Introduction: Vascular Access (VA) is essential in a patient with End Stage Renal Disease (ESRD) in a Hemodialysis program, determining the survival of a patient on the long-term. As a result of better hemodialysis programs, our patients achieve longer survival. Hence vascular exhaustion or End-Stage VA Failure (ES-VAF) is becoming more frequent. There are scarce reports on patients in ES-VAF catheter management. The objective is to show the experience of placement and patency days of trans-lumbar and intra-atrial catheters.
Methods: We included all patients with attempted placement of intra-atrial catheter (IAC) and trans-lumbar catheter (TLC) from 2014 -2019. We analyzed the outcome of catheter patency days, complications, morbidity and mortality. By using non-parametric statistics.
Results: A total of 58 cases were assessed for eligibility, 31 of them being included for analysis; 20 IAC and 11 TLC. The median of primary patency days in IAC was 102.5 and median to secondary patency was 285.5 days; TLC had a median of 475 days of primary patency, were there were no interventions to reestablish functionality in this group. The standard overall mortality rate: TLC 27% and IAC 35%. Hospital average length of stay (ALOS): IAC 20.50 and TLC 7 days. The most common complications were cardiovascular events in IAC and in TLC were VA related infections.
Conclusions: TLC were a superior alternative to IAC with longer primary patency days, less hospital average length of stay days, and lower associated mortality. When TLC placement due to technical difficulty is not a possibility by the interventionist, it must be then considered IAC approach.
vascular access depletion, unconventional vascular access, trans-lumbar catheter and intra-atrial catheter, end stage renal disease
Hemodialysis (HD) is the most commonly used Renal Replacement Therapy (RRT) worldwide, and it is the main modality in most cases on prolonged RRT. In some countries, almost 100% of the population receive HD as in Japan. In countries such as the United States, although it is a dominant therapy, however many questions do exist about its high cost. To achieve a good quality of HD therapy, that allow our patients a greater survival, substantially depends on adequate functionality of the vascular access (VA). While it is an essential piece in all clinical practice guidelines, it is the most expensive individual portion of RRT. In the United States, maintaining a VA includes up to 30% of the total cost per year of an HD program [1].
Conventional VA are divided into 3 types: 1) autologous arterio-venous fistulas [(AVF); being the best alternative in relations to complications and survival]. 2)arterio-venous grafts [(AVG); prosthetic VA’s with synthetic material] and 3) central venous catheter [(CVC); placed in a necessarily high caliber vein (jugular, femoral or subclavian veins being usual)] [2-4].
The VA has a direct relationship with patient mortality, and in order to obtain an optimal HD treatment that will allow our patients to achieve a longer survival, the patient needs a functional VA to guarantee effective blood flow (Qb); The participation of the interventionalist and cardiothoracic surgeon are essential in the placement of NCVAs, highlighting in the procedure 3 elements: the selection of the patient, execution of the procedure and monitoring of the patient [5]. The clinical records of a VA must also include indication, type of device, site of access, duration of use, reason for withdrawal and complications. Any change of site may limit the sites available for future VA’s [6].
The use of CVC in Mexico has increased, contrary to the recommendations of international guidelines such as The Spanish Society of Nephrology (SEN) or The Kidney Disease Outcomes Quality Initiative (KDOQI) [7-8]. This unfortunately is without a singular reason; however, part of this population with increase in CVC use are elderly patients, diabetics, or fistula failures (dysfunction, thrombosis, infection, etc.). This increase in CVC use is causing an unexpected rise in vascular access exhaustion in our patients [9,10]. As a result, more Non-Conventional Vascular-Access (NCVA) are necessary to guarantee a VA with effective Qb. Currently there is no clear consensus about NCVA’s [11].
As a result of this vascular exhaustion, when there is no possibility of VA placement into any of the central veins due to stenosis or thrombosis, there is a new diagnose term coined by Shakarachi; End-Stage Vascular Access Failure (ES-VAF) [12]. In these dire clinical situations, we must start considering NCVA’s, that can range from grafts that bridge the sites of stenosis or CVC’s in non-conventional locations, such as Trans-hepatic, intra-atrial, brachiocephalic vein, cephalic, hemiazygos, trans-lumbar, and trans-renal catheters [13-21]. These NCVA’s are considered to be a heroic measure because of their high mortality [22,23] and have shown limited utility according to some reports worldwide [24-29]. Trans-Lumbar, Intra-Atrial and Trans-Hepatic Catheters have shown better overall results in groups with a NCVA´s [30-35].
Historically there have been a low number of patients with ESVAF, therefore the number of reports considering these alternatives are scarce and with a limited number of patients. The literature is mainly case-reports or case-series and limited to high-specialty reference centers [36-39]. One of the first authors that described the use of intra-atrial catheters (IAC) was Chavanon et al. [37] in 1999. Lund et al. [39] and 7] Gupta et al. [40] in 1995 are among the first studies with reports on alternative TLC in patients with ES-VAF.
The objective of this study is to determine the primary patency, primary assisted patency and secondary patency days of intra-atrial and translumbar catheters placed in patients with ESVAF as well as the morbidity, mortality and complications presented in our reference center.
Design. We conducted a retrospective, observational study in which the information was obtained from the clinical records at our center. The information was obtained between January 1st 2014 to December 31st 2018 from the Nephrology Service in Hospital La Raza "Antonio Fraga Mouret" National Health Care Medical Center in Mexico City.
Patients. We analyzed the database of patients in our hospital, which included all patients with ESRD and ES-VAF diagnosis. The total population was 58 patients; and out of which, 27 patients were excluded, 13 of them were excluded because the initial installation was not possible, they were excluded from patency analysis but not for complications analysis, the rest of excluded patients (14) due to incomplete information in clinical records. The final cohort included in the analysis of patency time was 31 patients, 20 of whom were installed IAC and 11 with TLC placement. In all of them the primary outcome of primary patency, assisted primary patency and secondary patency days were obtained. Complications, morbidity, and mortality causes were also registered for analysis. Catheter placement was made by the specialists; TLC by interventionalists guided by fluoroscopy and IAC by Cardiothoracic surgeons approached through Minithoracotomy.
Data collection: relevant demographic and clinical data were obtained from each patient. Inclusion Criteria. Right-holders of the Mexican Social Security Institute (IMSS) over 18 years of age, with a diagnosis of ESRD in Hemodialysis, and ES-VAF (> 4 accesses, with stenosis or thrombosis of central veins, confirmed by imaging). Patients that underwent NCVA placement (IAC or TLC) from 2014 to 2019 and had any registered information about primary patency, primary assisted patency and secondary patency outcomes. Clinical records were obtained within this period of time. Any complication was included as well. Exclusion criteria: patients with incomplete information in clinical records about primary outcome, no clear data of complications and no exact date of placement and/or withdrawal. There was no clear information in the clinical records regarding assisted primary patency of NCVA’s, so this information was not included in our analysis. The project was approved by the local research and ethics committee.
Statistics: Non-parametric statistics were used. Data are presented as median and interquartile range. The difference between the groups was analyzed with Students’ t-test and chi-squared test, as well as Kaplan-Meier survival curves of NCVA’s. To establish risk, a logistic regression analysis of bivariate and multivariate type with a significant value of p less than 0.05 was performed. The statistical package SPSS version 25 was used.
We had a base of 58 NCVAs, 45 of them IAC, and 13 TLC, after exclusion criteria we had a final group of 20 IAC and 11 TLC for the survival analysis.
Our initial group
- Baseline characteristics and background
Intra-atrial catheters (IAC’s): In this group 45 patients were assessed for eligibility and 25 of them were excluded for the following reasons: 11 for incomplete information, 14 for failed placement (Figure 1). Finally, only 20 patients were included in this group for analysis; Non-parametric statistics were used and the data are expressed as median with interquartile range (IQR); 60% were women (12 patients). The age was 39.5 (IQR: 27.5 to 51.7) (Table 1).
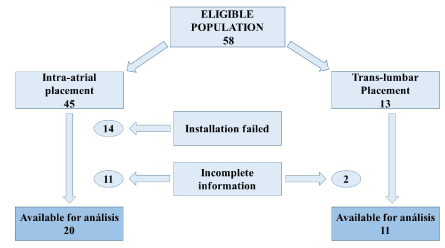
Figure 1. Eligible population
Table 1. Baseline characteristics of NCVA patients (n=31)
|
Intra-atrial (n=20) |
Translumbar (n=11) |
Variable |
Median |
Minimum-Maximum |
Median |
Minimum-Maximum |
Age (years) |
39.50 |
18-65 |
39.36 |
21-76 |
No. of Prev. Access |
8 |
5-24 |
5 |
3-9 |
Total primary patency days |
102.50 |
3-934 |
475 |
2-956 |
Total secondary patency days |
258.50 |
11-1755 |
n/a |
n/a |
ALOS |
20.50 |
1-44 |
7.00 |
3-70 |
NCVA: Non-conventional vascular access. n/a: not applicable |
ALOS: Average length of stay (days) |
Patency days: The median of primary patency days was 102.5 (IQR: 16 to 302.75). Out of the 20 patients with IACs, 45% required a second re-placement (9 patients) and 15% (3 patients) a third. There were no data in the clinical records to calculate the median time of assisted primary patency. The median of secondary patency days was 258.5 (IQR: 30 to 681).
The number of VA placed before the diagnosis of ESVAF was 8 [IQR: 7 to 10, minimum-maximum range (mMR): 5 to 24] (Table 1). Analysis of patients’ catheter history is described in Table 2.
Table 2. Type of VA placed previous to NCVA
|
|
Intra-atrial (n=20) |
Translumbar (n=11) |
VA Access type |
No. of Access |
No. of patients (%) |
No. of patients (%) |
Transhepatic |
1 |
1 (5) |
0 |
Translumbar |
1 |
1 (5) |
0 |
Intra-atrial |
1 |
NA |
2 (18.2) |
VA: Vacular access; NCVA: Nonconventional vacular access; No: Number.
Previous sites of VA’s before IAC: From the 20 successful IAC, the recommendation according to the guidelines for catheter placement in the internal jugular vein as the first option was not followed in catheter history of 4 patients, representing 20%.
Comorbidities: Of the 20 patients, 50% (10 patients) had hypertension; 10% (2 patients) Diabetes Mellitus and 5% (1 patient) had Bone Marrow Transplant secondary to hematologic malignancy. In 7 patients the etiology of ESRD was unknown.
The complications of IAC placement that occur within the primary patency time. Are divided by early (<30 days) and late complications (>30 days). Early complications were immediate dysfunction (7), Hemorrhagic shock (5), nosocomial pneumonia (4), hemotórax (2), atrial rupture (1), great cava vein tear lesion (1), pericardial effusion (1), atrial fibrillation (1) sepsis (1), cardiac tamponade (1) and catheter poorly positioned (1). A group of 7 patients died from cardiovascular events derived from surgical complications. Late complications were vascular access infection (6), accidental output without hemodynamic instability (4), thrombosis (2), Catheter fracture (1), endocarditis (1) (Table 3). Only 13 patients had primary patency with no secondary patency and 7 patients achieved secondary patency.
Table 3. Early and late complications related to NCVA (frequency)
Type of NCVA |
Early (< 30 days) |
Late (> 30 days) |
Intra-atrial n=20 |
Immediate dysfunction (7) |
Vascular access infection (6) |
| |
Hemorrhagic shock (5) |
Accidental Output (4) |
| |
Nosocomial pneumonia (4) |
Thrombosis (2) |
| |
Death related to placement (7)
Hemothorax (2) |
Fracture of catheter (2) Endocarditis (1) |
| |
Atrial rupture (1) |
|
| |
Great cava vein lesion (1) |
|
| |
Cardiac tamponade (1) |
|
| |
Pericardial effusion (1) |
|
| |
Atrial fibrillation (1) |
|
| |
Sepsis (1) |
|
Translumbar
n= 11 |
Hemothorax (2)
Retroperitoneal Hematoma (2)
Death related to placement (2) |
Vascular access infection (3) Accidental output (2) Thrombosis (1) |
| |
Hemorrhagic shock (1) |
|
| |
Nosocomial pneumonia (1) |
|
|
Great cava vein lesion (2) |
|
NCVA= Non-conventional vascular access.
Catheters used were tunneled in all patients. |
The average length of stay (ALOS) in patients with IAC was 20.5 days. (IQR 12.75 to 35.75).
Translumbar catheters (TLC): 13 patients were screened; 2 patients were excluded for incomplete information. Only 11 patients were included for primary outcome analysis. Baseline characteristics; 63.6% were men (7 patients), the age was 39.36 years (IQR 29 to 46). For the rest of the data refer Table 1.
Patency days: The median primary patency days was 475 (IQR: 111 to 676). There was no information in the clinical records about assisted primary patency or secondary patency in this group. The ALOS was 7 days (RIQ: 4 to 15). The number of previous VA was 5 (IQR: 4 to 8, mMR: 3 to 9).
Comorbidities and complications: Of the 11 patients, only 2 patients had coronary syndrome as the principal comorbidity.
The complications found within primary patency time of the TLC are divided by early (<30 days) and late complications (>30 days): Early complications were hemothorax (2), Hemorrhagic shock (1), retroperitoneal hematoma (2), cava vein tear (2), nosocomial pneumonia (1). Late complications were vascular access infection (3), accidental output without hemodynamic inestability (2), thrombosis (1).
- Comparison between both forms of NCVA: TLC vs IAC
The overall median primary patency days of the 31 NCVA’s (IAC and TLC) installed in ESRD patients with ESVAF was 311 days (IQR of 33 to 676, mMR 2-1755). In women, the median primary patency days was 192 (IQR 17.25 to 519.5, mMR 2 -1542). In men 475 days (IQR 111 to 1275, mMR 5-1755), with significant statistical difference (Figure 2).
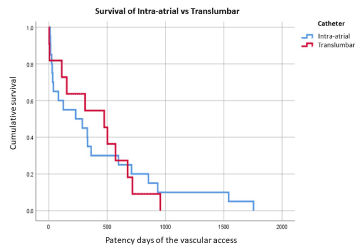
Figure 2. Kaplan-Meier curves for Survival of Intra-Atrial vs Trans-lumbar catheter
The ALOS of patients with IAC was 20.5 days (IQR 12.75 to 35.75). The reason for the prolonged ALOS was related to direct complications of VA placement (mainly bleeding) and indirect complications related to hospitalization (mainly nosocomial pneumonia). The ALOS for patients with TLC was 7 days. In this group the ALOS were related to non-direct complications of catheter placement, mainly of nosocomial infections.
We found an overall incidence of 20% catheter related infections that presented with bacteremia and required hospitalization for management. Of these, the most frequent agent found was S. Aureus in 20%, S. Epidermidis in 15%, E. Coli in 5% and P. Aeuruginosa in 5%. The rate of global NCVA infection per 1,000 days of catheter patency days was 2.1. In order of frequency of complications, we found that the most frequent was dysfunction of any cause including thrombosis in 30 to 35%, the second most frequent was hemorrhagic shock related to placement in 25%, in third place catheter related infection in 20%, and lastly accidental output 15%.
Patency between groups: The median primary patency days in IAC was 120. The median secondary patency days in IAC was 258.5 days (IQR from 30 to 681 days, mMR from 11 to 1755 days). The TLC median primary patency days was 475 days (IQR from 111 to 676 days, mMR from 2 to 956 days). We report the patients with the longest patency days in each group; TLC one patient with 956 days of primary patency and in IAC one patient with 1755 (3) days with primary assisted patency, with 3 surgical manipulations to maintain/restore patency. The difference in patency days between TLC vs IAC groups was 79%, TLC: 475 days versus 102.5 days of IAC, when comparing with primary patency days of both groups, in favor of TLC. And even 46% difference if we consider secondary patency days of the IAC (TLC 475 days of primary patency vs IAC 258.5 days of secondary patency) in favor of TLC.
Complications related to VA are responsible for 25 to 30% of hospitalizations and a quarter of the total cost for ESRD [10,40]. As a result of the progress in nephrological care, we have improved the survival of HD patients. Unfortunately, we have more recurrent access failure that culminates in exhaustion of standard options of VA resulting in a rise in the use of NCVA. This has shown to prolong survival in HD patients with ES-VAF. It is important to know the background characteristics and complications of these types of VA, as every year the incidence and prevalence is growing.
Starting with the demographic analysis, the average age with IAC and TLC was 39.3 and 39.5 years respectively, compared to other studies where they find an average of 60 years [30,33,41].
Diabetes mellitus (DM) in our series was 10% as the etiology of ESRD, different from Santos A. and colleagues who reported higher 31%, influenced by the age of cohorts in another countries [33]. Diabetes status influences the overall survival of the patient due to associated complications.
Regarding the history of the number of VA’s, the TLC is 5 (IQR 4-8) and in the case of IAC is 8 (IQR from 7 to 10), different from Power and collaborators with a median of 4.2 [33]. The prevalence of AVF creation prior to the diagnosis of ESVAF was 77% (70% in IAC and 91% in TLC) close to that described also by Power et al. [33] being in its study of 81%.
In our series, IAC had patency of 102.5 days (3.4 months) without considering replacement (RIQ of 16 to 302.75 days). A total of 45% of patients in IAC group required a second placement and 15% a third re-placement. Total patency days adding the re-placement was 258.5 days (8.6 months; RIQ from 30 to 681 days); with one IAC that reached a maximum of 1755 days (58.5 months). Comparing IAC patency days with other series we found that Lund et al. [36] reported 52% of functional catheters at 6 months and 17% at 12 months. Other reports included Biswal et al. 250 days [42,43], Moura et al. [34] of 315.5 days with an IQR 65 to 631 and Kade et al. [31] with an average of 261 days.
Regarding complications in IAC, Pereira et al. [42] reported a case-series of 7 patients with bleeding in 85% of cases, infection in 42%, and a 57% mortality rate. Our study had a mortality of 35%, 50% of them related to vascular complications associated with the procedure. the latter complication compared to the study by Oguz et al. [44], where they report 11% of thrombosis related dysfunction. Unfortunately, these dysfunctions mostly led to catheter replacement despite medical management.
The rest of the complications in IAC were late (> 30 days) in our study: 5% fracture, VA infection 30%, endocarditis or infected thrombus 5%, The accidental output is reported in other studies close to 15% [42], in our series it is 20%, related to the technique of performing catheter tunnel. The repositioning due to dysfunction is an expected complication in IAC. Pereira et al. [42] reported that 28% of their patients required up to 3 relocations. In our case series of 20 patients, 9 patients required a second IAC re placement intervention (45%) and in 3 patients (15%) a 3rd IAC re-placement to ensure VA for HD session.
In TLC, Rodríguez et al. [30] reported a mortality of 61.5% (37.5% etiology by sepsis, cerebral vascular event 25%, respiratory failure 25%, myocardial infarction 12.5%). In our group of patients TLC we found 27.3% (3 of 11 patients) of related mortality, with 2 cases directly related to the placement (18.2%) due to tear of cava vein with retroperitoneal bleeding and hypovolemic shock. There was 1 case (9.1%) that died secondary to nosocomial pneumonia. These complications were minor in our study due to shorter ALOS which was 7 days (with a range of 4 to 15 days), reducing complications of infections and/or thrombosis associated to in hospital stay days. Finally, the accidental output is 12.5% for Rodríguez et al. [30], but in our group it was 18.2% (4 of 11 patients). A patient scheduled for removal of the translumbar catheter due to dysfunction developed a hematoma requiring a transfusion.
The overall primary patency was 311 days (RIQ of 33 to 676 days, with a minimum of 2 days and a maximum of 1755 days). In this group of patients, mortality due to associated chronic comorbidities was not affected.
NCVAs are the last option for our patients, as a result we need to improve our approach to have better survival rate. TLC is a better option over IAC because they had less complications and the procedure could be improved if we add CT in the approach. IAC could be considered a second option in the guidelines because of higher ALOS and bleeding complications but with excellent patency days. This group of patients with ESVAF represent a nephrology emergency, and prompt action of health care providers with clear team guideline instructions is needed to optimize time. The NCVAs need special care to avoid accidental output with special attention in skin traction, excessive body movement, tight clothing, and aggressive cleaning.
It was a retrospective study with the information in the clinical records being incomplete to evaluate primary assisted patency in most of the cases, and we could not obtain additional data related to complications in the clinical records because our patients receive surrogate hemodialysis, especially those related to late complications as infections or partial dysfunctions, therefore, it is possible that we might be reporting less late complications. We did not intentionally evaluate the functionality in hemodialysis.
In patients with ES-VAF, TLC was a superior alternative to IAC with greater patency days, shorter ALOS, fewer complications, and lower associated mortality. Our series contained fewer adverse events associated with catheter placement and hospitalization than most of the series reported today. This provides some hope to patients who, at other times or places, were considered out of vascular access option. Complications related to placement, could be addressed by modifying the technique of positioning, or using computed tomography scan in the context of TLC.
However, clinicians must be aware of this growing cohort of patients. We issue a strong call for attention to angio-access placement programs not only in Mexico, but throughout the world. Since in many cases the placement was not planned, as well as the KDOQI and SEN guidelines were not performed in an orderly manner, leading to non-conventional venous accesses earlier in the life of a patient. These catheters are more of a heroic act than a true alternative for the management of patients with hemodialysis.
Therefore, we consider limiting their use on patients who have truly been diagnosed with ESVAF in order to avoid the unfortunate complications of large blood loss, morbidity in the short and longer term, and increased costs to the patients and healthcare systems.
- Allon M (2019) Vascular access for hemodialysis patients. CJASN 14: 1-8.
- Sociedad Española de Nefrología, Sociedad Española de Angiología y Cirugía Vascular, Sociedad Española de Radiología Vascular Intervencionista, Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, Sociedad Española de Enfermería Nefrológica. Guías de acceso vascular en hemodiálisis. 2004. Disponible en: http://www.codeinep.org/control/guia_acc.pdf.
- Tordoir J, Canaud B, Haage P, Konner K, Basci A, et al. (2007) EBPG on vascular access. Nephrol Dial Transplant 22: 88-117.
- National kidney foundation (2006) KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis 48: S1-322.
- Lewis C, Allen T, Burke D, Cardella J, Citron S, et al. (2003) Quality improvement guidelines for central venous access. Vasc Interv Radiol 14: S231-S235.
- Silberzweig J, Sacks D, Khorsandi A, Bakal C (2003) Reporting standards for central venous access. J Vasc Interv Radiol 14: S443-S452.
- Guías SEN de Centros de Hemodiálisis. Nefrología 2006; 6 (Supl. 8).
- National kidney foundation (2001) KDOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis 37: S7-S64.
- Navarro L, Reula A, Martínez EM, Martínez A, Ortells R, et al. (2012) Dificultad para conseguir un acceso vascular para hemodiálisis: caso clínico: 9 años en Hemodiálisis, 15 accesos vasculares. Enferm Nefrol 15: 219-221.
- Crehuet I, Méndez P (2011) Supervivencia de un catéter: un reto y un logro de enfermería. Rev Soc Esp Enferm Nefrol 14: 189-194.
- Gameiro J, Fonseca J, Jorge S, Lopes J (2018) Management of end - stage vascular access failure patients: a retrospective analysis. Port J Neprhol Hypert 32: 357-363.
- Al Shakarchi J, Nath J, McGrogan D, Khawaja A, Field M, et al. (2015) End-stage vascular access failure: can we define, and can we classify? Clin Kidney J 8: 590-593.
- Lorenz J (2006) Unconventional Venous Access Techniques. Semin intervent Radiol 23: 279-286.
- Agrawal A, Alaly J, Misra M (2009) Intracardiac access for hemodialysis; A case series. Hemodialysis International 13: S18 -S23.
- Crehuet I, Mendieta S, Briso Montiano M, González R (2008) Catéter translumbar en vena cava inferior: última Opción de acceso vascular para hemodiálisis. Rev Soc Esp Enferm Nefrol 11: 238-241.
- Lorenz J, Regalado S, Navuluri R, Zangan S, Vanha T, et al. (2010) Trnashepatic guidance of translumbar hemodialysis Catheter placement in the setting of chronic infrarenal IVC Occlussion. Cardiovasc Intervent Radiol 33: 635-638.
- Herscu G, Woo K, Weaver L, Vincent L (2013) Use of unconventional dialysis access in patients with no viable alternative. Am Vasc surg 27: 332-336.
- Jaber M, Thomson M, Smith D (2008) Azygos vein dialysis catheter placement using the translumbar approach in a patient with inferior vena cava occlusion. Cardiovasc Intevent Radiol 31: s206-S208.
- Kariya S, Tanigawa N, Kojima H, Komemushi A, Shomura Y, et al. (2009) Percutaneous translumbar inferior vena cava cannulation under computed tomography guidance. Jpn J Radiol 27: 176-179.
- Yasa H, Lafci B, Tetik O, Ozsoyler I, Ergunes K, et al. (2007) Placing of permanent catheter trhoyug right anterior mini thoracotomy in patients with chronic renal failure. EJVES 6: 90-91.
- El-Sabrout RA, Duncan JM (1999) Right atrial bypass grafting for central venous obstruction associated with dialysis access: another treatment option. J Vasc Surg 29: 472-478.
- Chemla ES, Korrakuti L, Makanjuola D, Chang AR (2005) Vascular access in haemodialysis patients with central venous obstruction or stenosis: one center’s experience. Ann Vasc Surg 19: 692-698.
- Murthy R, Arbabzadeh M, Lund G, Richard H, Levitin A, et al. (2002) Percutaneous transrenal hemodialysis catheter insertion. J Vasc Interv Radiol 13: 1043-1046.
- Gallichio M, Kahn D, Lempert N, Conti D (1994) Placement of a double lumen silastic catheter for hemodialysis access through the cephalic vein. J Am Coll Surg 178: 171-172.
- Lau T, Kinney T (2001) Direct US-guided puncture of the innominate veins for central venous access. J Vasc Interv Radiol 12: 641-645.
- Younes HK, Pettigrew CD, Anaya-Ayala JE, Soltes G, Saad WE, et al. (2011) Transhepatic hemodialysis catheters: functional outcome and comparison between early and late failure. J Vasc Interv Radiol 22: 183-191.
- Curtas S, Bonaventura M, Meguid M (1989) Cannulation of inferior vena cava for long term central venous access. Surg Gynecol Obstet 168: 121-124.
- Patel N (2000) Percutaneous translumbar placement of a Hickman catheter into the azygous vein. AJR Am J Roentgenol 175: 1302-1304.
- Rodríguez J, Ramírez J, Oviedo P, Mora P, Llaro M, et al. (2018) Catéteres translumbares y transhepaticos para hemodiálisis. Una opción viable. Nefrología 470: 1-3.
- Kade G, Les J, Buczkowa M, Labus M, Ninemczyck S, et al. (2014) Percutaneous translumbar catheterization of the inferior vena cava as an emergency access for hemodialysis. 5 years of experience. J Vasc Access 15: 306-310.
- Loskutov A, Dave A, Gooden C, Saucier N, Cho K, et al. (2018) Vascular acces via translumbar Hemodialysis Reliable Outflow dialysis catheter in a case of severe central venous occlusion. The Journal of Vascular Access.
- Power A, Singh S, Ashby D, Hamady M, Moser S, et al. (2010) Translumbar central venous catheters for long therm Hemodialysis. Nephrol Dial Transplan 25: 1588-1595.
- Moura F, Leite F, Dantas Y, Helena A, Azevedo R, et al. (2018) Translumbar hemodialysis long- term catheters: An alternative for vascular Access failure. Bras J Neprhol 2018: 1-6.
- Vanholder R, Hoenich N, Ringoir S (1987) Morbidity and mortality of central venous Catheter Hemodialysis a review of 10 years’ Experience. Neprhon 47: 274-279.
- Solak Y, Tekinalp M, Atalay H, Kayrak M, Yeksan M (2010) A rare but ominous association: Intracardiac Trhombus and vegetation simultaneously in a hemodialysis patient. Hemodialysis International 14: 341-342.
- Santoro D, Benedetto F, Mondello P, Pipito N, Barilla D, et al. (2014) Vascular Access for hemodialysis: current perspectives. International Journal of Nephrology and Renovascular Disease 7: 281-294.
- Chavanon O, Maurizi-Balzan J, Chavanis N, Morel B, Blin D (1999) Successful prolonged use of an intracardiac catheter for dialysis. Nephrol Dial Transplant 14: 2015-2016.
- Lund G, Scott O, Trerotola S, Scheel P (1995) Percutaneous translumbar Inferior Vena Cava Cannulation for Hemodialysis. American Journal of Kidney disease 25: 732-737.
- Gupta A, Karak P, Saddekni S (1995) Translumbar inferior vena cava catheter for long-tem hemodialysis. J Am Soc Nephrol 5: 2094-2097.
- Punzi M, Ferro F, Petrosino F, Masiello P, Villari V, et al. (2003) Use of an intra-aortic tesio catheter as vascuilar access for haemodialysis. Neprhol Dial Transplant 18: 830-832.
- Pereira M, Lopez N, Godinho I, Jorge S, Nogueira E, et al. (2017) Life-saving vascular access in vascular capital exhaustion: single canter experience in intra atrial catheters for hemodialysis. J Bras Nefrol 39: 36-41.
- Biswal R, Nosher JL, Siegel RL, Bodner LJ (2000) Translumbar placement of paired hemodialysis catheters (Tesio catheters) and follow-up in 10 patients. Cardiovasc Intervent Radiol 23: 75e8.
- Oguz E, Ozturk P, Erkul S, Calkavur T (2012) Right intra-atrial catheter placement for hemodialysis in patients with multiple venous failure. Hemodial Int 6: 306-309.
- Falk A (2006) Use of the brachiocephalic vein for placement of tunneled hemodialysis catheters. AJR Am J Roentgenol 187: 773-777.
Editorial Information
Editor-in-Chief
Ying-Fu Chen
Kaohsiung Medical University, Taiwan
Article Type
Research Article
Publication history
Received date: January 11, 2021
Accepted date: February 04, 2021
Published date: February 08, 2021
Copyright
©2021 Vera AVV. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Vera AVV, López MJP, Martínez CA, Arellanes FEH, Menjivar CM (2021) Patency of trans-lumbar and intra-atrial catheters in patients with end stage renal disease and end stage vascular access failure. Trends Med 21: DOI: 10.15761/TiM.1000267


