovascular (CV) disease in AS is 10-30% and includes aortic valve regurgitation, aortitis, atrioventricular (AV) and/or bundle branch block. In PsA and IBD, there is increased risk of CV events, because high levels of cytokines promote atherosclerosis. Furthermore, the persistence of systemic inflammation promotes the development of myocardial inflammation
Magnetic Resonance Imaging (MRI) can identify inflammation in the early stage of SpA, which usually occurs years before the development of structural lesions. Bone marrow edema (BME) has been detected not only at sacroiliac joints, but also at the spine, and it is considered as the hallmark of inflammation. Cardiovascular magnetic resonance (CMR) allows function and tissue characterization and detects subclinical cardiac lesions, including myocardial oedema, myocarditis, replacement and diffuse myocardial fibrosis, despite the presence of well controlled musculoskeletal disease.
Considering that bone radiographic, cardiac echocardiographic findings and serum biomarkers are late markers of bone and heart involvement, MRI/CMR can play an indispensible role for early diagnosis/follow up of bone/heart disease in SpA.
magnetic resonance imaging; spondyloarthropathies; bone lesions; cardiac lesions; heart failure
The term of spondyloarthropathies (SpA) describes a group of inflammatory arthritides encompassing ankylosing spondylitis (AS), reactive arthritis, arthritis/ spondylitis associated with psoriasis (PsA) and arthritis/spondylitis associated with inflammatory bowel diseases (IBD). The human leukocyte antigen (HLA)-B27 positivity, peripheral joint involvement mainly of the lower extremities, sacroiliitis, spondylitis, enthesitis, dactylitis, uveitis, enteric mucosal lesions and skin lesions are the classic manifestations of these diseases [1-3].
The recently developed Assessment of SpondyloArthritis International Society (ASAS) classification criteria proposed the classification of SpA according to the main clinical manifestations including either predominantly axial or predominantly peripheral presentation, with or without coexisting psoriasis, IBD or preceding infection [4-8]. Inflammatory back pain (IBP), the leading symptom of the SpA, is due to inflammation of sacroiliac joints, spine and spinal entheses. However, the sensitivity and specificity of IBP for diagnosis of axial SpA is only 80% [6,7]. HLA B-27 positivity is an important index for the early diagnosis of SpA. Five to 10% of the population is HLA B-27 positive. However, in patients with AS and other SpA the positivity of HLA B-27 reaches 70% - 95% and 70%, respectively [8].
Type of spondyloarthropathies
Ankylosing spondylitis (AS) is the commonest expression of SpA. It is 2-3 times more common in men than women. IBP in a young patient is the most frequent symptom, but peripheral arthritis and/or enthesopathy may be found in some patients. Uveitis, positive family history for AS, impaired spinal mobility or chest expansion, all supports the diagnosis of AS [1].
Axial involvement is one of the disease characteristics and 90% of patients have radiographic sacroiliitis during the course of the disease. Currently, a patient can be classified as having definite AS, if at least one clinical criterion (IBP, limitation of lumbar spine or limitation of chest expansion) plus a radiologic criterion (bilaterally grade 2 or unilateral grade 3-4 sacroiliitis) are fulfilled [9].
Axial spondyloarthritis: Unfortunately, sacroiliitis appears on plain radiographs years after the onset of IBP. Therefore, in 2009, ASAS developed two sets of criteria for classification of axial SpA that include patients without definite radiographic sacroiliitis [10]. The new criteria include a ‘clinical arm’ and an ‘imaging arm’. The positivity of both “arms” had 82.9% sensitivity and 84.4% specificity, while the positivity of the ‘imaging arm’ alone had a sensitivity of 66.2% and specificity of 97.3%. ASAS criteria are simple and easily applicable in every day clinical practice [11-15].
Peripheral spondyloarthritis: After the development of ASAS criteria for axial SpA, ASAS experts developed criteria for SpA patients with predominant peripheral symptoms. The sensitivity of the criteria was 77.8% and the specificity was 82.2%. The new ASAS classification criteria for peripheral arthritis perform better than the previously used criteria [10].
Psoriasis is a common disease affecting nearly 1% -2% of the population. In some forms of SpA, concurrent psoriasis may also occur. Psoriasis may precede, occur in parallel, or appear years after the onset of arthritis. In latter cases, patients may be misdiagnosed as having other types of arthritis, such as seronegative RA or reactive arthritis; however, positive family history for psoriasis is of great help in these cases. Patients with arthritis should be also evaluated for potentially “hidden” psoriatic lesions, located under the breasts, around the umbilicus or anus, over the hairline, nasal cleft or nails [11].
Patients with PsA usually present inflammatory axial involvement similar to AS. However, there are several differences compared to AS [11] including the presence of asymmetrical sacroiliitis, non-marginal syndesmophytes, asymmetrical syndesmophytes and more frequent involvement of the cervical spine [11].
- Arthritis in Inflammatory bowel disease
Two types of joint involvement occur in patients with IBD including a) arthritis (inflammation) and b) arthralgia (pain without inflammation). Arthralgia is more common in IBD, occurring in 40-50% of patients, a rate similar to that of the general population. Arthritis occurs in 15-20% of Crohn’s disease (CD) patients and approximately 10% of ulcerative colitis (UC) [12].
Approximately 60–70% of arthritis in IBD has the characteristics of peripheral oligo-arthritis with<5 joints affected. The most affected joints are the knees, ankles, wrists, elbows and hips. A smaller proportion of IBD patients have symmetrical polyarthritis of the small joints of the hands. Finally, 1–6% of all IBD patients develop AS affecting the sacroiliac joints and the spine. While large joint arthritis is almost always associated with active IBD, AS and small joint polyarthritis appear independently of the patient’s IBD [12].
Cardiovascular involvement in SpA
The incidence of cardiovascular (CV) involvement in AS ranges between 10% to 30% [13] and may presented as aortic valve regurgitation, aortitis of the ascending aorta, atrioventricular (AV) and/or bundle branch block. The most frequently observed complications are conduction defects and aortic regurgitation. Mitral regurgitation in AS is rare but may lead to heart failure (HF) [14-17]. CVD is more frequent in patients with long-term AS and peripheral joint involvement [18].
The incidence of aortic dissection has been reported about 2.9 in 100,000 [19] and represents the most fatal complication of the disease [20]. The most frequently observed symptom in AS patients is chest pain; however, it may have atypical findings including lack of pain or development of cardiac, neurological symptoms or evidence of ischemic extremities [21,22]. Aortic dissection is generally observed between the ages of 50-70 years and the ratio of men to women is 2:1 [21].
The main sources of stroke in AS are the lesions of the proximal aorta, but paraplegia may develop, due to distal lesions involving the spinal arteries. Although stroke and paraplegia develop rarely, their incidence in cases of aortic dissection is ranged from 2% to 8% [23].
- Psoriatic Arthritis (PsA)
PsA has an increase in risk of clinical and subclinical CVD, mainly due to accelerating atherosclerosis. Both conventional and nonconventional CV risk factors contribute to increase atherosclerosis with consequent increase of CV risk. Although conventional risk factors occur more frequently in patients with PsA, they can only partially explain the excess of CV risk. It seems that inflammation is an important precipitating factor for the increased CV risk in PsA. Inflammation can act either by directly promoting the development of atherosclerosis or by increasing the effect of established conventional CV risk factors [24]. Furthermore, systemic inflammation can also precipitate myocardial inflammation [24].
- Inflammatory bowel disease
Patients with IBD are at increased risk of CV events. High levels of cytokines, C-reactive protein (CRP) and homocysteine in IBD may contribute to endothelial dysfunction finally leading to atherosclerosis. Although IBD patients do not present the typical risk factors for CVD, changes in lipid profiles similar to those seen in patients with CV events have been reported. Furthermore, increased levels of coagulation factors, frequently occurring in IBD, may predispose to arterial thromboembolic events. Finally, the gut itself may have an impact on atherogenesis through its microbiota. Microbial products are released from the inflamed intestinal mucosa into the circulation through a leaky barrier. As a consequence, the increase of proinflammatory cytokines contributes to endothelial damage and CV events [25].
The link between bone and cardiac inflammation
There is a link between bone inflammation in SpA and CVD development which was usually attributed to coronary artery disease (CAD). However, the commonest CVD expression in SpA is heart failure (HF), which may start at diagnosis and increases proportionally to duration and severity of the systemic inflammation. However, it should be noted that HF in SpA is not the result of epicardial coronary artery disease, but is related to myocardial inflammation, coronary microvascular dysfunction and fibrosis, all of them leading to heart failure with preserved ejection fraction (HFpEF) [26].
Pro-inflammatory mediating factors that are characteristic of systemic inflammation such as leptin, aldosterone and neprilysin can cause expansion and biological transformation of epicardial adipose tissue (EAT) leading to coronary microvascular injury and myocardial fibrosis [27]. In patients with expanded EAT, the release of leptin and aldosterone promote myocardial inflammation, microcirculatory dysfunction and fibrosis [27]. These effects are opposed to the anti-inflammatory and anti-fibrotic actions of endogenous natriuretic peptides [28] and lead to impairment in the distensibility of the left ventricle (LV). Therefore, blood volume is regulated only due to an increase in cardiac filling pressures. This finally leads to exertional dyspnoea and HF. EAT volume is increased in SpA and 30–50% of affected patients have subclinical cardiac inflammation, microcirculatory dysfunction and fibrosis finally leading to HFpEF.
SpA patients are characterized by increased levels of aldosterone in blood and inflamed tissues [29] and therefore, spironolactone, an aldosterone antagonist, has been proposed as a treatment for these arthritides. At the same time, the increased levels of leptin in blood and synovial fluid, found in SpA, are associated with the extent of joint/bone involvement and linked with patient-reported symptoms and disease activity [30]. Interestingly, high levels of leptin may identify SpA patients, who respond poorly to anti-inflammatory treatment [31].
The currently used anti-inflammatory agents in the treatment of SpA have the potential to reduce the risk of cardiac involvement and HFpEF [32]. In addition, it is possible that drugs modulating the leptin-aldosterone-neprilysin axis could modify the CV consequences of systemic inflammation and change the clinical course of HFpEF in these patients [33].
The indispensible role of magnetic resonance imaging
The pathophysiologic mechanisms, already discussed, make clear the correlation between the systemic inflammation and the development of CVD and emphasize the need for a concurrent assessment of both bone and cardiac inflammation. In this context, the role of a sensitive and reproducible imaging modality that can identify early the presence of inflammation is of great value for both bones and heart. The role of various modalities in the early inflammation identification in bones and heart is described below
- Role of imaging for bone/joint assessment in SpA
The imaging of the sacroiliac joints and the spine plays an important role in the diagnosis and monitoring of SpA. Sacroiliitis on conventional radiography was used as an important criterion for AS diagnosis. Usually, bilateral grade ≥ 2 or unilateral grade ≥ 3 sacroiliitis are considered typical for the diagnosis of AS [13]. However, radiographic sacroiliitis reflects changes late in the course of the disease and only in a subgroup of SpA patients [12].
The introduction of magnetic resonance imaging (MRI) in the clinical practice has changed dramatically the diagnostic algorithm of SpA, because MRI-detected sacroiliitis has been included in the new Assessment of SpondyloArthritis Society (ASAS) classification criteria for axial SpA, as a basic diagnostic tool [34]. Prior to this innovation, radiological sacroiliitis was diagnosed on the basis of x-rays, where changes can be detected only at advanced stages of the disease and therefore, they have low specificity for patients in early stages of the disease. In contrast, MRI can identify inflammation in the early stage of SpA, which usually occurs years before the development of structural lesions. Bone marrow edema (BME) has been detected not only at sacroiliac joints in axial SpA, but also at the spine, and it is considered as the hallmark of inflammation (Figure 1&2). Among inflammatory lesions that can be detected on MRI at the sacroiliac joints, only BME has been considered as a reliable index in the identification of active sacroiliitis [35]. This finding is thought to reflect the presence of osteitis and correlates with histologically documented inflammation. Bollow, et al. demonstrated an inflammatory infiltrate of T-cells, macrophages and scarce B-cells in biopsy of patients with MRI-detected sacroiliitis [36]. Moreover, they found a higher number of inflammatory cells in patients with active sacroiliitis on MRI, but not in patients with chronic sacroiliitis. Recent studies support that not only the presence, but also the severity of BME is important for the disease diagnosis. In a prospective study of 40 patients with IBP, the presence of severe sacroiliitis in association with HLA-B27 positivity was highly predictive of a diagnosis of AS at 8 years, with a positive likelihood ratio of 8.0. In contrast, patients with mild or no BME had a low likelihood to develop AS [37]. In another study comparing patients with IBP with patients with mechanical back pain or healthy volunteers, BME of the sacroiliac joints was found also in the control group, although it was less frequent compared to patients with IBP. The very early IBP of SpA can be differentiated from non-SpA, based on BME severity [38].

Figure 1: Semicoronal STIR image shows bone marrow edema especially at the sacral side of the left sacroiliac joint and moderate synovium edema.
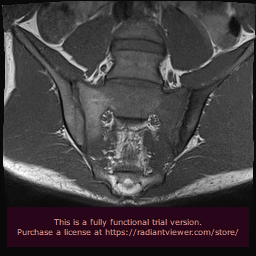
Figure 2: T1w image shows low signal abnormalities due to marrow edema and mild sclerosis of the iliac side endplate.
MRI can identify active inflammation at sacroiliac joints and spine in established or early axial disease, independently of disease stage [10]. ASAS classification criteria for axial SpA have 2 arms: an imaging and a clinical arm. The imaging arm includes either sacroiliitis on conventional radiography or on MRI, which is of indispensible value for the recognition of pre-radiographic changes in early SpA [4]. Regarding spondylitis, which may also occur before sacroiliitis, the diagnosis of a “positive MRI” for spinal inflammation is also needed [39]. However, there are insufficient data regarding the use of spinal MRI and the specificity of spinal findings in the axial SpA diagnosis [40].
Active inflammatory lesions (bone marrow edema, osteitis, synovitis, enthe-sitis, capsulitis) and structural damage (sclerosis, erosions, fat deposition, ankylosis) can be detected by MRI. ASAS/OMERACT imaging group defined that a minimum amount of BME in one lesion of at least two adjacent slices or more than one lesion at least in one slice is required for the definitive diagnosis of sacroiliitis [41].
MRI can also assess the evolution of spinal damage of AS. Usually, it begins with bone inflammation that may be visible as osteitis, typically seen on a short tau inversion recovery (STIRT2) image of the MRI [42,43]. To repair the tissue, subchondral bone marrow is replaced with fatty metaplasia, which presents as a fatty lesion on a T1 weighted MRI scan [42,43]. Inflammation or intermediate stage fatty lesions may play an important role in the development of new bone formation (structural damage), emphasizing the need for early deterioration of inflammation to reduce risk of further bone damage [43]. Almost 60% to 70% of AS patients develop irreversible structural changes leading in spinal fusion and reduced spinal mobility [43,44]. Although AS patients usually present reduced physical/spinal function, due to structural lesions and inflammation, in patients with non-radiologic positive SpA (nr-axSpA), this functional disability is typically due to inflammation alone [44]. Risk factors for progression of spinal disease in AS patients include male sex, presence of syndesmophytes at baseline, elevation of CRP and smoking (in men) [45].
Recently, several studies have examined the MRI characteristics of new bone formation in axial skeleton in patients with SpA and assessed findings that showed potential markers of disease progression [46-52]. The intra-articular high signal intensity on T1-weighted MR images known as “Backfill” with high signal intensity similar to adipose tissue on T1-weighted MR images in the sacroiliac joint space was considered as a metaplastic tissue refilling of the eroded subchondral bone [46,50,51]. There is no consensus about the clinical value of this finding, because until now there is no histopathological evaluation of this lesion [50]. However, this intra-articular high signal intensity on T1-weighted MRI images of the sacroiliac joints has been documented in 38–63% of SpA patients ≤ 45 years old and had a high diagnostic value for this disease [46,50]. There is also supported that this finding should be considered stronger that the classic ASAS criteria for SpA sacroiliitis, even if there is no concurrent BME [51].
Ankylosis of the sacroiliac joints is the typical finding of end-stage axial SpA. This may appear as low signal intensity obliteration of articular cortical margins, on most MRI sequences, but it may present high signal intensity on T1-weighted MR images, when the subarticular bone marrow crossing the sacroiliac joint has high-fat content [50,51].
The presence of discal high signal intensity on T1-weighted MR images, which has only been evaluated in a limited number of patients, has high signal intensity similar to adipose tissue on T1-weighted images within the intervertebral disc and was considered as an early discal calcification [52-58]. In a recent study, it was considered as highly specific for SpA, although it needs further validation [52].
Syndesmophyte formation is defined as bony growth originating from the Sharpey fibers of the annulus fibrosus [52]. On sagittal spinal MRI, syndesmophytes will be observed as longitudinal bony outgrowths at the anterior and posterior corners of the vertebral bodies, oriented craniocaudally. The signal intensity on T1-weighted images is isointense or hyperintense to red bone marrow—in case of presence of fatty bone marrow [52]. However, non-bridging syndesmophytes in the absence of other findings of new bone formation were only seen in patients without SpA [21] and therefore, they should not be used for SpA diagnosis or follow up. Vertebral corner bridging also known as “bridging syndesmophytes” or “ankylosis within the annulus fibrosus” [52] is observed in the anterior or posterior corners of the vertebral bodies, at the Sharpey fibers of the annulus fibrosus of the intervertebral disc [52]. The signal intensity on T1-weighted images is isointense or hyperintense to red bone marrow, in case of fatty bone marrow [52]. In contrast to non-bridging syndesmophytes, this MRI feature is specific for SpA [52].
Transdiscal ankylosis, also known as “non-corner ankylosis” [52], is defined as bone fusion crossing the vertebral joint space through the expected location of the nucleus pulposus in the intervertebral disc, with obliteration of the cortical margins of the vertebral body [52]. Similar to syndesmophytes, the signal intensity on T1-weighted images is isointense or hyperintense to red bone marrow, in case of presence of fatty bone marrow [52]. This finding is specific for axial SpA [52] and is considered a marker of late disease, because axial SpA almost always starts in the sacroiliac joints, typically leaving the spine unaffected for long time. However, it may also be found in younger patients with more severe disease [52].
Role of imaging for cardiac assessment in SpA
The main imaging modalities used for evaluation of myocardial status in SpA include echocardiography and cardiovascular magnetic resonance (CMR). Subclinical cardiac dysfunction was identified in AS patients despite well controlled musculo-skeletal disease, using echocardiography [59]. Furthermore, aortic stiffness and left ventricular global longitudinal strain (LVGLS) were increased in AS patients [59]. In another echocardiographic study, early, subclinical myocardial dysfunction was observed in inflammatory joint disease (IJD) patients with preserved left-ventricular ejection fraction, but without traditional CV risk factors. In these patients, disease activity was the main predictor of myocardial strain impairment [60]. In another study, it was documented that AS patients had lower LVGLS compared with controls, independently of confounders. Furthermore, lower LVGLS was associated with larger aortic root diameter [61]. Finally, most asymptomatic SpA patients have concentric LV remodeling, which is closely associated with subclinical left ventricular systolic dysfunction (LVSD) [62]. Echocardiography, although it is an easy, low cost and widely available modality, it has the limitations of operator and acoustic window dependency and cannot perform cardiac tissue characterization [63].
In contrast to echocardiography, CMR is operator and acoustic window independent and can provide function and tissue characterization information noninvasively in the same examination [63]. A CMR study, including AS patients with abnormal findings on screening echocardiography, identified that aortic arch pulse wave velocity (PWV) was significantly higher in the AS group compared with the controls [64]. Higher PWV in the aortic arch was associated with functional disability, the presence of non-ischemic hyperenhancement and reduced LV systolic function [64]. In another CMR study in AS patients, global LV dysfunction and focal areas of hyperenhancement was identified. Myocardial extracellular volume fraction (ECV) was associated with the degree of disease activity [65]. Furthermore, occult CMR lesions, including oedema, myocarditis (Figures 3&4), diffuse subendocardial fibrosis and myocardial infarction are not unusual in treatment naïve SpA patients and may be reversed with appropriate treatment [66]. Recently, anti-TNF treatment, applied in SpA patients, reduced subclinical myocardial inflammation and improved CV function proving that CMR may have a place to assess CV disease progression and response to treatment [67].
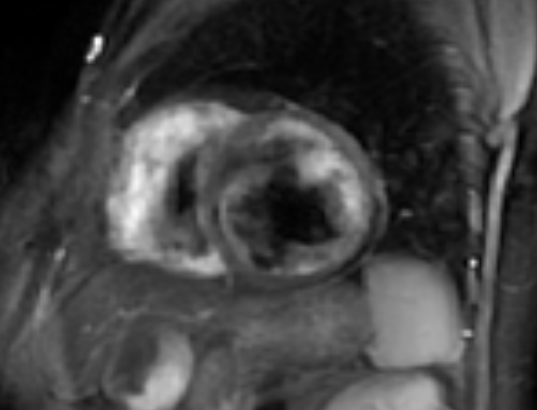
Figure 3: STIRT2 image of the heart showing myocardial oedema in a patient with AS.
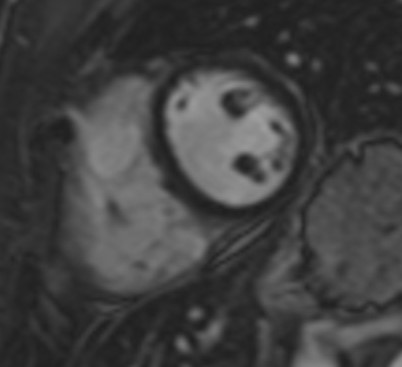
Figure 4: Inversion recovery image of the heart showing subepicardial LGE in the lateral wall in a patient with PsA.
MRI versus biomarkers in SpA
The evaluation of biomarkers in SpA is of particular interest, because the two most commonly used biomarkers, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are of very low sensitivity and specificity. The second reason is the need for cost-effectiveness assessment after treatment of SpA patients with the very expensive tumor necrosis factor-alpha (TNF-alpha) blockers. In these cases, the clinicians need to be much more accurate in predicting disease progression, evaluating disease activity and monitoring therapeutic efficacy [68]. Furthermore, according to the results from the SpondyloArthritis Caught Early (SPACE) cohort the disease process in axial spondyloarthritis is not reflected by alterations in blood inflammatory indices such as CRP, ESR and Calprotectin. Additionally, serum levels of interleukin-27 (IL-27), human β-defensin-2 (hBD-2) and lipcolin-2 (LCN-2) were also not elevated. In contrast, the direct visualization of the inflammatory process might be a more successful approach to identify imaging biomarkers for axial SpA [69].
Regarding the cardiac evaluation, troponin and BNP are the most commonly used biomarkers. In the appropriate patient, an elevated troponin indicates heart damage and should prompt urgent inpatient investigations, while a normal troponin indicates low risk, but not absence of risk. Furthermore, troponins may be falsely elevated in certain situations and do not always indicate heart attack. These situations include myocarditis, Takotsubo syndrome, pulmonary embolism and trauma including also cardioversion or defibrillation [70]. Finally, the prevalence of an increased troponin T in biopsy-proven myocarditis, is only 35–45% [71].
More specifically in patients with axial SpA, both reduced longitudinal strain (LS) and elevated serum high-sensitivity troponin I (hsTnI) are promising independent predictors for CV events and those with LS ≥ - 17.5% and hsTnI ≥ 3.0 pg/ml are at the highest risk of CV events [72].
It is impressive that although MRI has been used very early in the assessment of bone/joints, its application on cardiac evaluation of SpA patients has been only recently applied. There are many reasons for this including:
- At the very first moment of its application MRI was considered as the ideal tool to assess bones and soft tissues. Therefore, it was introduced very early in the diagnostic algorithm of SpA [34]
- Cardiovascular magnetic resonance (CMR) is a more overdemanding application that has become only recently available to cardiologists. Additionally, cardiologists are “obsessed” [63] with echocardiography, which is a modality unsuitable to detect myocardial inflammation, because it cannot perform tissue characterization.
Under these circumstances, only parameters available by echocardiography such as aortic dilatation or diastolic dysfunction were considered as serious cardiac complications in SpA. Furthermore, although HFpEF is the main cause of CV death in SpA, information regarding acute myocardial inflammation/fibrosis was completely ignored, due to the inability of echocardiography to detect these phenomena [63]. Finally, the knowledge that CV involvement may exist before or at diagnosis of various SpA [66], suggests that CMR should be considered in the diagnostic algorithm of CV assessment of SpA. At the moment published literature regarding CMR in SpA is poor. However, the need to assess myocardial inflammation/fibrosis as an effort to prevent the development of HFpEF in SpA patients should motivate further multicenter studies to establish the role of CMR in the SpA guidelines for CV evaluation.
Inflammation is the fundamental pathophysiologic background of bone/heart lesions in SpA patients. Since blood inflammatory indices are of limited value to assess bone/heart disease activity, there is a tremendous need for a targeted imaging approach. Bone MRI has been already included in the diagnostic algorithm of SpA, but the assessment of myocardial inflammation/ fibrosis, using CMR, is still not included in the routine evaluation of SpA. However, the fact that HFpEF represents the main cause of CV mortality in SpA, suggests that a reconsideration of cardiac evaluation in SpA is needed.
- Khan MA (2002) Update on spondyloarthropathies. Ann Intern Med 136: 896–907. [Crossref]
- Sieper J, Rudwaleit M, Khan MA, Braun J (2006) Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol 20: 401–417. [Crossref]
- Braun J, Sieper J (2002) Building consensus on nomenclature and disease classification for ankylosing spondylitis: results and discussion of a questionnaire prepared for the International Workshop on New Treatment Strategies in Ankylosing Spondylitis, Berlin, Germany, 18-19 January 2002. Ann Rheum Dis 61: iii61–iii67. [Crossref]
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, et al. (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68: 777–783. [Crossref]
- Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, et al. (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70: 25–31. [Crossref]
- Rudwaleit M, Metter A, Listing J, Sieper J, Braun J (2006) Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 54: 569–578. [Crossref]
- Sieper J, van der Heijde D, Landewé R, Brandt J, Burgos-Vagas R, et al. (2009) New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 68: 784–788. [Crossref]
- Rostom S, Dougados M, Gossec L (2010) New tools for diagnosing spondyloarthropathy. Joint Bone Spine 77: 108–114. [Crossref]
- van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27: 361–368. [Crossref]
- Rudwaleit M (2010) New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr Opin Rheumatol 22: 375–380. [Crossref]
- Helliwell PS, Taylor WJ (2005) Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis 64: ii3–ii8. [Crossref]
- Rodríguez-Reyna TS, Martínez-Reyes C, Yamamoto-Furusho JK (2009) Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol 15: 5517-5524. [Crossref]
- Kazmierczak J, Peregud-Pogorzelska M, Biernawska J, Przepiera-Bedzak H, Goracy J, et al. (2007) Cardiac arrhythmias and conduction disturbances in patients with ankylosing spondylitis. Angiology 58: 751-756. [Crossref]
- Khan MA (1994) Ankylosing spondylitis. Clinical features. In: Klippel JH, Dieppe PA. Rheumatology. St. Louis: Mosby 25: 1-10.
- Calin A (1998) Ankylosing spondylitis. In: Maddison PJ, Isenberg PA, Woo P, Glass DN. Oxford Textbook of Rheumatology. Oxford: Oxford University Press, 1058-1070.
- Inman RD (1997) Ankylosing spondylitis. In: Klippel JH. Primer on the Rheumatic Disease. Atlanta: Arthritis Foundation, 189-195.
- Ozkan Y (2016) Cardiac Involvement in Ankylosing Spondylitis. J Clin Med Res 8: 427-430. [Crossref]
- Ryall NH, Hellivvell PS (1998) A critical review of ankylosing spondylitis. Critical Rev Phys Med Rehab 10: 265-301.
- Meszaros I, Morocz J, Szlavi J, Schmidt J, Tornoci L, et al. (2000) Epidemiology and clinicopathology of aortic dissection. Chest 117: 1271-1278. [Crossref]
- Sorensen HR, Olsen H (1964) Ruptured and Dissecting Aneurysms of the Aorta. Incidence and Prospects of Surgery. Acta Chir Scand 128: 644-650. [Crossref]
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, et al. (2000) The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 283: 897-903. [Crossref]
- Farina GA, Kwiatkowski T (2003) Aortic dissection. Prim Care Update Ob/Gyns 10: 161-166.
- Zull DN, Cydulka R (1988) Acute paraplegia: a presenting manifestation of aortic dissection. Am J Med 84: 765-770. [Crossref]
- Zhu TY, Li EK, Tam LS (2012) Cardiovascular risk in patients with psoriatic arthritis. Int J Rheumatol 2012: 714321. [Crossref]
- Schicho R, Marsche G, Storr M (2015) Cardiovascular complications in inflammatory bowel disease. Curr Drug Targets 16: 181-188. [Crossref]
- Kibari A, Cohen AD, Gazitt T, Bitterman H, Lavi I, et al. (2019) Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study. Clin Rheumatol 38: 2069-2075. [Crossref]
- Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, et al. (2015) Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132: 2134–2145. [Crossref]
- Tamura N, Ogawa Y, Chusho H, Nakao K, Suda M, et al. (2000) Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97: 4239–4244. [Crossref]
- Guy A, Sharif K, Bragazzi NL, Krosser A, Gilburd B, et al. (2018) Low levels of renin and high aldosterone-to-renin ratio among rheumatoid patients and ankylosing spondylitis patients: a prospective study. Isr Med Assoc J 20: 632–636. [Crossref]
- Hartl A, Sieper J, Syrbe U, Listing J, Hermann K, et al. (2017) Serum levels of leptin and high molecular weight adiponectin are inversely associated with radiographic spinal progression in patients with ankylosing spondylitis: results from the ENRADAS trial. Arthritis Res Ther 19: 140. [Crossref]
- Hambardzumyan K, Bolce RJ, Wallman JK, van Vollenhoven RF, Saevarsdottir S (2019) Serum biomarkers for prediction of response to methotrexate monotherapy in early rheumatoid arthritis: results from the SWEFOT trial. J Rheumatol 46: 555–563. [Crossref]
- Heslinga SC, Van Sijl AM, De Boer K, Van Halm VP, Nurmohamed MT (2015) Tumor necrosis factor blocking therapy and congestive heart failure in patients with inflammatory rheumatic disorders: a systematic review. Curr Med Chem 22: 1892–1902. [Crossref]
- Merrill M, Sweitzer NK, Lindenfeld J, Kao DP (2019) Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail 7: 228–238. [Crossref]
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, et al. (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68: 777-783.
- Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, et al. (2009) Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/ OMERACT MRI group. Ann Rheum Dis 68: 1520-1527.
- Bollow M, Fischer T, Reisshauer H, Backhaus M, Sieper J, et al. (2000) Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis - cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 59: 135-140. [Crossref]
- Bennett AN, McGonagle D, O’Connor P, Hen-sor EM, Sivera F, et al. (2008) Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 58: 3413-3418. [Crossref]
- Marzo-Ortega H, McGonagle D, O’Connor P, Hensor EM, Bennett AN, et al. (2009) Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann Rheum Dis 68: 1721-1727. [Crossref]
- Braun J, Baraliakos X (2011) Imaging of axial spondyloarthritis including ankylosing spondylitis. Ann Rheum Dis 70 : i97–i103. [Crossref]
- van der Heijde D, Rudwaleit M, Landewé RB, Sieper J (2010) Justification for including MRI as a tool in the diagnosis of axial SpA. Nat Rev Rheumatol 6: 670–672. [Crossref]
- Rudwaleit M, Jurik AG, Hermann KG, Landewé R, van der Heijde D, et al. (2009) Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 68: 1520–1527.
- Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, et al. (2009) The Assessment of SpondyloArthritis International Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 68: ii1-ii44.
- Poddubnyy D, Sieper J (2017) Mechanism of new bone formation in axial spondyloarthritis. Curr Rheumatol Rep 19: 55. [Crossref]
- Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, et al. (2016) American College of Rheumatology /Sponylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 68: 282-298. [Crossref]
- Deminger A, Klingberg E, Geijer M, Göthlin J, Hedberg M, et al. (2018) A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther 20: 162. [Crossref]
- Weber U, Pedersen SJ, Østergaard M, Rufibach K, Lambert RG, et al. (2012) Can erosions on MRI of the sacroiliac joints be reliably detected in patients with ankylosing spondylitis? - a cross-sectional study. Arthritis Res Ther 14: R124. [Crossref]
- Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ (2014) Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol 66: 2958–2967. [Crossref]
- Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ (2015) Development and preliminary validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol 42: 79–86. [Crossref]
- de Hooge M, van den Berg R, Navarro-Compán V, Reijnierse M, van Gaalen F, et al. (2016) Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis 75: 1308–1314. [Crossref]
- Laloo F, Herregods N, Varkas G, Jaremko JL, Baraliakos X, et al. (2017) MR signal in the sacroiliac joint space in spondyloarthritis: a new sign. Eur Radiol 27: 2024–2030. [Crossref]
- Laloo F, Herregods N, Jaremko JL, Verstraete K, Jans L (2018) MRI of the sacroiliac joints in spondyloarthritis: the added value of intra-articular signal changes for a ‘positive MRI’. Skeletal Radiol 47: 683–693. [Crossref]
- Laloo F, Herregods N, Jaremko JL, Carron P, Elewaut D, et al. (2019) New bone formation in the intervertebral joint space in spondyloarthritis: an MRI study. Eur J Radiol 109: 210–217. [Crossref]
- Jans L, Coeman L, Van Praet L, Carron P, Elewaut D, et al. (2014) How sensitive and specific are MRI features of sacroiliitis for diagnosis of spondyloarthritis in patients with inflammatory back pain? JBR-BTR 97: 202–205. [Crossref]
- Major NM, Helms CA, Genant HK (1993) Calcification demonstrated as high signal intensity on T1-weighted MR images of the disks of the lumbar spine. Radiology 189: 494–496. [Crossref]
- Vignaux O, Sarrazin JL, Cordoliani YS, Cosnard G (1994) Hypersignal of the intervertebral disks in T1-weighted spin-echo MRI sequences. J Radiol 75: 363–367. [Crossref]
- Bangert BA, Modic MT, Ross JS, Obuchowski NA, Perl J, et al. (1995) Hyperintense disks on T1-weighted MR images: correlation with calcification. Radiology 195: 437–443. [Crossref]
- Tyrrell PN, Davies AM, Evans N, Jubb RW (1995) Signal changes in the intervertebral discs on MRI of the thoracolumbar spine in ankylosing spondylitis. Clin Radiol 50: 377–383. [Crossref]
- Malghem J, Lecouvet FE, François R, Berg BCV, Duprez T, et al. (2005) High signal intensity of intervertebral calcified disks on T1-weighted MR images resulting from fat content. Skeletal Radiol 34: 80–86. [Crossref]
- Ozen S, Ozen A, Unal EU, Tufekcioglu O, Ataman S, et al. (2018) Subclinical cardiac disease in ankylosing spondylitis. Echocardiography 35: 1579-1586. [Crossref]
- Lo Gullo A, Rodríguez-Carrio J, Aragona CO, Dattilo G, Zito C, Suárez A, et al. (2018) Subclinical impairment of myocardial and endothelial functionality in very early psoriatic and rheumatoid arthritis patients: Association with vitamin D and inflammation. Atherosclerosis 271: 214-222.
- Midtbø H, Semb AG, Matre K, Rollefstad S, Berg IJ, et al. (2019) Left Ventricular Systolic Myocardial Function in Ankylosing Spondylitis. Arthritis Care Res (Hoboken) 71: 1276-1283. [Crossref]
- Giollo A, Farina N, Cioffi G, Ognibeni F, Dalbeni A, et al. (2020) Concentric left ventricular remodelling is associated with subclinical systolic dysfunction in patients with psoriatic arthritis. Scand J Rheumatol 49: 389-396. [Crossref]
- Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, et al. (2016) Cardiovascular magnetic resonance in rheumatology: Current status and recommendations for use. Int J Cardiol 217: 135-148. [Crossref]
- Biesbroek PS, Heslinga SC, van de Ven PM, Peters MJL, Amier RP, et al. (2018) Assessment of aortic stiffness in patients with ankylosing spondylitis using cardiovascular magnetic resonance. Clin Rheumatol 37: 2151-2159. [Crossref]
- Biesbroek PS, Heslinga SC, Konings TC, van der Horst-Bruinsma IE, Hofman MBM, et al. (2017) Insights into cardiac involvement in ankylosing spondylitis from cardiovascular magnetic resonance. Heart 103: 745-752. [Crossref]
- Mavrogeni S, Markousis-Mavrogenis G, Koutsogeorgopoulou L, Dimitroulas T, Bratis K, et al. (2017) Cardiovascular magnetic resonance imaging pattern at the time of diagnosis of treatment naïve patients with connective tissue diseases. Int J Cardiol 236: 151-156. [Crossref]
- Ntusi NAB, Francis JM, Sever E, Liu A, Piechnik SK, et al. (2018) Anti-TNF modulation reduces myocardial inflammation and improves cardiovascular function in systemic rheumatic diseases. Int J Cardiol 270: 253-259. [Crossref]
- Chen CH, Yu DT, Chou CT (2009) Biomarkers in spondyloarthropathies. Adv Exp Med Biol 649: 122-32. [Crossref]
- Turina MC, Yeremenko N, van Gaalen F, van Oosterhout M, Berg IJ, et al. (2017) Serum inflammatory biomarkers fail to identify early axial spondyloarthritis: results from the SpondyloArthritis Caught Early (SPACE) cohort. RMD Open 3: e000319.
- Gupta S, de Lemos JA (2007) Use and misuse of cardiac troponins in clinical practice. Prog Cardiovasc Dis 50: 151-165. [Crossref]
- Lauer B, Niederau C, Kuhl U, Schannwell M, Pauschinger M, et al. (1997) Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 30: 1354–1359. [Crossref]
- Chen Y, Chan YH, Chung HY, Wu MZ, Yu YJ, et al. (2020) Cardiovascular events prediction by left ventricular longitudinal strain and serum high-sensitivity troponin I in patients with axial spondyloarthritis. Clin Rheumatol 39: 3373-3382. [Crossref]




