Treatment of type 1 diabetes is based on exogenous insulin injections and has drastically improved diabetes mortality and morbidity. Nevertheless, this therapy failed to control effectively and safely the glycaemic levels in some patients suffering from brittle diabetes. For these patients, morbidity and mortality remain high despite improved metabolic results, but transplantation development of pancreatic islets allowed to achieve optimal glycaemic control and insulin-independency (with an acceptable safety profile in comparison to pancreas organ transplantation).
Human islets are mainly transplanted by infusion in the portal vein. However, numerous factors limit the full development of this technique, such as: immune rejection, inflammation, and lack of oxygen and blood supply during the first days post-transplantation.
Although islets represent a small fraction of the pancreatic volume, they receive an abundant vascularization. The islet isolation process disrupts all the vascular connections and a delayed revascularization process occurs after grafting. During this period of revascularization, hypoxia and ischemia impair islet viability as well as functionality. During the early post-transplantation period, islets undergo central necrosis which leads to a huge loss of islet mass during this early post-transplantation period. Improving islet vascularization and oxygenation during this period remains a key challenge to enhance islet transplantation outcomes.
It is widely accepted that vascularization and oxygenation are two essential axes to address, but only few innovative strategies were clinically tested to optimise islet transplantation. We believe that improving at least one of these axes will greatly benefit the outcome of the pancreatic islets. In this review, we shed light on the current advances regarding islets’ vascularization and oxygenation, in an attempt to identify the most clinically relevant ones. We briefly review the concept of immunoisolation before developing the main axes regarding vascularization and oxygenation. We also mention some developments in bioengineering.
According to the World Health Organization, diabetes was in the top ten causes of death worldwide in 2016 [1]. In 2019, the International Diabetes Foundation estimates that 463 million people lived with diabetes [2] and that type 1 diabetes (T1D) represented 7% to 12% of the diabetic population [3]. This number is expected to rise to 700 million people by 2045 [2]. T1D is an auto-immune disease, characterized by a β-cell dysfunction. Physiopathology of T1D is complex and implies a cross-talk between injured or stressed β-cells and immune cells (macrophages and T cells) that lead to pancreas inflammation (insulitis) and β-cell apoptosis [4].
It provokes an absolute insulin deficiency which is responsible for chronic hyperglycaemia. It is well known that insulin deficiency leads to multiple complications such as retinopathy [5], nephropathy [6], neuropathy [7] and a major increase in cardiovascular risk [8]. Treatment of T1D is based on insulin replacement therapy through multi-daily injections or insulin pump. Optimal glycaemic control is necessary to reduce microvascular complications [9,10]. However, despite modern diabetes care strategies and treatment, patients with T1D fail to achieve optimal glycaemic control [11]. Moreover, a subpopulation develops a severe form of diabetes known as brittle diabetes [12]. Brittle diabetes is responsible for frequent, unpredictable severe metabolic events (keto-acidosis and/or severe hypoglycaemia) which dramatically impact the quality of life and vital prognosis [12]. For these patients, β-cells replacement (pancreas organ or islets transplantation) may serve as an alternative.
Data from the International Pancreas Transplant Registry showed improvement of pancreas transplantation over time: one year insulin independence rate is now of 80% for pancreas transplanted alone (PTA, i.e. without kidney graft association), 86% for simultaneous pancreas-kidney graft and 80% for pancreas after kidney transplantation (PAK) [13]. After five years of graft survival, insulin independence increases from 55 to 58% for PTA and 55% to 65% for PAK [13,14]. Despite these good metabolic results, whole PTA requires a complex surgery associated with a higher risk of complications such as pancreas thrombosis (10-35% according to literature) [15] responsible for early graft loss. Bleeding, graft pancreatitis, sepsis, and fistula may also occur [15].
To isolate pancreatic islets, pancreas from brain-dead donors are removed and undergo a two-step dissociation: an enzymatic dissociation followed by a mechanical dissociation [16]. Islets are then purified, using a density gradient centrifugation, and collected. The islet suspension is infused in the portal vein by percutaneous transhepatic approach. The complexity of the islet isolation procedure is multifaceted considering ischemia, the double dissociation and the density purification. However the islet transplant infusion procedure per say is less complex than PTA. Over the past two decades, results of islet transplantation have also improved: between 2007 and 2010, the graft survival (C-peptide ≥0.3 ng/mL) was close to PTA, with 92% at 1 year [17]. Moreover, 66% of patients transplanted were insulin independent at 1 year. Results from the GRAGIL Network showed a 74% graft survival 5 years after islet transplantation, transplanted alone (ITA) or after kidney graft (IAK) [18]. In 2016, a prospective multicentric clinical trial confirmed the efficacy of islet transplantation on metabolic results [19]. Later, the first randomised controlled trial on islet transplantation showed an improvement of metabolic results at 6 months compared to intensive insulin therapy [20]. In addition, islet transplantation reduced severe hypoglycemic events [18–20] and improved Clarke and HYPO scores [19]. Taken together, these results demonstrate that islet transplantation is an efficient and rather lasting therapy to restore glycaemic control and to prevent severe hypoglycaemia in patients with severe glycaemic lability. Moreover, islet transplantation reduced retinopathy progression and did not deteriorate renal function [21].
Despite these convincing clinical results, major obstacles need to be overcome to further improve islet transplantation [22] including: shortage of donor pancreas, long-term toxicity of immunosuppressant therapy, poor engraftment linked to lack of oxygen, poor blood supply, inflammation, long-term immune rejection. At early stage post-intrahepatic transplantation, between 50 to 60% of the graft is lost [23,24] due to inflammation [25,26], cytokines exposure, hyperglycaemia [24] and hypoxia [27] impacting islet outcomes. Moreover, immunosuppressant therapy can expose recipients to severe complications (infection, cancer or renal function impairment) [28], which have led the way to the development of new research strategies such as immunoisolation. Islet immunoisolation currently implies using either macroencapsulation in which the whole islet preparation is encapsulated in a single device, or microencapsulation in which a single islet or a duet of islets are encapsulated in microcapsules. Historically, the first transplantation devices were intravascular hollow fibers [29–33] which were developed to allow islets to be close to arterial rich oxygenated blood (or in arteriovenous shunt). These macrodevices were successfully implanted in large animal models [34]. However, these devices provoked cloating [31] and preventive-heparin injection was often associated with massive haemorrhage. This approach was abandoned in the late 90’s and meanwhile, clinical intraportal transplantations of free islets were increasing [16,35], encouraging the development of macro and microencapsulation. Macroencaspulation often involves alginate sheets or other planar devices, now developed for extravascular use [36-39]. Microencapsulation [40-44] consists of several capsules containing islets, often composed of alginate formed with an air-jet syringe pump extrusion [45-48].
However, encapsulation leaves unsolved the problems of delayed graft vascularization and oxygenation, and of central necrosis [49]. Indeed, if macroencapsulation offers several advantages (islets are in a single device, which can be easily and totally removed if needed), vascularization and oxygen supply to islets within macrodevices remain a challenge [50]. To provide sufficient nutrient and allow oxygen diffusion, the total volume of islets in a sheet cannot exceed 40% of the size of the device [36]. Clinical trials using such devices and stem derived β-cells are currently ongoing (NCT02239354, NCT02939118, NCT03163511, NCT03162926) and preliminary results presented at conferences did not show restoration of insulin secretion [51].This could be due to the foreign body response and also certainly due to central islet necrosis triggered by low oxygen diffusion through the device, partially due to fibrotic overgrowth surrounding the macrodevices [51]. The few published clinical studies on macrodevices showed disappointing metabolic results [52,53]. Microencapsulation could theoretically help to enhance oxygen diffusion by reducing the size between the oxygenated environment and the islet core: however, data on animal models showed that microencapsulated islets are also prone to central necrosis and oxygenation problems [54,55]. Clinical trials with human microencapsulated islets demonstrated a good feasibility and safety but metabolic outcomes were contrasted depending on the study [56–59].
Consequently, strategies have been developed to improve engraftment of islets and encapsulated islets outcome. Recently, a review by Papas, et al. nicely summarised the oxygenation strategies [60], but in this review, we wish to take this reflexion a step further by focusing on the current advances regarding both islets’ vascularization and oxygenation. We believe both these aspects should be considered in order to try to identify the most clinically relevant strategies. We aim at summarizing and comparing the various strategies that have been developed so far to enhance vascularization and oxygen diffusion regarding islets or encapsulated islets transplantation. First, we briefly mention islet physiology and then we will summarize the existing devices, and the various transplantation sites, and their different issues focusing on vascularization and oxygenation.
Previously, Jansson and Carlsson have reviewed precisely the physiologic vascularization of pancreatic islets and the process of revascularization after graft [61]. In fact, islets are surrounded by a “glomerular-like” capillary network, that derive either from capillaries of the exocrine pancreas (for small islets) or directly from proper arterioles [61]. While islets represent 1 to 2% of pancreas, they receive between 10% to 20% of pancreatic blood flow, depending on species and age [62], thus maintaining adequate oxygen supply, to keep an oxygen tension around 30-40mmHg in pancreas [63,64]. Indeed, islets have one of the highest blood supplies in the human body [65]. During islet preparation, vascular connections are disrupted. After islet transplantation, oxygenation of islets during the early first days depends on the diffusion of oxygen according to a gradient between the core (less oxygenated) and the periphery (better oxygenated) of the islet [64]. It is estimated that after transplantation, 70% of islets are hypoxic [63], whichever the site of transplantation, with an oxygen tension around 5mmHg while it is around 40mmHg in normal pancreas [64,66]. The revascularization process starts a few days after islet transplantation, with an increase of vessels diameter from day 7 to day 14 [65]. During this period, oxygen and nutrient deprivation may contribute to the mass loss of islets partly because of central islet necrosis.
The effect of hypoxia on rat islets has been studied in normoxic conditions (pO2 = 160mmHg, reflecting atmospheric conditions): after 24h, the pO2 decreased in culture medium. After 24h of culture in hypoxic conditions (pO2 = 15mmHg), viability was reduced, insulin secretion was impaired and levels of Hypoxia inducible factor-1α (HIF-1α) mRNA increased [67]. De Groot, et al. showed that the viability of encapsulated rat islets decreased in hypoxic conditions, mainly due to an increase of necrosis [68]. High density seeding decreased viability of free islets and impaired secretion by inducing local hypoxia [69].
Muthyala, et al. [70] compared effects of 2-5% hypoxia on viability and on metabolic activity of free pig islets versus encapsulated islets. Under normoxia, no viability changes were observed over time, both in free and in encapsulated islets. Interestingly, viability of encapsulated islet was maintained in hypoxic conditions, compared to normoxic conditions and decreased only at day 6 [70]. However, the encapsulation did not improve the insulin secretion nor the metabolic activity. Thus, even if the study of Muthyala, et al. was performed in pig islets (which have a different metabolic profile than human islets), encapsulation in these conditions seems to confer a protection to hypoxia, giving hope for future explorations in rodent and in human islets.
Hals, et al. compared the effect of an 8h-hypoxia followed by a 14h-18h reoxygenation period on human encapsulated islets: they showed that viability decreased in hypoxic conditions versus normoxic conditions but interestingly, they did not find differences in viability between encapsulated islets and free islets in hypoxic conditions [71].
The ideal site for islet transplantation is described as requiring a rich arterial and venous vascularization in order to provide nutrients oxygen supply and to normalise blood glucose after release of insulin, respectively [72]. The ideal site should also be easily accessible with a minimally invasive procedure [72]. An easy access to islets would enable the monitoring of islets’ viability and allow to retrieve the graft if necessary [73,74] without triggering early inflammation, thrombosis and instant blood mediated inflammatory reaction (IBMIR).
Finding a transplantation site satisfying all these criterions simultaneously remains impossible so far. We will detail several sites of transplantation which are still competing as “the ideal site”. Transplantation sites [75,76] and oxygen partial pressure in various potential transplantation sites [60] have been previously reviewed, here we will discuss the interest of each transplantation site in terms of oxygenation and vascularization.
Nowadays, 90% of clinical islet transplantations in human take place in the intraportal site [77], due to the arterial supply and venous drainage, and the good accessibility to inject islets being minimally-invasive. However, drawbacks are numerous [73] as islets are exposed in the portal vein to high concentrations of glucose, as well as high toxic drug concentrations compared to the whole pancreas and the direct contact with the intravascular system triggering IBMIR [63,73]. Finally, the hepatic oxygen tension (5 -10 mmHg) is lower than that of the pancreas (40mmHg), but to date it remains the site with the best clinical results in human [78].
Despite a low oxygen tension [79], the kidney capsule is often used in rodents, since it enables morphological and histological follow up of the transplant by biopsy, restores normoglycemia and allows better engraftment than the liver [75]. In human, the risk of altering the kidney in a population of patients who are susceptible to renal impairment makes the kidney capsule inappropriate for clinical islet transplantation [75].
The pancreas site has surprisingly been less evaluated than other sites, despite being the native place for islets, providing ideal conditions of oxygenation, microenvironment, and exposure to glucose. This is probably due to the invasive surgery required to implant islets, the poor knowledge regarding the risk of recurrence of auto-immunity and the destruction of the graft [74,75].
The peritoneum cavity and the omentum represent an attractive alternative to the intraportal site. Indeed, the accessibility in surgery without an invasive technique and the size allow large infusion of islets [75]. The rich vascularization of this site, the physiological function as fibrotic inhibition makes it a good candidate to compete with the intraportal site [80]. Indeed, the omentum seems to provide a faster revascularization and oxygenation compared with intraportal route: vascular density was better in human islets transplanted in mice omentum and the number of hypoxic β-cells decreased during a 7 day-period, while it remained unchanged for islets transplanted in the intraportal site [81]. Other preclinical studies validated the omentum as a safe transplantation site for microencapsulated islets or seeded islets in biologic scaffold, with faster revascularization [82], better differentiation [83], lower stress-markers [83] and good glycaemic results [82,84]. In 2017, encouraging results were obtained through the transplant of islets within a bioengineered omental pouch in a type 1 diabetic patient [85]. In 2018, Stice, et al. published four cases of patients that underwent a total pancreatectomy and islet autotransplantation and who could not have a complete intraportal islet transplantation (12 to 36% of islets have been transplanted in an omental pouch): the glycaemic results were equivalent to a control population (i.e. receiving all the transplant by intraportal route). No patient had intraoperative complications. The postoperative complications reported (anemia, nosocomial pneumonia, etc.) were not specifically related to the omental pouch technique.
A clinical trial involving the omental site for islet transplantation was recently completed (NCT02821026) but no results were published so far.
The subcutaneous site is often used in rodents, mostly for implantation of macrodevices [75]. The main advantage is the easy and rapid access, performed under local anesthesia, authorizing full graft removal but also the possibility to reload the device if necessary [74]. However, the engraftment is limited by the low vascularization [75,86] and in order to overcome this, prevascularization strategies have been developed such as the preimplantation of an empty microdevice showing encouraging results [87–89]. More recently, the inguinal subcutaneous white adipose tissue have shown promising results in mice, since the site is correctly vascularized by the epigastric inferior artery [90]. However, most subcutaneous tissues lack rich vascularization, thus inadequate for both the good engraftment and the development in human. Also, the volume of islets to inject seems to be too important regarding the capacity of this site: a recent clinical trial showed the safety of macroencapsulation in subcutaneous space in human but failed to restore metabolic control [53].
The splenic site would expose islets to the same environment as the pancreas, with a good vascularization and delivery of insulin in the splenic vein, thus in the portal venous system [77]. Advantages are similar to the intraportal site, but the major drawback is the risk of massive haemorrhage [77] although surgical procedure advances could minimize the risk of haemorrhage [91].
Gastric submucosal space [92], bone marrow [93], epididymal [94] and mammary fat pads [95], muscle [96,97] have the advantage of good vascularization, but further studies need to confirm the potential of these sites. Epididymal and mammary fat pads being murine specific tissues, they have no human relevance. Two recent clinical trials showed the feasibility and safety of islet transplantation in the bone marrow, but the graft survival was less than 4 months [98]. More recently, it has been established that lymph nodes allowed survival of functional pancreatic islets in mice [99] and rats [100] but the site has not been explored further.
It seems to us that the omental site pouch is the most promising alternative to the hepatic site due to its richer vascularization. The results of the recent clinical trial NCT02821026 are not yet published, but they will enable to determine if these favorable vascularization conditions offered by the omental site clearly improve islet transplantation outcomes.
Oxygenation of islets has been an issue for a long time and particularly since the 90’s. In 1991, Dionne, et al. presented a perifusion system to assess effect of pO2 on insulin secretion [101]. Then, Küthreiber, et al. determined pO2 in vivo in diffusion chambers seeded with islets [102], Schrezenmeir, et al. assessed the pO2 of free versus microencapsulated islets [103], Wu, et al. developed an electrolyzer for generating oxygen in situ by electrolysis of water, which can be associated with a diffusion chamber [104]. Carlsson, et al. evaluated the oxygen tension in transplanted rats and human islets [66,105,106].
Two methods have been identified to improve islet oxygenation: to supply O2 or to improve O2 diffusion. The first can be achieved either by O2 carriers or using abilities of membranes to carry O2, or even by direct perfusion of O2 in the site of islet transplantation. The second, by changing the macrodevice conformation, opting for a planar conformation or using small islets. The use of medium supplemented with oxygen-precharged perfluorocarbons (PFC) or directly into the encapsulation material to increase its oxygen permeability have been widely explored [107]. Some strategies with enriched culture medium, bioengineered capsules or O2 have been tested but only in vitro so far. For example: the use of a marine haemoglobin able to bind about 150 oxygen molecules [67], the enrichment of medium with micro/nanobubbles filled with pure O2 [108], co-encapsulation with haemoglobin [109], co-encapsulation with algae as photosynthetic generator [110]. Improving conditions of shipment by gas-permeable culture bags [111] or culture islets in gas-permable silicone rubber membrane vessels [112–114] have shown promising results on oxygen consumption rate (OCR) in vitro. Islet culture in bioreactors, allowing remodeling of islets with the formation of channels with external openings that could allow a better oxygen diffusion were tested. These studies showed that islets had a preserved viability and function, but so far none have assessed oxygen parameters [115,116]. In this review, we focus on oxygen supply techniques that have already been tested in vivo ((Table 1 and Figure 1).
Figure 1. Current developments to enhance the oxygen supply of isolated islets
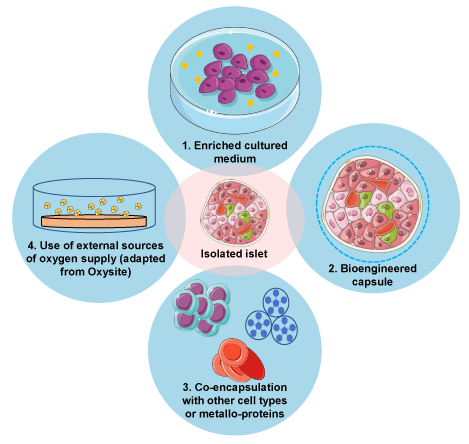
Four different strategies to improve transplanted islets’ oxygenation are illustrated.
Table 1. Strategies to improve oxygen delivery to islets
Reference |
Agent or method tested |
Strategy |
Model |
Result |
Carlsson, et al. [53] |
βAir |
Macroencapsulation device |
Phase I study in human |
Safety of the device but limited metabolic effects. |
Stokes, et al. [117] |
DFO |
Enriched medium +/- intraperitoneal injection |
Human islets, in vivo (mice) |
DFO increased the β-cell mass at day 28. |
Fraker, et al. [121] |
PFD/PDMS Culture dishes |
Bioengineered pre-culture device |
Human islets, in vitro and in vivo (mice) |
In vitro: increased O2 diffusivity, better OCR rate, better Glucose Stimulated Insulin Release Index, and a protection against hypoxia in PFD/PDMS group
In vivo: higher percentage of diabetes reversal and earlier reversal, but no significant |
Lee, et al. [122] |
20% PFD enriched microcapsules |
Bioengineered microcapsules |
Rats islets and mice, in vitro and in vivo (mice) |
In vitro, PFD microcapsules decreased cell death and ROS production. Correction of hyperglycemia when transplanted in mice, no differences in insulin secretion but higher fold increase insulin secretion for PFD microcapsules. |
Vériter, et al. [123] |
BmMSCs and aMSCs |
Macroencapsulation device |
- Pig islets alone, naked or in macroencapsulation device in vitro and in vivo (rats)
- BmMSCs or aMSCs alone |
Macrodevice seeded with aMSC had higher O2 concentration than BmMSCs. |
Vériter, et al. [124] |
BmMSCs and aMSCs |
Co-encapsulation in macrodevice |
Pig islets + BmMSCs or aMSCs, in vitro and in vivo (rats) |
In vitro: islets+ aMSCs, islets+BmMSCs and aMSCs alone had higher oxygen consumption than control islets in hyperglycemic condition. aMSCs+islets had higher insulin stimulation index than BmMSCs+islets.
In vivo: islets+ aMSCs had higher oxygen consumption than islets+ BmMSCs |
Lebreton, et al. [129] |
Human amniotic epithelial cells |
Co-encapsulation (shielded agarose islets) |
Rats or human islets, in vitro and in vivo (mice) |
Decreased cell death and maintained insulin secretion in hypoxic conditions. |
Coronel, et al. [133] |
OxySite |
Enriched medium |
Non primate human islets, in vitro and in vivo (rats) |
Increase of pO2 of culture medium, increase of islets viability, increase of stimulation index in vitro. Restauration of euglycemia when transplanted in rats compared to control islets. |
Coronel, et al. [134] |
OxySite |
Macroencapsulation device |
MIN-6 cell line and rat islets, in vitro and MIN-6 cell line, in vivo (mice) |
Increased metabolic activity of β-cells in vitro and restored euglycemia in vivo compared to control islets. |
Liang, et al. [135] |
OxySite |
Bioscaffold |
Rat islets, in vitro and in vivo (rats) |
In vitro: maintain constant oxygen release for 20 days.
In vivo: improves glycaemic control in rats transplanted with lower purity islets. |
Lee, et al. [136] |
PDMS + CaO2 |
Macroencapsulation device |
MIN-6 cell line and pig NPCCs, in vitro and in vivo (mice) |
Increased viability in hypoxia, decreased number of hypoxic cells, decreased ROS. Good tolerance in vivo. |
Ravazi, et al. [137] |
Collagen based cryogel bioscaffold + CaO2 |
Bioscaffold |
Mice islets, in vitro and in vivo (mice) |
In vitro: increased viability, insulin secretion and insulin stimulation index for islets seeded into scaffold+0.25wt%CaO2 versus naked islets. A higher concentration of CaO2 (0.5wt% or 1wt%) decreased viability.
In vivo: increase metabolic control for islets in scaffold+0.25wt%CaO2 versus islets alone or islets in scaffold without CaO2. |
Evron, et al. [138] |
Synechococcus lividus |
Macroencapsulation device |
Rat islets, in vitro and in vivo (rats) |
Maintained O2 production rate for 30 days, restored euglycemia when implanted in rats. |
Ludwig, et al, [139] |
βAir |
Macroencapsulation device |
Pig islets, in vivo (mini pig) |
Preserved islet integrity, increased OCR of islets 13 days after implantation. |
Neufeld, et al. [140] |
βAir |
Macroencapsulation device |
Rat islets, in vivo (mini pig) |
Maintained pO2 in the device for 75 days, restored euglycemia in mini pigs. |
Komatsu, et al. [141] |
Oxygen inhalation |
Oxygen inhalation
Enriched medium |
Rat islets, in vitro and in vivo (rats) |
In vitro: Islets cultured in medium with 140mmHg O2 had better viability, better stimulation index.
In vivo: Inhalation of 50% O2 (ambient air in cage or directly with reservoir mask) led to increase of pO2 in subcutaneous transplant site, improved islets survival and decreased the number of islets necessary to reverse diabetes. |
Hughes, et al. [142] |
Oxygen inhalation
Hyperbaric oxygen therapy |
Oxygen inhalation |
Rat islets, in vivo (rats) |
Hyperoxia (housing in 100% O2) allowed to achieve normoglycemia with reduced number of islets. No effect of hyperbaric oxygen therapy. |
Pre-transplantation step: enriched culture medium or bioengineered capsule
Adjonction of Deferoxamine (DFO, iron chelator activating HIF-1α) in islet culture medium prior to transplantation increased the β-cell mass at day 28 compared to control, in a HIF-1α-dependent mechanism, suggesting that increase of HIF-1α is a protective factor for islets [117]. HIF-1α seems to be a protective factor in islet transplantation, required for successful islet transplant outcomes [117]. Despite this protective aspect, some studies have also showed negative effects regarding transplant outcome, for example in hematopoietic stem cell transplantation [118]. These contradictory results could be due to the antecedent of islet stress induction, which then activate HIF-1α [119,120].
Fraker, et al. pre-cultured islets in a PFC/ Polydimethylsiloxane (PDMS) culture dish allowing islets to receive O2 from both the top and the bottom: in vitro, they showed an increase of O2 diffusivity, a better OCR rate, a better glucose stimulated insulin release index, and protection against hypoxia. In vivo data showed that the percentage of diabetes reversal in mice transplanted with human islets cultured in standard dishes or in the PFC/PDMS dishes was improved and seemed to appear sooner [121].
In encapsulated islets, the use of 20% Perfluorodecalin enriched microcapsules increased islets viability for 2 days, limited the Reactive Oxygen Species (ROS) production and maintained longer euglycemia in rats compared to alginate microcapsules and naked islets [122].
Co-transplantation or co-encapsulation of islets with others cellular types or with metalloproteins to improve oxygenation of islets.
Islets can be transplanted along with another cell type, so that the latter encourages the development of a vascular network. However, current transplantation strategies require a retrievable transplant for patients. This major limitation has encouraged macrodevices developments. In fact, macrodevices implanted in subcutaneous site and seeded with sole adipocyte mesenchymal stem cells (aMSCs) were significantly better oxygenated than those seeded with bone marrow mesenchymal stem cells bone marrow mesenchymal stem cells (bmMSCs) [123]. This proof of concept has been supported by subsequent results regarding co-encapsulation with islets [124]: in rats, pig islets co-encapsulated with aMSCs had a better oxygenation. The benefit of the MSCs is certainly due to the secreting capacity of these cells.
In the meantime, microdevices were developed with a different strategy, trying to maintain proximity of co-culture and limit the fibrosis which often occurs with larger devices. MSCs have been encapsulated by microfluidics and have proven to improve islet survival [125], but no O2 results were published so far. Development of a new generation of Haemoglobin Based Oxygen Carrier is currently in progress [126–128], but in vitro application and co-encapsulation with cells have not been tested so far. More recently, promising data on co-encapsulation of rat islets with human amniotic epithelial cells showed an improvement of viability and maintained glucose response under hypoxic conditions [129]. Hemarina, a French company, has developed a universal oxygen carrier which has been used for therapeutic purposes including Covid-19 [130,131]. We believe this new oxygen carrier could be of great interest for future co-encapsulation approaches and look forward to further investigations.
Use of external sources of oxygen supply
Hydrolytic activation dissociates calcium peroxide and therefore provides an O2 supply [132]. However, this reaction is extremely rapid and leads to hyperoxide condition, with the formation of hydroxyl radical, a ROS, toxic for islets [132]. Encapsulation of solid calcium peroxide (CaO2) within a polydimethylsiloxane (PDMS) disk tempers the hydrolitic activity, enabling slow water infiltration to dissociate the calcium peroxide [132]. This device, called “OxySite”, increases pO2 in culture as long as in contact with water, benefiting non-encapsulated rat islets. It also significantly increased the metabolic activity compared to control hypoxic islets and control normoxic islets [133]. Implantation of a macrodevice containing islets and an OxySite disk in immunocompetent diabetic mice allowed to restore normoglycemia [134]. Recently, OxySite has managed to miniaturised microbeads from 10mm disks to 220µm, directly integrated into a PDMS scaffold [135]. The OxySite scaffold was able to release oxygen during 20 days in vitro, suggesting that it should theoretically maintain islets oxygenation during the 14-day period of transplant revascularisation. Moreover, the OxySite maintained graft efficacy of lower purity islets in vivo: with 99%-purity islets, all diabetic recipients reached normoglycemia, whether they were transplanted with OxySite or control scaffold; but with 80%-purity islets, only recipients with the OxySite scaffold reached normoglycemia [135].
A similar device was developed by another team, with PDMS+CaO2, which improved viability of MIN-6 cells and of pig Neonatal Pancreatic Cell Clusters (NPCCs) particularly in hypoxic conditions [136]. During hypoxia, levels of caspase 3 and 7 were reduced compared to control and ROS production was decreased in PDMS+CaO2 device compared with PDMS alone and control group, while insulin secretion was significantly higher for NPCCs in PDMS+CaO2 group compared to PDMS alone [136]. More recently, a study demonstrated the effect of oxygenation for islet transplantation, as well as O2 toxicity by excessive oxygenation [137]. In addition to developing a new oxygen–generating bioscaffold facilitating islet survival and function, they also showed the appropriate O2 range for islets which may be narrower than expected.
Evron, et al. reported a successful implantation of a macrodevice made of several layers, in which several immobilized Synechococcus lividus (active thermophilic cyanobacteria) produce oxygen by photosynthesis when illuminated by a light source integrated to the device [138]. However, pO2 furnished by this device was under the 50mmHg required and the authors estimated that for the application of this device in human, regarding the number of islets requested, the size would have to approach 450 cm².
A macrodevice with an oxygen chamber with daily refuelling (βAir device) showed good results in a proof-of-concept study [139]: explantation of the device at day 13-post transplantation showed no fibrosis and no inflammatory reaction around the device, and morphologically intact islets and OCR increased from 0.86 ± 0.16 pmol/min/IEQ before transplantation to 1.85 ± 0.06 at day 13 [139]. Study on mini-pigs was successful with a reversal of diabetes [140] ; moreover, the oxygen concentration in the gas chambers was constantly > 300mmHg, allowing the constitution of an oxygen gradient between the gas-chamber and the islet module and thus ensuring an adequate oxygen supply for the islets [140]. However, even if first phase 1 studies demonstrated a good safety profile of the βAir device, the clinical outcomes were disappointing, probably due to the low number of IEQ transplanted [52,53]. Implantable Electrochemical Oxygen Generator is currently in development, in the proof-of-concept state, and should be able to continuously generate and supply oxygen, compared to the βAir device that needs daily refuelling [60].
More recently, simple oxygen inhalation with 50% O2 in rats increased the subcutaneous pO2 from 45mmHg to 140mmHg, both in prevascularized subcutaneous site and in native subcutaneous site [141]. It led to a functional graft reversing diabetes and maintaining viability of islets > 150µm compared to rats in normoxia conditions [141]. In 2003, a study demonstrated that hyperoxia improved the survival of intraportally transplanted syngeneic pancreatic islets [142]. This finding suggests that O2 inhalation could be rather simple and clinically applicable. However, this technique should be adjusted since hyperoxia could also cause brain and lung damage in human. Indeed, hyperoxia may aggravate oxidative stress and exacerbate inflammatory response. Hyperoxia increases the production of peroxynitrite and apoptosis (mediated by caspases), impact gene regulation implied in cell death (such as c-myc or bax) and can be responsible of vasoconstriction, leading to abnormalities in microcirculation [143]. In patients, high O2 concentration can be used in intensive care unit: conflicting clinical data exist on hyperoxia, but for stroke, there does not seem to be any benefit from hyperoxia or even possible adverse effects [143]. Indeed, hyperoxia can reduce the blood flow for acute myocardial infarction and thus increase myocardial ischemia; in sepsis a study showed an association between hyperoxia and mortality [143].
Beside the enhancement of oxygen delivery, strategies to support and enhance islet or encapsulation device vascularization have been developed. Historically, a significant part of research on vascularization concentrated on biomaterials, such as synthetic scaffolding or membrane [144], with research on ideal pore size [145]. More recent approaches evaluated the potential of extra-cellular matrix [146–149], prevascularization of transplantation site [89,150,151], delivery of pro-angiogenic factors [152–154] and bioengineering of macro or microcapsule to improve neovascularization [155,156]. Bowers, et al. recently reviewed the physical properties of devices such as pore size, surface roughness and stiffness of devices, extracellular matrix scaffolding, and the prevascularization of transplanting sites allowing better vascularization [157]. For example, pre-implantation of the macrodevice increases vascular profiles, with a more homogeneous distribution of the vessels [158] and increases endocrine tissue volume within the microdevice [89]. Design of a macrodevice appears particularly important since micropatterned lid with MSCs seems to facilitate prevascularization in vivo [159] whereas meshes [160] encourage cells to organize and create new vessels. Bowers, et al. also reviewed the way to release pro-angiogenic factors such as Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor (FGF) [157]. In this review, we will focus mainly on two ways improving vascularization: co-injection with other cell types and systemic injection of pharmacological agents (Table 2 and Figure 2).
Figure 2. Current developments to enhance the vascularization of isolated islets
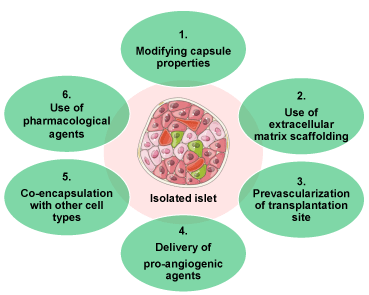
Six different strategies to improve transplanted islets’ vascularization are shown.
Table 2. Strategies to improve vascularization of transplant
Reference |
Agent or method tested |
Strategy |
Model |
Result |
Vériter, et al. [123] |
BmMSCs and aMSCs |
Macroencapsulation device |
- Pig islets alone, naked or in macroencapsulation device in vitro and in vivo (rats)
- BmMSCs or aMSCs alone |
In vitro, hypoxia in normoglycemic condition decreased VEGF secretion of naked pig islets. In hyperglycemia, no change in VEGF secretion between hypoxic and normoxic conditions.
Increase of VEGF secretion in vitro in hypoxic conditions and in hyperglycemia for both MSCs type.
No difference in number of vessels between bmMSCs or aMSCs but higher VEGF-positive cells for aMSCs. |
Vériter, et al. [124] |
BmMSCs and aMSCs |
Co-encapsulation in macrodevice |
Pig islets + BmMSCs or aMSCs, in vivo (rats and primates) |
In vivo, increase of vessels number for islets+aMSCs and islets+BmMSCs compared to islets alone, but higher VEGF-positive cells for islets+aMSCs. |
Ito, et al. [162] |
Co-infusion islets + MSCs |
Non-encapsulated cell |
In vivo (rats and mice) |
Increases of % of rats with euglycemia with MSCs, doubling of number of capillaries per β-cell in the MSCs group |
Xiang, et al. [164] |
MSCs |
Non-encapsulated cells |
In vitro and in vivo (mice) |
MSCs cultured in hypoxia produced more growth promoting factors (VEGF) than those cultured in normoxia. They reduced hypoxia-induced apoptotic rate of islets. |
Kim, et al. [165] |
PLGA device coated MSCs |
Bioengineered device for prevascularization |
In vivo (mice) |
MSCs pre-cultured in hypoxic conditions promoted neo-vascularization when transplanted in mice. Superior diabetic control in mice, after infusion of islets in prevascularized space with hypoxic MSCs |
Forbes, et al. [166] |
Human ombilical cord perivascular MSCs |
Non-encapsulated cells |
In vitro and in vivo (mice) |
Increases of vessel density, days to cure diabetes was reduced |
Oh, et al. [169] |
MSCs spheroid |
Non-encapsulated cells |
In vivo (mice and non-human primates) |
Increases vascular density, increase CD31+ cells, improve metabolic results |
Kogawa, et al. [167] |
MSCs CellSaic |
Micro + microencapsulated islets |
In vivo (mice) |
Increases vascular area, number of vessels and improve metabolic results |
Perez-Basterrechea, et al. [170] |
Fibroblasts |
Macroencapsulation |
In vivo (mice) |
Increases number of vessels per field, graft survival and insulin positive cells compared to control |
Cheng, et al. [172] |
Endothelial cells |
Non-encapsulated cells |
In vivo (rats) |
Increases microvessel density, of CD34 immunostaining, and improvement of metabolic results compared to control |
Grapensparr, et al. [173] |
Endothelial cell progenitors |
Non-encapsulated cells |
In vivo (mice) |
Increase graft blood perfusion, increase CD31 immunostaining, no experiment on metabolic results |
Vlahos, et al. [174] |
“Module” coated with endothelial cells |
Bioengineered device |
In vivo (mice) |
Improves time of revascularization (higher number of CD31+ cells for modules with endothelial cells at day 7 and 14 but not at day 21), improves metabolic results |
Samikannu, et al. [175] |
Sitagliptin |
Pharmacologic agent |
In vitro and in vivo (mice) |
Improves vascular density, transplant blood flow, VEGF secretion and metabolic results |
Jia, et al. [177] |
Exandin-4 |
Pharmacologic agent |
In vitro and in vivo (mice) |
Improves vascular profiles, HIF-1α, metabolic result, volume of endocrine cells, decreases necrotic cells and levels of caspase 3 |
Langlois, et al. [179] |
Liraglutide |
Pharmacologic agent |
In vitro and in vivo (mice) |
Improves number of CD31+ cells, VEGF release, HIF-1α and metabolic results |
Senior, et al. [185] |
Sitagliptin |
Pharmacologic agent |
Pilot clinical study |
Insulin independence achieved in 2/8 patients (25%) but no maintained effects after sitagliptin discontinuation. |
Johansson, et al, [186] |
Prolactin |
Pharmacologic agent |
In vitro and in vivo (mice) |
Increases of blood flow transplant, of vascular density, endocrine volume of graft |
Langlois et al, [187] |
DFO |
Pharmacologic agent |
In vitro and in vivo (rats) |
Improves VEGF expression during 3 days by increasing levels of HIF-1α |
Najdahmadi, et al. [188] |
H2S |
Pharmacologic agent |
In vivo (mice) |
Decreases number of CD31+ cells and vascular density |
Lee, et al. [189] |
Resveratrol |
Pharmacologic agent |
In vitro and in vivo (mice) |
Increases volume of vessels / islet, no difference in β-cell volume, decreases oxidative stress, decrease cell death in vitro in hypoxic conditions |
Min, et al. [190] |
Tocilizumab |
Pharmacologic agent |
In vivo (monkeys) |
Reduction of CD31+ cells in tozicilizumab group compared to control |
Menger, et al. [192] |
EPO |
Pharmacologic agent |
In vivo (mice) |
Improves functional capillary density, revascularized area, shortens engraftment |
Menger, et al. [193] |
DPO |
Pharmacologic agent |
In vivo (mice) |
Fails to improve revascularization process |
Co-transplantation with other cell types to improve vascularization.
We previously mentioned the benefit of co-transplantation with other cell types regarding oxygenation, and in this part we will discuss the capacity of other cell types to provide a supportive network and the secretion of pro-angiogenic factors.
Mesenchymal stem cells: MSCs promote angiogenesis by secreting pro-angiogenic factors [161]. Several studies investigated their benefit in islet graft revascularization. Vériter, et al. [123] evaluated the sole impact on vascularization of MSCs derived either from bone marrow or from adipocytes, without islets, seeded in a macrodevice and then implanted subcutaneously in Wistar rats. No differences were observed in terms of number of vessels surrounding the macrodevice, but a higher number of VEGF-positive cells was observed for the macrodevice seeded with adipocyte derived MSCs [123]. Interestingly, MSCs seem to improve graft function when co-transplanted with islets, partly by promoting revascularization [162,163]: co-transplantation of MSCs and naked islets under the renal capsules in Lewis rats significantly increased by two folds the capillaries in the group MSC+ [162].
A study showed that bone marrow MSCs pre-cultured in hypoxic conditions (hMSCs) [164] have adapted by secreting pro-angiogenic and anti-apoptotic factors such as VEGFA, IL-6, MCP-1 and MMP9: when co-cultured with islets in hypoxic conditions, MSCs improved viability compared to islets alone and MSCs pre-cultured in normoxic conditions (nMSCs) [164]. This observation was confirmed in vivo: mice transplanted with hMSCs had better glycemic control and less apoptotic rate than nMSCs group and control islet group, for non-encapsulated islets [164] or for islets encapsulated in a PLGA macrodevice previously coated with hMSCs [165]. MSCs derived from human umbilical cord also promoted neovascularization and transplant function [166].
Interestingly, Kogawa, et al. tried to combine micro and macroencapsulation to benefit from both techniques and to overcome their respective drawbacks: mice were co-transplanted subcutaneously with microencapsulateds islets and MSC-CellSaic (a bioresorbable scaffold seeded with MSCs), into a mesh bag [167]. Microencapsulated islets + MSC-CellSaic had better metabolic outcomes than microencapsulated islets alone, increased the vascular area and the number of vessels. When seeded without islets, MSC-CellSaic significantly improved the vascular area and the number of vessels compared to MSCs spheroids and controls [167].
Development of 3D-culture allows better interaction between cells and the formation of spheroid that could be used as a support for a vascularization network [168]. Intraportal infusion of islets co-transplanted with bone marrow mononuclear cell derived spheroid (BM-spheroid) in mice succeeded to reverse diabetes and improved vascularization compared to islets transplanted alone: vascular density was almost 3 times higher than in the control group, without increasing thrombogenicity [169]. MSC-spheroid, derived from mouse pancreatic MSCs, caused thrombosis, leading to liver necrosis but co-transplantation with islets was not tested in the study.
Fibroblasts: Co-transplantation of islets with fibroblasts is of great interest considering their capacity to secrete pro-angiogenic factors such as VEGF, HIF-1α or FGF. Fibroblasts seeded in a scaffold and grafted in the subcutaneous site significantly improved the vascularization of islets [170]. At day 1 and 3, revascularization (considering the number of vessels) was faster with fibroblasts, but on day 7 and 10 the rate of revascularization was similar between the two groups [170]. In this model, the fibrotic process allowed the creation of a new ECM with increased vascularization. This new organised and vascularised ECM improved islet engraftment as well as the long-term survival and function of the graft [170]. More recently, another study confirmed the importance of fibroblast-like population in the revascularisation process by helping reconstitute and organise ECM [171]. This highlights the importance of fibroblasts in future developments.
Endothelial cells and endothelial progenitors: Co-transplantation of naked islets under the kidney capsule with vascular endothelial cells transfected to increase the production of VEGF showed promising results in rats since the vessels density was 6-fold higher than the control groups [172]. Human islets coated with endothelial cell progenitors from human umbilical cord had a significantly higher vascular density, a higher blood oxygen tension and a higher blood perfusion compared with control at 1 month post transplantation in normoglycemic mice [173]. Glycaemic control was not assessed in this study. Interestingly, coating a collagen macrodevice containing rat islets with endothelial cells significantly increased the number of vessels after transplantation [174]. Even if the number of vessels was similar at day 21 between the module coated with endothelial cells and the control module, the difference at day 7 and 14 suggests a higher revascularization process which seems to limit ischemic effects [174].
Use of systemic pharmacologic agent
The use of GLP1 agonist or DPP4 inhibitor could be of great interest in future clinical practice in islet transplantation. For example, sitagliptin, a DPP4 inhibitor, significantly increased VEGFR-2 expression in islets transplanted under the kidney capsule in mice, but also increased the phosphorylation of cAMP response element-binding, finally leading to increased VEGF secretion [175]. Consequently, vascularization of islets increased, as shown by the increased proliferation of endothelial cells in the sitagliptin group in comparison with the control group. Sitagliptin also increased mTOR expression in transplanted β-cells, explaining the increase of expression of VEGFR-2 [175].
Mice transplanted with human islets under the kidney capsule and injected with either Exenatide, GLP1 or the oral administration of sitagliptin showed increased secretion of human C-peptide and improved post-prandial glycemia during an oral glucose tolerance test [176]. Exandin-4, a GLP1 receptor agonist, improved vascular proliferation around a macrovascular device transplanted under the kidney capsule in mice, in comparison with controls [177]. Authors showed that Exandin-4 significantly enhanced mRNA expression of Bcl-2 (antiapoptotic gene), Pdx-1 (gene encoding for proliferation and differentiation of β-cells) and HIF-1α (encoding for vessels proliferation) [177]. Exenatide, another GLP1 agonist, increased insulin secretion and glycaemic outcomes in patients transplanted in the intraportal site [178]. Langlois, et al. [179] demonstrated the angiogenic properties of liraglutide in vitro, with increased number of CD31 positive cells and increased staining intensity compared to control islets. The effect was at least partly mediated by HIF-1α since liraglutide induced a transient overexpression of HIF 1α after 12h in culture, before turning back to the control expression level, leading to the overexpression of VEGF gene, compared to control islets. VEGF gene overexpression is confirmed by elevated VEGF release at 48h. In vitro, viability was preserved at 48h compared to control, and insulin secretion was higher with liraglutide [179]. In vivo, liraglutide improved body weight gain and fasting glycaemia and immunohistological analysis of islets showed a significant higher number of endothelial cells around as well as inside transplanted islets when treated with liraglutide compared to the control group [179].
This encouraging effect of liraglutide on vascularization was not found in a previous study: Nishimura, et al. [180] transplanted mice islets into a dorsal skinfold chamber of recipient mice that were divided into a control group, a group with islets pre-cultured with liraglutide for 24h before transplantation and a group with subcutaneous injection of liraglutide for 8 days. There was no increase in the rate of newly formed vessels in any group. However, details are not given regarding the metabolic control of this transplantation.
Interestingly, in mice, the pre-treatment of mice islets for 10 min with liraglutide prior transplantation was more efficient to restore normoglycemia than systemic treatment with liraglutide [181]. In human, effect of liraglutide as an adjunctive treatment of type 1 diabetes remains disappointing [182–184]. A clinical trial on sitagliptin + pantoprazole in islet transplanted patients, with early graft insufficiency showed that sitagliptin allowed 2/8 patients to reached insulin independence (defined as: no insulin use for at least 1 week + HbA1c < 6.0% + fasting plasma glucose < 7mmol/L + C-peptide > 0.5nmol/L) [185]. Three months after stopping sitagliptin and pantoprazole, no patient reached the primary endpoint.
Other substances have been tested such as prolactin. Prolactin was used in vitro in a 24h pre-treatment in isolated islets and in vivo as injections during the 7 first days post transplantation under the renal capsule in mice. In both cases the blood flow increased by 40% at 1-month post transplantation [186]. The vascular density was markedly increased when islets were treated with prolactin compared with control mice and the O2 tension was increased in treated mice [186]. It also decreased the level of thrombospondin-1 mRNA, implied as a negative regulator of angiogenesis [186].
Pharmacological stimulation of VEGF production by DFO iron chelator led to VEGF overexpression for 3 days by inducing HIF-1α factor, whereas VEGF overexpression with an adenoviral transduction was maintained 14 days [187]. However, transduction with VEGF-adenovirus led to loss of functionality of islets, as opposed to the use of DFO [187]. Even if DFO had adverse effects (mimicking iron deficiency), this study constitutes an interesting proof of concept for pharmacological stimulation with a more specific drug [187].
Hydrogen sulfide (H2S) was previously described as an effective angiogenic stimulator. It has been tested in mice: animals implanted with a poly(D, L-lactide-co-ε-caprolactone) scaffold were intraperitoneally injected with sodium hydroxysulfide [188]. Unexpectedly, authors described a lower vascularization of the device and a lower CD31 immunostaining in mice with H2S treatment compared to control [188].
Resveratrol (RSV) has been tested in diabetic mice: animals were transplanted with 200 IEQ under the left kidney capsule and treated orally with RSV [189]: RSV increased glycaemic control, β-cell and vascular density after transplantation compared to control transplanted mice. Oxidative stress was reduced in RSV group. In hypoxic conditions, RSV pre-treatment of isolated islets prevented cell death and ROS increase.
A recent study with Tocilizumab, an IL6 blocker, expected to reduce initial inflammatory response and thus improve vessels formation, succeeded to avoid C-reactive protein peak after transplantation but lowered the revascularization in monkeys intraportally transplanted [190].
The protein tyrosine phosphatase 1B (PTP1B) regulates phosphotyrosine signaling in several pathways implied in differentiation, cell growth, metabolism or apoptosis. PTP1B inhibits phosphorylation of VEGFR2 and VE-Cadherin, and impairs stimulation of angiogenesis by VEGF-A [191]. In a recent proof-of-concept and mechanistic study, PTP1B-KO mice islets showed excellent results after transplantation in rodent recipients [191] allowing diabetes reversion, increasing the vascular density by 3 times compared to control mice and doubling vessels area at day 30 and drastically decreasing Caspase-3 positive cells. The PTP1B-KO mice islets increased VEGF-A in an independent HIF1α way. The development of pharmacological inhibitors of PTP1B and its effect on islet transplant would be interesting to test in the future.
Pre-treatment of diabetic mice with intraperitoneal injection of erythropoietin (EPO) [192] improved engraftment of islets, neovascularization and shortens the return to normoglycemia. Since pre-treatment with EPO might not be transferred to human subjects, a long-lasting analogue of EPO called darbepoetin-α treatment was tested but failed to accelerate revascularization process [193].
Despite its safety and efficacy, islet transplantation struggles to overcome oxygenation and vascularization challenges to fully develop. As we summarised in this review, limiting factors are well identified and several solutions have been proposed for each issue, but the pieces of the puzzle remain to be put together.
Concerning oxygenation, studies lack consensus on the main judgment criterion. For example, some studies evaluate the improvement in pO2, while others evaluate the impact on the viability and the insulinosecretion of islets. A variety of ways to evaluate oxygenation is found in the literature: some authors use pO2 while others refer to the oxygen consumption rate, or to the consumption of O2 per week. Recently, non-invasive method of oxygen measurement by Fluorine-19 magnetic resonance has been described allowing to measure pO2 [194] or dissolved oxygen concentration [195] in non-human primates. Besides, only few studies have managed to evaluate the effect of improved oxygenation in hypoxia condition. This achievement seems essential as improving the oxygenation of islets in normoxic conditions in vitro does not presume of an improvement in hypoxia in vivo. If improving oxygenation is a crucial aspect of islet transplantation, some concerns have been raised regarding the potential O2 toxicity, especially regarding oxidative metabolism. Some studies have nicely highlighted the paradoxical positive finding of the attenuation of hypoxia-induced effects which could be secondary to a protective effect of the hyperoxia induced reduction of oxidative metabolism [196,197.]
Concerning vascularization, several strategies of improvement are appearing especially co-transplantation with other cells types, use of a pharmacological agent, injection of a proangiogenic factor and bioengineering devices. However, studies lack consensus on the priority of evaluation criteria, either islet viability and functionality or the proliferation of vessels, with a common endpoint based on glycaemic control. In most studies, quantification of vessels is based on histological sections, with CD31 staining, and more recently lectin [106]. All these methods require explantation of islets or of the device, implying there is no dynamic study of the revascularization process in vivo. In 2016, Jansson, et al. reviewed the different techniques that can be used to measure pancreatic islets blood flow [65] to adapt to transplanted islets. Recently, Weaver, et al. performed an elegant quantitative approach to evaluate the vascularization of leading islet transplant extrahepatic tissue sites, with lectin, and nicely showed the integration of islets into the host vasculature [86]. Use of laser-scanning microscopy that have been previously reported to monitor in vivo islet graft in the anterior chamber of the eye appears as a nice tool to visualise vascularization process [171,198].
Clearly, vascularization and oxygenation are linked but authors often evaluate either one or the other criterion, while it seems that a global evaluation of both parameters would be interesting. We believe that recent advances in this field are extremely promising, and much remains to be done before giving a single direction regarding further developing islet transplantation with or without encapsulation. Interestingly, both oxygenation and vascularization issues remain a common concern regarding islet transplantation. As developed in this review, co-encapsulation with MSCs or oxygen nano-carriers seems especially promising, as well as the potential of some pharmacological agents, once further evaluated. The results of clinical trials regarding the omentum transplantation site will undeniably modify the future of islet transplantation. The idea of combining macro and microencaspulation in order to co-transplant islets with other cell types [167] is very original but is a double-edged sword. It allows to remove microencapsulated islets graft but it can dramatically deteriorate the functionality and viability of the islets: the encouraging results obtained in that study need to be further confirmed.
Other strategies diverging from islet transplantation are now rising such as injection of exosomes derived from MIN-6 cells (mouse insulinoma cell line) [199], development of in vitro model of spheroid and islets-on-chip to help create 3D-vascularization [200], reconstitution of pseudo-islets (disaggregated islets embedded into endothelialized collagen rods) [201] and changing the islet source by using of induced pluripotent stem cells [202] that are able to created viable vascularized transplants. Very recently, an important study reported the creation of organoids derived from the fusion of islet cells and functional blood vessels, with positive results on vascularization and impressive results on the numbers of islets needed to normalize glycemia [203]. All these new strategies give further hope for the improvement of islet transplantation technique. In particular, the organoids technology is very promising for regenerative and personalized medicine, but requires technical [204] and ethical [205,206] consensus.
Altogether, the future of islet transplantation remains an exciting perspective for type 1 diabetic patients in regards to the possible improvements in vascularization and oxygenation. Both short and long-term islet transplantation success will be improved and will further open-up this treatment strategy to more diabetic patients.
JC wrote the manuscript. ET, PYB and SL completed, discussed and reviewed the manuscript.
The authors declare that they have no competing interests. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
- https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, et al. (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157: 107843. [Crossref]
- Ogurtsova K, Fernandes JD da R, Huang Y, Linnenkamp U, Guariguata L, et al. (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128: 40-50. [Crossref]
- Eizirik DL, Pasquali L, Cnop M (2020) Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 16: 349-362. [Crossref]
- Singh RP, Elman MJ, Singh SK, Fung AE, Stoilov I (2019) Advances in the treatment of diabetic retinopathy. J Diabetes Complications 33: 107417. [Crossref]
- Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C (2017) Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev 2017: 33. [Crossref]
- Bondar A, Popa AR, Papanas N, Popoviciu M, Vesa CM, et al. (2021) Diabetic neuropathy: A narrative review of risk factors, classification, screening and current pathogenic treatment options (Review). Exp Ther Med 22: 690. [Crossref]
- Chalakova T, Yotov Y, Tzotchev K, Galcheva S, Balev B, et al. (2021) Type 1 Diabetes Mellitus - Risk Factor for Cardiovascular Disease Morbidity and Mortality. Curr Diabetes Rev 17: 37-54. [Crossref]
- The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, et al. (1993) The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med 329: 977-986. [Crossref]
- Nathan DM (2014) The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 37: 9-16. [Crossref]
- https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/national-diabetes-audit-report-1-care-processes-and-treatment-targets-2016-17
- Vantyghem MC, Press M (2006) Management strategies for brittle diabetes. Ann Endocrinol (Paris) 67: 287-296. [Crossref]
- Niclauss N, Morel P, Berney T (2014) Has the Gap Between Pancreas and Islet Transplantation Closed? Transplantation 98: 593-599. [Crossref]
- Gruessner RWG, Gruessner AC (2013) The current state of pancreas transplantation. Nature Reviews Endocrinology 9: 555-562.
- Dholakia S, Oskrochi Y, Easton G, Papalois V (2016) Advances in pancreas transplantation. J R Soc Med 109: 141-146. [Crossref]
- Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, et al. (2000) Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N Engl J Med 343: 230-238. [Crossref]
- Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, et al. (2012) Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 35: 1436-1445. [Crossref]
- Lablanche S, Borot S, Wojtusciszyn A, Bayle F, Tétaz R, et al. (2015) Five-Year Metabolic, Functional, and Safety Results of Patients With Type 1 Diabetes Transplanted With Allogenic Islets Within the Swiss-French GRAGIL Network. Diabetes Care 38: 1714-1722. [Crossref]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, et al. (2016) Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 39: 1230-1240. [Crossref]
- Lablanche S, Vantyghem MC, Kessler L, Wojtusciszyn A, Borot S, et al. (2018) Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 6: 527-537. [Crossref]
- Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, et al. (2008) A Multi-Year Analysis of Islet Transplantation Compared With Intensive Medical Therapy on Progression of Complications in Type 1 Diabetes. Transplantation 86: 1762-1766. [Crossref]
- Khosravi-Maharlooei M, Hajizadeh-Saffar E, Tahamtani Y, Basiri M, Montazeri L, et al. (2015) Therapy of endocrine disease: Islet transplantation for type 1 diabetes: so close and yet so far away. Eur J Endocrinol 173: R165-R183. [Crossref]
- Eich T, Eriksson O, Sundin A, Estrada S, Brandhorst D, et al. (2007) Positron emission tomography: a real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation 84: 893-898. [Crossref]
- Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, et al. (2002) β-Cell Death and Mass in Syngeneically Transplanted Islets Exposed to Short- and Long-Term Hyperglycemia. Diabetes 51: 66-72. [Crossref]
- Wilson JT, Chaikof EL (2008) Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev 60: 124. [Crossref]
- Bertuzzi F, Marzorati S, Maffi P, Piemonti L, Melzi R, et al. (2004) Tissue Factor and CCL2/Monocyte Chemoattractant Protein-1 Released by Human Islets Affect Islet Engraftment in Type 1 Diabetic Recipients. J Clin Endocrinol Metab 89: 5724-5728. [Crossref]
- Dionne KE, Colton CK, Yarmush ML (1993) Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42: 12-21.
- Katabathina V, Menias CO, Pickhardt P, Lubner M, Prasad SR (2016) Complications of Immunosuppressive Therapy in Solid Organ Transplantation. Radiol Clin North Am 54: 303-319. [Crossref]
- Tze WJ, Wong FC, Chen LM, O’Young S (1976) Implantable artificial endocrine pancreas unit used to restore normoglycaemia in the diabetic rat. Nature 264: 466-467. [Crossref]
- Sun A, Parisius W, Macmorine H, Sefton M, Stone R (1980) An Artificial Endocrine Pancreas Containing Cultured Islets of Langerhans. Artificial Organs 4: 275-278.
- Chick WL, Perna JJ, Lauris V, Low D, Galletti PM, et al. (1977) Whittemore AD, Like AA, Colton CK, Lysaght MJ. Artificial pancreas using living beta cells:. effects on glucose homeostasis in diabetic rats. Science 197: 780-782. [Crossref]
- Sullivan SJ, Maki T, Borland KM, Mahoney MD, Solomon BA, et al. (1991) Biohybrid artificial pancreas: long-term implantation studies in diabetic, pancreatectomized dogs. Science 252: 718-721. [Crossref]
- Maki T, Ubhi CS, Sanchez-Farpon H, Sullivan SJ, Borland K, et al. (1991) Successful treatment of diabetes with the biohybrid artificial pancreas in dogs. Transplantation 51: 43-51. [Crossref]
- Monaco AP, Maki T, Ozato H, Carretta M, Sullivan SJ, et al. (1991) Transplantation of islet allografts and xenografts in totally pancreatectomized diabetic dogs using the hybrid artificial pancreas. Ann Surg 214: 339-360. [Crossref]
- Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, et al. (190) Insulin Independence After Islet Transplantation Into Type I Diabetic Patient. Diabetes 39: 515-518. [Crossref]
- Storrs R, Dorian R, King SR, Lakey J, Rilo H (2001) Preclinical Development of the Islet Sheet. Annals of the New York Academy of Sciences 944: 252-266.
- Tatarkiewicz K, Hollister-Lock J, Quickel RR, Colton CK, Bonner-Weir S, et al. (1999) Reversal of hyperglycemia in mice after subcutaneous transplantation of macroencapsulated islets. Transplantation 67: 665-671. [Crossref]
- Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, et al. (2005) Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol 153: 419-427. [Crossref]
- Wang W, Gu Y, Hori H, Sakurai T, Hiura A, et al. (2003) Subcutaneous transplantation of macroencapsulated porcine pancreatic endocrine cells normalizes hyperglycemia in diabetic mice. Transplantation 76: 290-296. [Crossref]
- Lim F, Sun AM (1980) Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science 210: 908-10. [Crossref]
- O’Shea GM, Goosen MFA, Sun AM (1984) Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 804: 133-136.
- Sun AM, O’Shea GM (1985) Microencapsulation of living cells — A long-term delivery system. Journal of Controlled Release 2: 137-141.
- Soon-Shiong P, Feldman E, Nelson R, Komtebedde J, Smidsrod O, et al. (1992) Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation 54: 769-774. [Crossref]
- De Vos P, Haan BJD, Wolters GHJ, Strubbe JH, Schilfgaarde RV (1997) Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 40: 262-270. [Crossref]
- Lane WR (1947) A Microburette for Producing Small Liquid Drops of Known Size. Journal of Scientific Instruments 24: 98.
- Levvy GA (1947) The Delivery of Small Drops of Liquid. Journal of Scientific Instruments 24: 274.
- Ennis WB, James DT (1950) A Simple Apparatus for Producing Droplets of Uniform Size from Small Volumes of Liquids. Science 112: 434-436. [Crossref]
- Gröhn P, Klöck G, Schmitt J, Zimmermann U, Horcher A, et al. (1994) Large-scale production of Ba(2+)-alginate-coated islets of Langerhans for immunoisolation. Exp Clin Endocrinol 102: 380-387. [Crossref]
- Iwata H, Arima Y, Tsutsui Y (2018) Design of Bioartificial Pancreases From the Standpoint of Oxygen Supply. Artif Organs 42: E168-E185. [Crossref]
- Hwa AJ, Weir GC (2018) Transplantation of Macroencapsulated Insulin-Producing Cells. Curr Diab Rep 18: 50. [Crossref]
- https://viacyte.com/press-releases/two-year-data-from-viacytes-step-one-clinical-trial-presented-at-ada-2018/
- Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, et al. (2013) Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A 110: 19054-19058. [Crossref]
- Carlsson PO, Espes D, Sedigh A, Rotem A, Zimerman B, et al. (2018) Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant 18: 1735-1744. [Crossref]
- Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, et al. (2006) Survival of Microencapsulated Islets at 400 Days Posttransplantation in the Omental Pouch of NOD Mice. Cell Transplant 15: 359-365. [Crossref]
- Chae SY, Kim YY, Kim SW, Bae YH (2004) Prolonged Glucose Normalization of Streptozotocin-Induced Diabetic Mice by Transplantation of Rat Islets Coencapsulated with Crosslinked Hemoglobin. Transplantation 78: 392-397. [Crossref]
- Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, et al. (1994) Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343: 950-951. [Crossref]
- Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, et al. (2006) Microencapsulated Pancreatic Islet Allografts Into Nonimmunosuppressed Patients With Type 1 Diabetes. Diabetes Care 29: 137-138. [Crossref]
- Basta G, Montanucci P, Luca G, Boselli C, Noya G, et al. (2011) Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care 34: 2406-2409. [Crossref]
- Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, et al. (2009) Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32: 1887-1889. [Crossref]
- Papas KK, De Leon H, Suszynski TM, Johnson RC (2019) Oxygenation strategies for encapsulated islet and beta cell transplants. Adv Drug Deliv Rev 139: 139-156. [Crossref]
- Jansson L, Carlsson PO (2002) Graft vascular function after transplantation of pancreatic islets. Diabetologia 45: 749-763. [Crossref]
- Jansson L (1994) The regulation of pancreatic islet blood flow. Diabetes Metab Rev 10: 407-416. [Crossref]
- Carlsson PO (2011) Influence of microenvironment on engraftment of transplanted β-cells. Ups J Med Sci 116: 1-7. [Crossref]
- Lau J, Henriksnäs J, Svensson J, Carlsson PO (2009) Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant 14: 688-693. [Crossref]
- Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, et al. (2016) Pancreatic islet blood flow and its measurement. Ups J Med Sci 121: 81-95. [Crossref]
- Carlsson PO, Palm F, Andersson A, Liss P (2001) Markedly Decreased Oxygen Tension in Transplanted Rat Pancreatic Islets Irrespective of the Implantation Site. Diabetes 50: 489-495. [Crossref]
- Rodriguez-Brotons A, Bietiger W, Peronet C, Magisson J, Sookhareea C, et al. (2016) Impact of Pancreatic Rat Islet Density on Cell Survival during Hypoxia. J Diabetes Res 2016: 3615286. [Crossref]
- de Groot M, Schuurs TA, Keizer PPM, Fekken S, Leuvenink HGD, et al. (2003) Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant 12: 867-875. [Crossref]
- Brandhorst D, Brandhorst H, Mullooly N, Acreman S, Johnson PRV (2016) High Seeding Density Induces Local Hypoxia and Triggers a Proinflammatory Response in Isolated Human Islets. Cell Transplant 25: 1539-1546. [Crossref]
- Muthyala S, Safley S, Gordan K, Barber G, Weber C, et al. (2017) The effect of hypoxia on free and encapsulated adult porcine islets—an in vitro study. Xenotransplantation 24: 10.1111/xen.12275. [Crossref]
- Hals IK, Rokstad AM, Strand BL, Oberholzer J, Grill V (2013) Alginate microencapsulation of human islets does not increase susceptibility to acute hypoxia. J Diabetes Res 2013: 374925. [Crossref]
- Bartlett ST, Markmann JF, Johnson P, Korsgren O, Hering BJ, et al. (2016) Report from IPITA-TTS Opinion Leaders Meeting on the Future of β-Cell Replacement. Transplantation 100 Suppl 2: S1-44. [Crossref]
- Berman DM, O’Neil JJ, Coffey LCK, Chaffanjon PCJ, Kenyon NM, et al. (2009) Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 9: 91-104. [Crossref]
- Vériter S, Gianello P, Dufrane D (2013) Bioengineered Sites for Islet Cell Transplantation. Curr Diab Rep 13: 745-755. [Crossref]
- Zhu H, Li W, Liu Z, Li W, Chen N, et al. (2017) Selection of Implantation Sites for Transplantation of Encapsulated Pancreatic Islets. Tissue Eng Part B Rev 24: 191-214. [Crossref]
- Cantarelli E, Piemonti L (2011) Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 11: 364-374. [Crossref]
- Merani S, Toso C, Emamaullee J, Shapiro AMJ (2008) Optimal implantation site for pancreatic islet transplantation. British Journal of Surgery 95: 1449-1461.
- Liljebäck H, Espes D, Carlsson PO (2019) Unsurpassed Intrahepatic Islet Engraftment - the Quest for New Sites for Beta Cell Replacement. Cell Med 11: 2155179019857662. [Crossref]
- Carlsson PO, Palm F, Andersson A, Liss P (2000) Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation 69: 761-766. [Crossref]
- Contreras JL (2008) Extrahepatic transplant sites for islet xenotransplantation. Xenotransplantation 15: 99-101. [Crossref]
- Espes D, Lau J, Quach M, Ullsten S, Christoffersson G, et al. (2016) Rapid Restoration of Vascularity and Oxygenation in Mouse and Human Islets Transplanted to Omentum May Contribute to Their Superior Function Compared to Intraportally Transplanted Islets. Am J Transplant 16: 3246-3254. [Crossref]
- Bartholomeus K, Jacobs-Tulleneers-Thevissen D, Shouyue S, Suenens K, In’t Veld PA, et al. (2013) Omentum Is Better Site Than Kidney Capsule for Growth, Differentiation, and Vascularization of Immature Porcine β-Cell Implants in Immunodeficient Rats. Transplantation 96: 1026-1033. [Crossref]
- Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, et al. (2016) Bioengineering the Endocrine Pancreas: Intraomental Islet Transplantation Within a Biologic Resorbable Scaffold. Diabetes 65: 1350-1361. [Crossref]
- Pareta R, McQuilling JP, Sittadjody S, Jenkins R, Bowden S, et al. (2014) Long-Term Function of Islets Encapsulated in a Redesigned Alginate Microcapsule Construct in Omentum Pouches of Immune-Competent Diabetic Rats. Pancreas 43: 605-613. [Crossref]
- Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, et al. (2017) Bioengineering of an Intraabdominal Endocrine Pancreas. N Engl J Med 376: 1887-1889. [Crossref]
- Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, et al. (2017) Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Science Advances 3: e1700184.
- Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, et al. (2015) A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 33: 518-523. [Crossref]
- Komatsu H, Gonzalez N, Kandeel F, Mullen Y (2020) Intermittent normobaric oxygen inhalation enhances subcutaneous prevascularization for cell transplantation. Microvasc Res 132: 104070. [Crossref]
- Sörenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, et al. (2008) Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: studies in a rodent model. Transplantation 86: 364-366. [Crossref]
- Yasunami Y, Nakafusa Y, Nitta N, Nakamura M, Goto M, et al. (2018) A Novel Subcutaneous Site of Islet Transplantation Superior to the Liver. Transplantation 102: 945-952. [Crossref]
- Sakata N, Yoshimatsu G, Kodama S (2018) The Spleen as an Optimal Site for Islet Transplantation and a Source of Mesenchymal Stem Cells. Int J Mol Sci 19: 1391. [Crossref]
- Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, et al. (2009) Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant 9: 2485-2496. [Crossref]
- Cantarelli E, Melzi R, Mercalli A, Sordi V, Ferrari G, et al. (2009) Bone marrow as an alternative site for islet transplantation. Blood 114: 4566-4574. [Crossref]
- Chen X, Zhang X, Larson C, Chen F, Kissler H, et al. (2007) The epididymal fat pad as a transplant site for minimal islet mass. Transplantation 84: 122-125. [Crossref]
- Outzen HC, Leiter EH (1981) Transplantation of pancreatic islets into cleared mammary fat pads. Transplantation 32: 101-105. [Crossref]
- Rafael E, Tibell A, Rydén M, Lundgren T, Sävendahl L, et al. (2008) Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant 8: 458-462. [Crossref]
- Svensson J, Lau J, Sandberg M, Carlsson PO (2011) High vascular density and oxygenation of pancreatic islets transplanted in clusters into striated muscle. Cell Transplant 20: 783-788. [Crossref]
- Maffi P, Nano R, Monti P, Melzi R, Sordi V, et al. (2019) Islet Allotransplantation in the Bone Marrow of Patients With Type 1 Diabetes: A Pilot Randomized Trial. Transplantation 103: 839-851. [Crossref]
- Komori J, Boone L, DeWard A, Hoppo T, Lagasse E (2012) The mouse lymph node as an ectopic transplantation site for multiple tissues. Nature Biotechnology 30: 976-983.
- Veroux M, Bottino R, Santini R, Bertera S, Corona D, et al. (2019) Mesenteric lymph nodes as alternative site for pancreatic islet transplantation in a diabetic rat model. BMC Surg 18: 1-8. [Crossref]
- Dionne KE, Colton CK, Yarmush ML (1991) A microperifusion system with environmental control for studying insulin secretion by pancreatic tissue. Biotechnol Prog 7: 359-368. [Crossref]
- Kühtreiber WM, Lanza RP, Beyer AM, Kirkland KS, Chick WL (1993) Relationship between insulin secretion and oxygen tension in hybrid diffusion chambers. ASAIO 39: M247-251. [Crossref]
- Schrezenmeir J, Kirchgessner J, Gerö L, Kunz LA, Beyer J, et al. (1994) Effect of microencapsulation on oxygen distribution in islets organs. Transplantation 57: 1308-1314. [Crossref]
- Wu H, Avgoustiniatos ES, Swette L, Bonner-Weir S, Weir GC, et al. (1999) In Situ Electrochemical Oxygen Generation with an Immunoisolation Device. Annals of the New York Academy of Sciences 875: 105-125.
- Carlsson PO, Liss P, Andersson A, Jansson L (1998) Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 47: 1027-1032. [Crossref]
- Carlsson PO, Palm F, Mattsson G (2002) Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab 87: 5418-5423. [Crossref]
- Colton CK (2014) Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev 67-68: 93-110. [Crossref]
- Sayadi LR, Alexander M, Sorensen AM, Sarantopoulos N, Lau H, et al. (2019) Micro/nanobubbles: Improving Pancreatic Islet Cell Survival for Transplantation. Ann Plast Surg 83: 583-588. [Crossref]
- Schrezenmeir J, Hyder A, Vreden M, Laue C, Mueller-Klieser W (2001) Oxygen profile of microencapsulated islets: effect of immobilised hemoglobin in the alginate matrix. Transplantation Proc 33: 3511-3516. [Crossref]
- Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, et al. (2006) Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng 12: 337-344. [Crossref]
- Ichii H, Sakuma Y, Pileggi A, Fraker C, Alvarez A, et al. (2007) Shipment of Human Islets for Transplantation. American Journal of Transplantation 7: 1010-1020.
- Papas KK, Avgoustiniatos ES, Tempelman LA, Weir GC, Colton CK, et al. (2005) High-density culture of human islets on top of silicone rubber membranes. Transplantation Proc 37: 3412-3414.
- Avgoustiniatos ES, Hering BJ, Rozak PR, Wilson JR, Tempelman LA, et al. (2008) Commercially Available Gas-Permeable Cell Culture Bags May Not Prevent Anoxia in Cultured or Shipped Islets. Transplantation Proc 40: 395-400. [Crossref]
- Kitzmann JP, Pepper AR, Gala-Lopez B, Pawlick R, Kin T, et al. (2014) Human Islet Viability and Function Is Maintained During High-density Shipment in Silicone Rubber Membrane Vessels. Transplantation Proc 46: 1989-1991. [Crossref]
- Rutzky LP, Bilinski S, Kloc M, Phan T, Zhang H, et al. (2002) Microgravity culture condition reduces immunogenicity and improves function of pancreatic islets1. Transplantation 74: 13-21. [Crossref]
- Petry F, Weidner T, Czermak P, Salzig D (2018) Three-Dimensional Bioreactor Technologies for the Cocultivation of Human Mesenchymal Stem/Stromal Cells and Beta Cells. Stem Cells Int 2018: 2547098. [Crossref]
- Stokes RA, Cheng K, Deters N, Lau SM, Hawthorne WJ, et al. (2013) Hypoxia-Inducible Factor-1α (HIF-1α) Potentiates β-Cell Survival after Islet Transplantation of Human and Mouse Islets. Cell Transplant 22: 253-266. [Crossref]
- Yao Y, Wang L, Zhou J, Zhang X (2017) HIF-1α inhibitor echinomycin reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. J Transl Med 15: 28. [Crossref]
- Marshall D, Sabek O, Fraga D, Kotb M, Gaber AO (2005) Examination of the molecular signature associated with islet dysfunction. Transplant Proc 37: 1311-1312. [Crossref]
- Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, et al. (2006) Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets. Am J Transplant 6: 2636-2643. [Crossref]
- Fraker CA, Cechin S, Álvarez-Cubela S, Echeverri F, Bernal A, et al. (2013) A physiological pattern of oxygenation using perfluorocarbon-based culture devices maximizes pancreatic islet viability and enhances β-cell function. Cell Transplant 22: 1723-1733. [Crossref]
- Lee SH, Park HS, Yang Y, Lee EY, Kim JW, et al. (2018) Improvement of islet function and survival by integration of perfluorodecalin into microcapsules in vivo and in vitro. J Tissue Eng Regen Med 12: e2110-e2122. [Crossref]
- Vériter S, Aouassar N, Adnet PY, Paridaens MS, Stuckman C, et al. (2011) The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials 32: 5945-5956. [Crossref]
- Vériter S, Gianello P, Igarashi Y, Beaurin G, Ghyselinck A, et al. (2014) Improvement of Subcutaneous Bioartificial Pancreas Vascularization and Function by Coencapsulation of Pig Islets and Mesenchymal Stem Cells in Primates. Cell Transplant 23: 1349-1364. [Crossref]
- Laporte C, Tubbs E, Pierron M, Gallego A, Moisan A, et al. (2020) Improved human islets’ viability and functionality with mesenchymal stem cells and arg-gly-asp tripeptides supplementation of alginate micro-encapsulated islets in vitro. Biochemical and Biophysical Research Communications 528: 650-657.
- Bäumler H, Xiong Y, Liu ZZ, Patzak A, Georgieva R (2014) Novel Hemoglobin Particles—Promising New-Generation Hemoglobin-Based Oxygen Carriers. Artif Organs 38: 708-714. [Crossref]
- Kao I, Xiong Y, Steffen A, Smuda K, Zhao L, et al. (2018) Preclinical In Vitro Safety Investigations of Submicron Sized Hemoglobin Based Oxygen Carrier HbMP-700. Artif Organs 42: 549-559. [Crossref]
- Kloypan C, Prapan A, Suwannasom N, Chaiwaree S, Kaewprayoon W, et al. (2018) Improved oxygen storage capacity of haemoglobin submicron particles by one-pot formulation. Artif Cells Nanomed Biotechnol 46: S964-S972. [Crossref]
- Lebreton F, Bellofatto K, Wassmer CH, Perez L, Lavallard V, et al. (2020) Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am J Transplant 20: 1551-1561. [Crossref]
- Varney J, Rivera A, Dong V, Tieu P, Zia S, et al. (2020) Mini-review on the properties and possible applications of therapeutic oxygen carrier Hemarina-M101. Transfusion and Apheresis Science: Official Journal of the World Apheresis Association: Official Journal of the European Society for Haemapheresis. 21: 103016.
- Lupon E, Lellouch AG, Zal F, Cetrulo CL, Lantieri LA (2021) Combating hypoxemia in COVID-19 patients with a natural oxygen carrier, HEMO2Life® (M101). Med Hypotheses. 146: 110421. [Crossref]
- Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL (2012) Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences of the United States of America 109: 4245-4250.
- Coronel MM, Geusz R, Stabler CL (2017) Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 129: 139-151. [Crossref]
- Coronel MM, Liang JP, Li Y, Stabler CL (2019) Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials 210: 1-11. [Crossref]
- Liang JP, Accolla RP, Soundirarajan M, Emerson A, Coronel MM, et al. (2021) Engineering a macroporous oxygen-generating scaffold for enhancing islet cell transplantation within an extrahepatic site. Acta Biomaterialia.
- Lee EM, Jung JI, Alam Z, Yi HG, Kim H, et al. (2018) Effect of an oxygen-generating scaffold on the viability and insulin secretion function of porcine neonatal pancreatic cell clusters. Xenotransplantation 25: e12378. [Crossref]
- Razavi M, Primavera R, Kevadiya BD, Wang J, Buchwald P, et al. (2020) A Collagen Based Cryogel Bioscaffold that Generates Oxygen for Islet Transplantation. Adv Funct Mater 30: 1902463. [Crossref]
- Evron Y, Zimermann B, Ludwig B, Barkai U, Colton CK, et al. (2015) Oxygen supply by photosynthesis to an implantable islet cell device. Horm Metab Res 47: 24-30. [Crossref]
- Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, et al. (2010) A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res 42: 918-922. [Crossref]
- Neufeld T, Ludwig B, Barkai U, Weir GC, Colton CK, et al. (2013) The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PloS One 8: e70150. [Crossref]
- Komatsu H, Rawson J, Barriga A, Gonzalez N, Mendez D, et al. (2018) Posttransplant oxygen inhalation improves the outcome of subcutaneous islet transplantation: A promising clinical alternative to the conventional intrahepatic site. Am J Transplant 18: 832-842. [Crossref]
- Hughes SJ, Davies SE, Powis SH, Press M (2003) Hyperoxia improves the survival of intraportally transplanted syngeneic pancreatic islets. Transplantation 75: 1954-1959. [Crossref]
- Damiani E, Donati A, Girardis M (2018) Oxygen in the critically ill: friend or foe? Curr Opin Anaesthesiol 31: 129-135. [Crossref]
- Juang JH, Bonner-Weir S, Ogawa Y, Vacanti JP, Weir GC (1996) Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 61: 1557-1561. [Crossref]
- Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, et al. (1995) Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res 29: 1517-1524. [Crossref]
- Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, et al. (2013) Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34: 6760-6772. [Crossref]
- Guruswamy Damodaran R, Vermette P (2018) Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. Journal of Tissue Engineering and Regenerative Medicine 12: 1230-1237.
- Abualhassan N, Sapozhnikov L, Pawlick RL, Kahana M, Pepper AR, et al. (2016) Lung-Derived Microscaffolds Facilitate Diabetes Reversal after Mouse and Human Intraperitoneal Islet Transplantation. PloS One 11: e0156053. [Crossref]
- Llacua A, de Haan BJ, Smink SA, de Vos P (2016) Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J Biomed Mater Res A 104: 1788-1796. [Crossref]
- Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, et al. (2006) Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 81: 1318-1324. [Crossref]
- Smink AM, Li S, Hertsig DT, De Haan BJ, Schwab L, et al. (2017) The Efficacy of a Prevascularized, Retrievable Poly(D,L,-lactide-co-ϵ-caprolactone) Subcutaneous Scaffold as Transplantation Site for Pancreatic Islets. Transplantation 101: e112-e119. [Crossref]
- Brady AC, Martino MM, Pedraza E, Sukert S, Pileggi A, et al. (2013) Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue Eng Part A 19: 2544-2552. [Crossref]
- Najjar M, Manzoli V, Abreu M, Villa C, Martino MM, et al. (2015) Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol Bioeng 112: 1916-1926. [Crossref]
- Phelps EA, Templeman KL, Thulé PM, García AJ (2015) Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv Transl Res 5: 125-136. [Crossref]
- Weaver JD, Headen DM, Coronel MM, Hunckler MD, Shirwan H, et al. (2019) Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am J Transplant 19: 1315-1327. [Crossref]
- Weaver JD, Headen DM, Hunckler MD, Coronel MM, Stabler CL, et al. (2018) Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 172: 54-65. [Crossref]
- Bowers DT, Song W, Wang LH, Ma M (2019) Engineering the vasculature for islet transplantation. Acta Biomater 95: 131-151. [Crossref]
- Rafael E, Wu GS, Hultenby K, Tibell A, Wernerson A (2003) Improved survival of macroencapsulated islets of Langerhans by preimplantation of the immunoisolating device: a morphometric study. Cell Transplant 12: 407-412. [Crossref]
- Groot Nibbelink M, Skrzypek K, Karbaat L, Both S, Plass J, et al. (2018) An important step towards a prevascularized islet microencapsulation device: in vivo prevascularization by combination of mesenchymal stem cells on micropatterned membranes. J Mater Sci Mater Med 29: 174. [Crossref]
- Song W, Chiu A, Wang LH, Schwartz RE, Li B, et al. (2019) Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat Commun 10: 1-12. [Crossref]
- Tao H, Han Z, Han ZC, Li Z (2010) Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int 2016: 1314709. [Crossref]
- Ito T, Itakura S, Todorov I, Rawson J, Asari S, et al. (2010) Mesenchymal Stem Cell and Islet Co-Transplantation Promotes Graft Revascularization and Function. Transplantation 89: 1438-1445. [Crossref]
- Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E (2010) Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation 89: 686-693. [Crossref]
- Xiang C, Xie QP (2018) Protection of mouse pancreatic islet function by co-culture with hypoxia pre-treated mesenchymal stromal cells. Mol Med Rep 18: 2589-2598. [Crossref]
- Kim JS, Jung Y, Kim SH, Shin JS, Kim SH, et al. (2019) Vascularization of PLGA-based bio-artificial beds by hypoxia-preconditioned mesenchymal stem cells for subcutaneous xenogeneic islet transplantation. Xenotransplantation 26: e12441. [Crossref]
- Forbes S, Bond AR, Thirlwell KL, Burgoyne P, Samuel K, et al. (2020) Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization. Sci Transl Med 12: eaan5907. [Crossref]
- Kogawa R, Nakamura K, Mochizuki Y (2020) A New Islet Transplantation Method Combining Mesenchymal Stem Cells with Recombinant Peptide Pieces, Microencapsulated Islets, and Mesh Bags. Biomedicines 8: 299. [Crossref]
- Oh BJ, Jin SM, Choi JM, Oh SH, Shim W, et al. (2015) Improved Revascularization of Islet Grafts Using an Angiogenic Monocyte Subpopulation Derived From Spheroid Culture of Bone Marrow Mononuclear Cells. Am J Transplant 15: 1543-1554. [Crossref]
- Oh BJ, Jin SM, Hwang Y, Choi JM, Lee HS, et al. (2018) Highly Angiogenic, Nonthrombogenic Bone Marrow Mononuclear Cell-Derived Spheroids in Intraportal Islet Transplantation. Diabetes 67: 473-485. [Crossref]
- Perez-Basterrechea M, Esteban MM, Alvarez-Viejo M, Fontanil T, Cal S, et al. (2017) Fibroblasts accelerate islet revascularization and improve long-term graft survival in a mouse model of subcutaneous islet transplantation. PloS One 12: e0180695. [Crossref]
- Nilsson J, Fardoos R, Hansen L, Lövkvist H, Pietras K, et al. (2020) Recruited fibroblasts reconstitute the peri-islet membrane: a longitudinal imaging study of human islet grafting and revascularisation. Diabetologia 63: 137-148. [Crossref]
- Cheng Y, Liu YF, Zhang JL, Li TM, Zhao N (2007) Elevation of vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World J Gastroenterol 13: 2862-2866. [Crossref]
- Grapensparr L, Christoffersson G, Carlsson PO (2018) Bioengineering with Endothelial Progenitor Cells Improves the Vascular Engraftment of Transplanted Human Islets. Cell Transplant 27: 948-956. [Crossref]
- Vlahos AE, Cober N, Sefton MV (2017) Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci U S A 114: 9337-9342. [Crossref]
- Samikannu B, Chen C, Lingwal N, Padmasekar M, Engel FB, et al. (2013) Dipeptidyl peptidase IV inhibition activates CREB and improves islet vascularization through VEGF-A/VEGFR-2 signaling pathway. PloS One 8: e82639. [Crossref]
- Luo J, Nguyen K, Chen M, Tran T, Hao J, et al. (2013) Evaluating insulin secretagogues in a humanized mouse model with functional human islets. Metabolism 62: 90-99. [Crossref]
- Jia X, Sharma A, Kumagai-Braesch M, Wernerson AM, Sörenby AK, et al. (2012) Exendin-4 Increases the Expression of Hypoxia-Inducible Factor-1α in Rat Islets and Preserves the Endocrine Cell Volume of Both Free and Macroencapsulated Islet Grafts. Cell Transplant 21: 1269-1283. [Crossref]
- Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, et al. (2017) Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation 83: 24-28. [Crossref]
- Langlois A, Mura C, Bietiger W, Seyfritz E, Dollinger C, et al. (2016) In Vitro and In Vivo Investigation of the Angiogenic Effects of Liraglutide during Islet Transplantation. PLoS One 11: e0147068. [Crossref]
- Nishimura R, Ushiyama A, Sekiguchi S, Fujimori K, Ohuchi N, et al. (2013) Effects of Glucagon-Like Peptide 1 Analogue on the Early Phase of Revascularization of Transplanted Pancreatic Islets in a Subcutaneous Site. Transplantation Proceedings 45: 1892-1894. [Crossref]
- Lee SM, Kim D, Kwak KM, Khin PP, Lim OK, et al. (2020) Comparison of the Effects of Liraglutide on Islet Graft Survival Between Local and Systemic Delivery. Cell Transplant 29: 0963689720971245. [Crossref]
- Pozzilli P, Bosi E, Cirkel D, Harris J, Leech N, et al. (2020) Randomized 52-week Phase 2 Trial of Albiglutide Versus Placebo in Adult Patients With Newly Diagnosed Type 1 Diabetes. J Clin Endocrinol Metab 105: e2192-e2206. [Crossref]
- Johansen NJ, Dejgaard TF, Lund A, Schlüntz C, Frandsen CS, et al. (2020) Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 8: 313-324. [Crossref]
- Ballav C, Dhere A, Kennedy I, Agbaje OF, White S, et al. (2020 Lixisenatide in type 1 diabetes: A randomised control trial of the effect of lixisenatide on post-meal glucose excursions and glucagon in type 1 diabetes patients. Endocrinology, Diabetes & Metabolism 3: e00130.
- Senior PA, Koh A, Yau J, Imes S, Dinyari P, et al. (2017) Sitagliptin plus pantoprazole can restore but not maintain insulin independence after clinical islet transplantation: results of a pilot study. Diabet Med 34: 204-212. [Crossref]
- Johansson M, Olerud J, Jansson L, Carlsson PO (2009) Prolactin Treatment Improves Engraftment and Function of Transplanted Pancreatic Islets. Endocrinology 150: 1646-1653. [Crossref]
- Langlois A, Bietiger W, Seyfritz E, Maillard E, Vivot K, et al. (2011) Improvement of Rat Islet Viability During Transplantation: Validation of Pharmacological Approach to Induce VEGF Overexpression. Cell Transplant 20: 1333-1342. [Crossref]
- Najdahmadi A, Smink AM, de Vos P, Lakey JRT, Botvinick E (2020) Non-Invasive Monitoring of Oxygen Tension and Oxygen Transport Inside Subcutaneous Devices After H2S Treatment. Cell Transplant 29: 0963689719893936. [Crossref]
- Lee EM, Park I, Lee YJ, You YH, Kim JW, et al. (2018) Effect of resveratrol treatment on graft revascularization after islet transplantation in streptozotocin-induced diabetic mice. Islets 10: 25-39. [Crossref]
- Min BH, Shin JS, Kim JM, Kang SJ, Kim HJ, et al. (2018) Delayed revascularization of islets after transplantation by IL-6 blockade in pig to non-human primate islet xenotransplantation model. Xenotransplantation 25: e12374. [Crossref]
- Figueiredo H, Figueroa ALC, Garcia A, Fernandez-Ruiz R, Broca C, et al. (2019) Targeting pancreatic islet PTP1B improves islet graft revascularization and transplant outcomes. Sci Transl Med 11: eaar6294. [Crossref]
- Menger MM, Nalbach L, Roma LP, Körbel C, Wrublewsky S, et al. (2020) Erythropoietin accelerates the revascularization of transplanted pancreatic islets. Br J Pharmacol 177: 1651-1665. [Crossref]
- Menger MM, Nalbach L, Wrublewsky S, Glanemann M, Gu Y, et al. (2020) Darbepoetin-α increases the blood volume flow in transplanted pancreatic islets in mice. Acta Diabetologica 57: 1009-1018. [Crossref]
- Safley SA, Graham ML, Weegman BP, Einstein SA, Barber GF, et al. (2020) Noninvasive Fluorine-19 Magnetic Resonance Relaxometry Measurement of the Partial Pressure of Oxygen in Acellular Perfluorochemical-loaded Alginate Microcapsules Implanted in the Peritoneal Cavity of Nonhuman Primates. Transplantation 104: 259-269. [Crossref]
- Mitchelson F, Safley SA, Gordon K, Weber CJ, Sambanis A (2021) Peritoneal dissolved oxygen and function of encapsulated adult porcine islets transplanted in streptozotocin diabetic mice. Xenotransplantation 28: e12673. [Crossref]
- Komatsu H, Cook C, Wang CH, Medrano L, Lin H, et al. (2007) Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PloS One 12: e0183780. [Crossref]
- Ma Z, Moruzzi N, Catrina S-B, Grill V, Björklund A (2014) Hyperoxia inhibits glucose-induced insulin secretion and mitochondrial metabolism in rat pancreatic islets. Biochemical and Biophysical Research Communications 443: 223-228.
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, et al. (2008) Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 14: 574-578. [Crossref]
- Sun Y, Mao Q, Shen C, Wang C, Jia W (2019) Exosomes from β-cells alleviated hyperglycemia and enhanced angiogenesis in islets of streptozotocin-induced diabetic mice. Diabetes Metab Syndr Obes 12: 2053-2064. [Crossref]
- Rambøl MH, Han E, Niklason LE (2020) Microvessel Network Formation and Interactions with Pancreatic Islets in Three-Dimensional Chip Cultures. Tissue Eng Part A 26: 556-568. [Crossref]
- Vlahos AE, Kinney SM, Kingston BR, Keshavjee S, Won SY, et al. (2020) Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy. Biomaterials 232: 119710. [Crossref]
- Dadheech N, James Shapiro AM (2019) Human Induced Pluripotent Stem Cells in the Curative Treatment of Diabetes and Potential Impediments Ahead. Adv Exp Med Biol 1144: 25-35. [Crossref]
- Nalbach L, Roma LP, Schmitt BM, Becker V, Körbel C, et al. (2021) Improvement of islet transplantation by the fusion of islet cells with functional blood vessels. EMBO Mol Med 13: e12616. [Crossref]
- Xinaris C (2019) Organoids for replacement therapy: expectations, limitations and reality. Curr Opin Organ Transplant 24: 555-561. [Crossref]
- Bredenoord AL, Clevers H, Knoblich JA (2017) Human tissues in a dish: The research and ethical implications of organoid technology. Science 355: eaaf9414. [Crossref]
- Baertschi B, Atlan H, Botbol-Baum M, Bed’hom B, Combrisson H, et al. (2021) Organoids Research: What are the ethical issues? HAL 20: 1-17.


