Abstract
Background and aim: The present work addresses both the early and late risk factors that affect the outcomes of the graft and recipient of living donor liver transplantation (LDLT) as experienced in the biggest center in the world as a step to improve graft and patient survival in the future
Patients and methods: During the period from 1999 to 2004, 505 patients underwent 518 LDLT in the Department of Liver Transplantation and Immunology, Kyoto University Hospital, Japan. The data was collected and analyzed retrospectively
Results: The recipient gender was 261 males (50.4%) and 257 females (49.6%). Pediatric patients (<18 years old) were 230 (44.4%) and adult patients (≥18 years old) were 288 (55.6%). Graft failure occurred in 48/518 patients (9.3%). From the patients with graft failure, 28/48 patients (58.3%) died. Retransplantation was performed in 14/48 patients (29.2%) and 6/48 (12.5%) are waiting for retransplantation. The total number of deaths was 110/518 patients (21.2%). The causes of death were sepsis, septic shock and multiple organ failure (MOF) in 79/110 patients (71.8%), disseminated intravascular coagulopathy (DIC) in 3/110 patients (2.7%) and graft failure in 28/110 patients (25.5%).
Conclusion: Graft recipient body weight ratio (GRWR) <1.0% and recurrence of the previous disease were found to be significant factors leading to graft failure following LDLT. Adult recipients, retransplantation, FHF in pediatrics, UNOS status 2A, right lobe graft, bacterial infection and graft failure have significant effects on patients’ mortality.
Key words
Outcome of LDLT, risk factors, morbidity, mortality, graft failure, retrospective analysis
Introduction
Liver transplantation proved to be a real breakthrough in surgery as the only effective intervention to deal with otherwise fatal liver diseases [1,2]. In Western countries, most of the organs used for transplantation are obtained from brain stem-dead, heart-beating cadaveric donors. However, the number of organs required to satisfy the needs of transplantation far exceeds the number of cadaveric organs available [1,3,4]. This has prompted a relaxation in deceased donor selection criteria and the use of organs from so called "marginal donors". This expansion could not solve the donor shortage and may increase recipient morbidity and mortality [4-7]. The need to resort to living-donor liver donation arose as a natural response to a growing demand for liver transplantation and a constant undersupply of grafts from brain-dead donors [5]. This need is even greater in countries where deceased donors is not allowed based on religious, legal or social basis, such as in many Asian countries [7-10]. The largest series in LDLT all over the world come from Asian centers especially the center in University of Kyoto where most of the innovations and techniques of LDLT are perfected [11].
Patients and methods
Study patients: During the period from 1999 to 2004, 505 patients underwent 518 living donor liver transplantation (LDLT) in the Department of Liver Transplantation and Immunology, Kyoto University Hospital, Japan. The data was collected and analyzed retrospectively.
Preoperative workup of all donors: A meticulous and extensive workup for the donors was done and was reported previously [12]. High resolution Duplex to detect the vascular anatomy, the spiral computed tomography (CT) with intravenous contrast to determine the graft volume and hepatic venous and portal vein anatomy. The anatomy of the biliary tree was provided in all donors by operative cholangiogram which provided sufficient information for safe surgery in most cases. Magnetic resonance (MR) cholangiography was indicated to diagnose bile duct anomalies in patients proved to have portal venous variants by the 3D CT portography. Assessment of the liver for steatosis by CT by measuring the liver-to-spleen (L/S) CT value ratio. Steatosis is suspected in when the L/S ratio is ≤ 1.1 [13,14].
The perioperative management of the recipient and surgical techniques: have been reported elsewhere [15].
Statistical analysis
Statistical calculations for mean values and standard deviations were performed using the SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA). Results were expressed as the mean and standard deviation (SD) after verification of normal distribution or median (interquartile range) for quantitative variables. ANOVA procedure and Pearson correlation coefficient were used to compare between different values. Risk factors for graft loss (early and late) were submitted to Univariate analysis. Categorical variables were analyzed by the chi-square test and numerical variables by the student t-test. A P value of <0.05 was considered statistically significant.
Results
Recipients’ characteristics: The recipient gender, preoperative performance status, Child-Pugh classification, United Network for Organ Share (UNOS) status, ABO blood type compatibility are listed in Table 1. The indications for LDLT were listed in Table 2.
Donors’ characteristics: The number of donors was 519 (one recipient received a dual graft from his twin daughters). Donor’s demographics, donor’s gender, donor/recipient relationship, liver/spleen (L/S) ratio, body mass index (BMI), graft characteristics and graft recipient body weight ratio (GRWR) were expressed in Table 3. Donors with L/S ratio ≤1.1 were considered to have mild to moderate steatosis.
Operative characteristics: (Table 4)
Postoperative complications: The median hospital stay was 52 days, range (18-371). The median period of follow up was 30 months, range (12-72).
Complications were divided into those occurred during the hospital stay, those occurred within 3 months from discharge and those occurred after 3 months from discharge. They were either technical (vascular and biliary) or non-technical (immunological and infectious).
A-Vascular complications: During hospital stay, vascular complications occurred in 33/518 patients (6.4%); hepatic artery thrombosis (HAT) occurred in 18 patients (3.5%), portal vein thrombosis (PVT) occurred in 7 patients (1.4%) and hepatic vein thrombosis (
HVT
) occurred in 6 patients (1.1%). HAT and PVT occurred in 2 patients (0.4%). Within 3 months from discharge, vascular complications occurred in 13 patients (2.5%); PVS occurred in 8 patients (1.5%), PVT in 3 patients (0.6%) and HVS in 2 patients (0.4%). After 3months from discharge, vascular complications occurred in 31 patients (6.0%); PVT occurred in 12 patients (2.3%), PVS in 12 patients (2.3%), HVS in 5 patients (1.0%), both PVS and HVS occurred in one patients (0.2%), and
IVC
stenosis occurred in one patient (0.2%).
Management of vascular complications: The management of HAT was surgical revision in the form of thrombectomy and reanastomosis in 2 patients (0.4%) and balloon angioplasty was the treatment in 16 patients (3.1%). Failure of treatment of HAT occurred in 3 patients who developed graft failure. Two underwent retransplantation and one died. The success rate of the management of HAT was 15/18 patients (83.3%). PVT was treated by continuous injection of urokinase for 48-72 hours. PVS was treated by balloon dilatation followed by systemically injection of heparin for 48 hours. Failure of treatment of portal vein complications occurred in one patients with PVT who developed graft failure and waiting for retransplantation. The success rate of management of portal vein complications was 41/42 patients (97.6%). Combined HAT and PVT which occurred in two patients had resulted in graft failure and death of the two patients.
HVT
was managed by the same way as adopted in PVT while HVS was treated by balloon dilatation angioplasty. Failure of the treatment of hepatic vein complications occurred in two patients that developed graft failure, one with
HVT
died and one with HVS underwent retransplantation. The success rate of management of hepatic vein complications was 11/13 patients (84.6%).
B-Biliary complications: During hospital stay, biliary complications occurred in 99 patients (19.1%); biliary leakage in 74 patients (14.3%), biloma in 7 patients (1.4%), biliary leak followed by biliary stricture in 6 patients (1.1%) and biliary stricture in 12 patients (2.3%). Within 3months from discharge, biliary complications occurred in 36 patients (6.9%); biliary leakage occurred in 3 patients (0.6%), biloma in 2 patients (0.4%), biliary leak followed by stricture in 2 patients (0.4%) and biliary stricture in 29 patients (5.6%). After 3months from discharge, biliary complications occurred in 67 patients (13.0%); biliary leakage in 2 patients (0.4%), biloma in 4 patients (0.8%), biliary leakage followed by biliary stricture in one patient (0.2%) and biliary stricture in 60 patients (11.6%).
Management of biliary complications: Biliary leakage occurred in 79/518 patients (15.4%). Simple follow-up was adopted in 50/79 patients (63.2%). Endoscopic nasobiliary drainage (ENBD) and tube insertion was resorted to in 16/79 patients (20.3%), but failed in one patient who needed Roux-en-Y operation. Roux-en-Y operation was the treatment in 4/79 patients (5.1%). External diversion of already made choledochjejunostomy (external stoma) in 9/79 patients (11.4%). Failure of the treatment of biliary leakage occurred in 7/79 patients (8.9%) who developed graft failure. Six died from septic cholangitis, septicemia and septic shock and one underwent retransplantation. The success rate of management of biliary leakage was 72/79 patients (91.1%). Biliary stricture occurred in 110/518 patients (21.1%), 9 of them were preceded by biliary leak. Simple follow-up was adopted in 2/110 patients (1.8%) who showed no progressive intra-hepatic biliary dilatation as detected by follow-up US. Percutaneous transhepatic cholangiography (
PTC
) with balloon dilatation and drainage (PTCD) and insertion of a tube (external or internal) was adopted in 62/110 patients (56.4%). This management failed in 3 patients who managed by Roux-en-Y operation. Endoscopic retrograde cholangiography (ERC) with bile duct dilatation (ERBD) and stent insertion was resorted to in 45/110 patients (40.9%). This management failed in one patient and was managed by Roux-en-Y operation. Biloma occurred in 13/518 patients (2.5%). They were managed by US guided drainage (percutaneous drainage), which was successful in all patients.
C-Immunological complications: During hospital stay, immunological complications occurred in 177 patients (34.2%); humoral rejection (HR) in 3 patients (0.6%), acute cellular rejection (
ACR
) in 170 patients (32.8%),
ACR
followed by chronic rejection (CR) in 3 patients (0.6%) and
ACR
followed by graft versus host disease (GVHD) in one patients (0.2%). Within 3months from discharge, immunological complications occurred in 30 patients (5.8%);
ACR
occurred in 23 patients (4.4%),
ACR
followed by CR in 2 patients (0.4%), CR in 3 patients (0.6%) and GVHD in 2 patients (0.4%). After 3months from discharge, immunological complications occurred in 37 patients (7.1%);
ACR
occurred in 28 patients (5.4%),
ACR
followed by CR in 3 patients (0.6%) and CR in 6 patients (1.1%)
Management of Immunological complications: HR occurred in 3 patients (0.6%), two of them responded to the conventional immunosuppressive therapy protocol, while one patient did not respond and developed graft failure and died.
ACR
occurred collectively in 230/518 patients (44.4%). The conventional immunosuppressive therapy protocol was successful in the management of
ACR
in 221/230 patients (96.1%). Eight patients with
ACR
ended by CR and one patient developed GVHD. CR occurred collectively in 17/518 patients (3.3%), eight of which were preceded by a history of
ACR
. All patients of CR did not respond to the conventional immunosuppressive therapy protocol and ended by graft failure. Three patients died, 9 patients underwent retransplantation and 5 patients are waiting for retransplantation. GVHD occurred in 3/518 patients (0.6%), one of which was preceded by a history of
ACR
. All patients of GVHD did not respond to the conventional immunosuppressive therapy protocol and ended by graft failure and death. Collectively, immunological complications resulted in graft failure in 21 patients, which denotes that immunosuppressive therapy protocol adopted in our center was successful in 223/244 patients of immunological complications with success rate (91.4%).
D-Infectious complications: During the hospital stay, bacterial infections occurred in 144/518 patients (27.8%). They included bacterial cholangitis in 52 patients, and other bacterial infections (such as wound infection, peritonitis, intra-abdominal abscesses and pneumonia) in 92 patients. They occurred more with patients who underwent Roux-en-Y choledochojejunostomy, patients with prolonged operative time, patients on heavy immunosuppression due to rejection, patients with arterial and biliary complications especially biliary stricture, patients with preoperative FHF and patients admitted in the ICU before the operation. The management of bacterial infections comprised specific antibiotics and operative intervention in cases of peritonitis and intra-abdominal abscesses. Combined bacterial and viral infections occurred in 9/518 patients (1.7%). Combined bacterial and fungal infections occurred in 7/518 patients (1.3%). Specific viral infections occurred in 105/518 patients (20.4%). Cytomegalovirus (CMV) infection occurred in 78/518 patients (15.1%). Epstein Barr virus (EBV) infection occurred in 18/518 patients (3.5%). Combined CMV and EBV infections occurred in 7/518 patients (1.6%). Other herpes viruses occurred in 2/518 patients (0.4%) and responded to acyclovir treatment. Within 3months from discharge, bacterial infections occurred in 7/518 patients (1.3%). They included bacterial cholangitis in 4 patients and other bacterial infections in 3 patients. Combined bacterial and fungal infection occurred in 1/518 patient (0.2%). Specific viral infections occurred in 12/518 patients (2.3%), of which CMV infection occurred in 7/518 patients (1.3%), EBV infection occurred in 4/518 patients (0.8%) and both CMV and EBV infections occurred in 1/518 patient (0.2%). After 3 months from discharge, bacterial infections occurred in 15/518 patients (2.9%). Cholangitis occurred in 11 patients and other bacterial infections occurred in 4 patients. Combined bacterial and viral infections occurred in 1/518 patient (0.2%). Combined bacterial and fungal infections occurred in 1/518 patient (0.2%). Specific viral infections occurred in 11/518 patients (2.1%). All viral infections were EBV infection, two of which (0.4%) were associated with posttransplant lymphoproliferative disease (PTLD). Collectively bacterial infections occurred in 185/518 patients (35.7%) and were responsible for 68 deaths. The risk of mortality from bacterial infection was 68/185 (36.8%) which is significant with (p value 0.001). Collectively specific viral infections occurred in 128/518 patients (24.8%) and were associated with 11 deaths. The risk of mortality from viral infection was 11/128 (8.6%) which is significantly low with (p value 0.0001).
E-Recurrence of the original disease: Hepatitis C virus (HCV) infection recurrence occurred in 33/86 patients. Only one patient developed HCV-related cirrhosis with graft failure and underwent retransplantation. Hepatitis B virus (HBV) infection recurrence was not detected in any of the patients with HBV infection. Hepatocellular carcinoma (
HCC
) recurrence occurred in 9/96 patients (9.4%). Two patients were detected within 3 months from discharge and 7 patients were detected after 3 months from discharge. All the patients died from graft failure and metastases. Autoimmune hepatitis (AIH) recurrence occurred in 2/3 patients (66.7%). One was detected within 3 months from discharge and the other was detected after 3 months from discharge. Both patients are under medical treatment and did not develop graft failure during the period of follow up. Primary sclerosing cholangitis (
PSC
) recurrence occurred in 3/19 patients (15.8%). They were detected after 3 months from discharge, two of them developed graft failure and died. Primary biliary cirrhosis (PBC) recurrence occurred in 1/31 patient (3.2%) and was detected after 3 months from discharge.
F-Graft failure: (Table 5 and Figure 1)
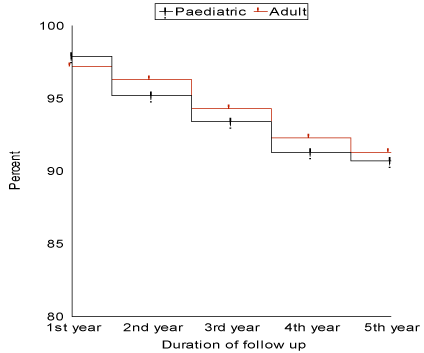
Figure 1: Graft survival curve for pediatrics and adults during the period of follow-up.
Graft failure occurred in 48/518 patients (9.3%), with 1-year, 3-year and 5-year survival of 97.9%, 93.4% and 90.7% respectively. Graft failure occurred during the first year in 11/48 patient (22.9%), during the second year in 12/48 patient (25.0%), during the third year in 11 patients (22.9%), during the fourth year in 10/48 patients (20.8%) and during the fifth year in 4/48 patients (8.4%). Early graft failure (during the first month from the operation) occurred in 10 patients (20.8%), the median was 18 days, range (7-29) and mean ± SD (16.6 ± 6.93). Late graft failure (after the first month from the operation) occurred in 38 patients (79.2%), the median was 5.15 months, range (1.15-54) and mean ± SD (8.71 ± 10.4). Graft failure due to vascular causes occurred in 8/48 patients (16.7%), due to biliary causes 7/48 patients (14.6%), due to immunological causes in 21/48 patients (43.7%) and due to recurrence of the original disease 12/48 patients (25.0%). From the patients with graft failure, 28/48 patients (58.3%) died, of which 17/28 patients (60.7%) died during their hospital stay, 5/28 patients (17.9%) died during the first year from discharge, 4/28 patients (14.3%) died during the second year from discharge and 2/28 patients (7.1%) died during the third year from discharge. Retransplantation was performed in 14/48 patients (29.2%) and 6/48 patients (12.5%) are waiting for retransplantation. Univariate analysis showed two factors to have significant effect in causing graft failure. They are GRWR <1.0% (p value 0.03) and recurrence of the original disease (p value 0.0001).
G-Mortality: (Figure 2)
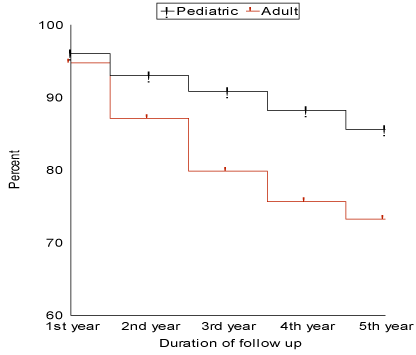
Figure 2: Patient survival curve for pediatrics and adults during the period of follow-up.
The total number of deaths was 110/518 patients (21.2%), with 1-year, 3-year and 5-year survival were 95.25%, 84.36% and 78.22% respectively. The number of deaths in pediatric age group was 33/230 patients (14.3%) while in adults it was 77/288 patients (26.7%) which is statistically significant (p value 0.0002). The causes of death were sepsis, septic shock and multiple organ failure (MOF) in 79/110 patients (71.8%), disseminated intravascular coagulopathy (DIC) in 3/110 patients (2.7%) and graft failure in 28/110 patients (25.5%). The number of deaths that occurred during the hospital stay was 84/110 deaths (76.4%). The median of their hospital stay was 37 days, range (2-510). The causes of death during hospital stay were sepsis and MOF in 64/84 patients (76.2%), DIC in 3/84 patients (3.5%) and graft failure in 17/84 patients (20.3%). The number of deaths occurred after the patient discharge was 26/110 deaths (23.6%). The causes of death were sepsis and MOF in 15/26 patients (57.7%) and graft failure in 11/26 patients (42.3%). The number of deaths that occurred during the first year of the study was 24 (21.8%), during the second year was 29 (26.4%), during the third year was 26 (23.6%), during the fourth year was 18 (16.4%) and during the fifth year was 13 (11.8%). The number of deaths in patients with BA was 16/149 patients (10.8%) which was found to be significantly low (p value 0.002), on the other hand, the number of deaths in other cholestatic liver diseases was 18/71 patients (25.4%) which although higher than in BA, yet, is not significant (p value 0.41). The number of deaths in patients with
HCC
was 26/96 patients (27.08%) and in patients with other tumors was 2/14 patients (14.3%). The number of deaths in patients with chronic hepatocellular diseases was 14/69 patients (20.3%). The number of deaths patients with FHF was 14/59 patients (23.8%). The number of deaths in patients with metabolic liver diseases was 3/21 patients (14.0%). The number of deaths in patients with of retransplantation was 17/37 patients (46.0%), which was found to be significantly high (p value 0.01). The number of deaths in UNOS status 1 was 11/50 (22.0%), UNOS status 2A was 35/131 (26.7%) which is significantly high (p value 0.007), UNOS status 2B was 54/259 (20.8%) and UNOS status 3 was 10/78 (12.8%) which is significantly low (p value 0.03). The number of deaths in patients who received right lobe grafts was 68/259 (26.3%) which was significantly high (p value 0.02) while the number of deaths in patients who received left lateral segment grafts was 20/150 (13.3%) which was significantly low (p value 0.02). The number of deaths complicated by bacterial infections was 68/185 (36.8%) which was significantly high (p value 0.001) while the number of deaths complicated by viral infection was 11/128 (8.6%) which is significantly low (p value 0.0001). The number of deaths in patients who developed graft failure was 28/48 (58.3%) which was significantly high (p value 0.003). Univariate analysis revealed that adult recipients, retransplantation, FHF in pediatrics, UNOS status 2A, right lobe graft, bacterial infection and graft failure have significant effects on patients’ mortality.
Discussion
Liver transplantation (LT) represents the only curative treatment for patients with end-stage liver disease. LT from living donors served to solve cadaveric graft shortage by increasing the donor pool and reducing waiting list mortality [16]. Adequate selection of both the donor and the recipient for LDLT is very important factor to prevent mortality and morbidity including graft failure and the need for retransplantation [2,5]. Few studies have investigated factors leading to graft failure especially with LDLT [17]. Several risk factors for graft loss after LDLT were identified by researchers as donor age [18], MELD score [19-23], intraoperative blood loss [23-25], warm ischemic time [25], and small for size syndrome [26-29]. Other studies investigated the factors responsible for graft loss and retransplantation as hepatic artery thrombosis [30,31], primary non-function [30] and hyperacute rejection [32]. Although improving outcomes and survival after LDLT with meticulous selection criteria [33,34], still no definite criteria can predict graft dysfunction or failure.
After the extension of the indications for LDLT to adults, the problem of "small-for-size graft" was encountered. This problem was either due to small size of the graft "small-for-size graft" or lower quality of the graft "small-for-size syndrome" [9]. In Kyoto University, several techniques are being explored and innovated in an attempt to ameliorate the impact of small-for-size syndrome. These procedures include, auxiliary partial orthotopic living donor liver transplantation (APOLT) [35], but it has a lot of complications and dual liver grafts [36,37], but it needs the presence of two available donors which is not always feasible. Other procedures included the middle hepatic vein (MHV) to the right lobe graft which may not add liver volume but can improve the graft function by prevention of the congestion of the anterior segment [38]. Control of portal pressure and graft perfusion may be adopted to prevent graft injury. This was achieved by innovative techniques such as splenic artery ligation (SPL) [39,40], or permanent portacaval (PC) shunt [41,42]. The present study showed that the graft recipient body weight ratio (GRWR) <1.0% is a significant risk factor for graft failure.
Hepatic steatosis evolved as a risk factor in LDLT. In cadaveric liver transplantation, primary non-function (PNF) occurs in as many as 80% of patients with severe steatosis and therefore grafts with more than 60% steatosis represent a contraindication to transplantation [43-45]. Initial poor function occurs in approximately 30% of patients receiving livers with moderate steatosis [45]. Several groups have shown that grafts with less than 30% steatosis have results similar to those of transplantation with non-steatotic livers [46-49]. In LDLT, Hayashi found that grafts with mild to moderate steatosis demonstrated slight disturbances in early graft function, but were similar to controls [50]. Grafts with severe steatosis were associated with poor function and outcome [50]. Similarly, Soejima evaluated the impact of the degree of steatosis in 60 consecutive donors and recipients. One-year graft survival in the non, mild and moderate steatosis groups was comparable (85.9%, 80.7%, and 80.0%). He concluded that grafts with moderate steatosis (<50%) can be used if the residual volume in the donor is at least 40% to avoid additional risk related to steatosis [51]. In the present study, although the results showed increased risk of graft failure in steatotic grafts, yet, the difference is not statistically significant (p value is 0.42 in BMI ≥25 and 0.31 in L/S ratio ≤1.1). In the light of these results, although the use of grafts with mild to moderate steatosis yielded comparable results with those without steatosis, it appears risky to use such grafts on a routine basis. Their use are justified when they are not associated with other risk factors. Liver biopsy is recommended in countries where there is lack of expertise in the evaluation and diagnosis of hepatic steatosis by radiological means [52]. It also serves to detect other pathological conditions in the donor such as hepatitis, fibrosis or cirrhosis, which may be prevalent in these countries. PNF, which is common in cadaveric liver transplantation, did not occur in the present study most likely due to the short cold ischemic time (CIT) in LDLT.
Hepatic grafts from ABO-incompatible donors are considered as a risk factor in LDLT because of the risk of hyperacute rejection mediated by preformed anti-ABO antibodies [53]. Yandza demonstrated that children less than 2 years old had lower anti-ABO antibodies titers and lower morbidity compared to adults [54]. Gugenheim suggested that ABO-incompatible liver transplantation is only justifiable in adult recipients as an emergency [55]. On the contrary, Hanto reported encouraging results in adults [56]. Gordon found reduced graft survival rate in ABO-incompatible liver transplantation from cadaveric donors. Farges has reported that hyperacute rejection is a complication in adult patients undergoing ABO-incompatible cadaveric liver transplantation [57]. A difference in outcome between adult and pediatric cadaveric liver transplantation has been reported, with pediatric transplants being more successful [58,59]. The reasons for more favorable outcome in children may be related to lower anti-ABO antibodies levels [54], or to an immature complement system [60], thus, the factors that initiate hyperacute rejection are absent during early infancy. The insignificant difference in the results of ABO-incompatible and ABO-compatible grafts as regards the graft failure in the present study can be attributed to the ABO-incompatibility protocol adopted in Kyoto University [61-64]. Although ABO incompatible LDLT may be carried out with relative safety in infants <1 year old using standard immunosuppression, yet, it carries increased risk of graft failure in older patients and should be used only in urgent cases and/or when they are the only available donors.
The donor age and sex as risk factors in liver transplantation were extensively studied in world literatures. Pittsburgh group studied the effect of donor age and sex on the outcome of grafts in cadaveric liver transplantation. They found that the effect of donor age became evident only when they were older than 45 years. They also found that livers from female donors yielded significantly poorer results, with 2-year graft survival of female donor to male recipient, 55% (range 45% to 67%); female donor to female recipient, 64% (range 54% to 77%); male donor to male recipient, 72% (range 66% to 78%); and male donor to female recipient, 78% (range 70% to 88%) [65]. Ikegami found that liver regeneration occurs earlier and proceeds more rapidly in younger livers than in older livers [66]. The results in the current study showed that there is no significant impact of the living donor age on graft survival but suggests better outcome from grafts obtained from younger donors. Grafts obtained from older living donors should be considered as a risk factor and can be used in the absence of other risk factors. As regards the effect of donor sex on graft outcome, the present study showed marginal significance between male and female donors on the graft outcome. Based on our results, old age and female gender should be considered as risk factors in LDLT. They are considered more risky if they are additive such as in old female donors. However, they should not be discarded from donation in the face of shortage of liver donors.
The recipient status at the time of transplantation was studied as a risk factor for both graft and patient survival. Fulminant hepatic failure (FHF), which belongs to United Network for Organ Sharing (UNOS) status 1, has reported mortality rate between 70-95% in children depending on the cause of the disease and the age of the patient [67]. It is crucial that early LDLT be performed without excessive delay in waiting for cadaveric grafts. LDLT as a mode of therapy in FHF in children was first attempted by Tanaka in 1994 [68] who reported on 3 pediatric patients with FHF, all of them received left lobe liver grafts estimated to be 0.8-1.0% of the body weight and were successfully discharged from the hospital. In 1998, the same group reported their results in a series of 11 children with survival rate of 73.0% after a mean follow-up of 28 months (range 13-67 months) [69]. Similar results were reported in pediatric patients from both Eastern [70], Western centers [71]. Mack in 2001 reported a retrospective study on 19 pediatric patients with FHF associated with MOF comparing the results of LDLT to a similar group of patients who received cadaveric allograft donation (CAD). Patients in the LDLT group had markedly improved survival compared with the CAD group. Thirty-day and six-month survival rates of the LDLT group were 88.0% and 63.0% compared with 45.0% and 27.0% in the CAD group, respectively. He suggested that the difference in survival outcomes was related to the fact that LDLT recipients had decreased waiting times for transplantation and decreased cold ischemia time as compared with the CAD recipients [72]. In the lights of these studies, it appears that, the results of LDLT in adult patients with FHF were superior to those in pediatric patients. The difference may be related to the cause of the disease, incidence of rejection and the rate of postoperative complications. Although our results showed worse outcome of grafts in FHF than in cholestatic hepatic diseases (CHD), yet, the difference is insignificant. Our results showed that age is a significant factor in graft failure in patients with FHF. Patients listed as UNOS status 2A showed poor survival outcomes after LDLT. Testa reported the results of 7 patients who had acute-on-chronic liver failure and underwent urgent LDLT using right lobe grafts. Patient and graft survival rates were only 43.0% at a mean follow-up of 15.1 months [73]. Our results showed that UNOS status is not a significant factor in graft failure but is a significant factor in patient mortality after LDLT.
Regarding MELD score as a risk factor for hepatic graft failure. Freeman in 2003 [74], showed that little lifetime benefit for the recipient is achieved with MELD scores less than 10 and perhaps less than 14. The relative risk for post-transplantation mortality starts to increase for candidates with MELD score greater than 25 at the time of transplantation. Therefore, candidates with MELD score between 14-25 would appear to derive the most lifetime benefit. These would seem to be the ideal candidates for adult LDLT. MELD score was applied in recipient selection in the present work. It was found that patients with graft failure had a median MELD score of 21, while those with successful grafts had a median score of 17. Although these values show worse graft outcome in patients with higher MELD score, yet, the difference is marginally significant.
LT in patients with HBV-related liver diseases is followed by a high incidence of recurrent graft infection and subsequent graft failure [75]. After the introduction of the prophylaxis protocol against HBV recurrence using a combination of high-dose of HBIG and lamivudine by Markowitz, the results began to improve [76]. So far, no recurrence of HBV was encountered among survivors in the present study. Therefore, it appears that, the adoption of prophylaxis using lamivudine and post-transplant lamivudine/HBIG is successful in the prevention of HBV recurrence. LT in patients with chronic end-stage liver disease caused by chronic HCV-related cirrhosis are reported to be followed by severe graft damage in cadaveric liver transplantation and even more in LDLT [77,78]. An analysis of the United Network for Organ Sharing database demonstrated significantly diminished 5-year survival after primary transplantation in HCV-positive patients [79]. The transplant group in the University of California at Los Angeles (UCLA) observed recurrent hepatitis in 86.0% of HCV-infected LDLT recipients compared with only 30.0% of cadaveric transplant recipients. The mean time to HCV recurrence was 4.75 months [77]. Similar outcomes were reported from Colombia University group who reported 80.0% of LDLT recipients developed recurrent HCV compared with 58.0% of cadaveric recipients (p value <0.05) with mean follow-up of 19 months [78]. In order to decrease the rate of recurrence and progression of HCV, Kyoto group began a protocol of steroid free immunosuppression in cases of HCV end-stage liver failure recipients. A monotherapy of tacrolimus without mycophenolate mofetil was used because it has been demonstrated that the administration of mycophenolate mofetil could result in a more sever recurrence [80,81]. In the present series, 33 patients of HCV developed HCV recurrence during the period of follow up. Only one patient developed liver cirrhosis with graft failure and underwent retransplantation. These results, in contrast to those published in the world literatures, are considered satisfactory. It is suggested that treatment of LDLT recipients before transplantation may prevent HCV recurrence after transplantation [82]. LT in patients with HCC is a logical approach as it can potentially cure both cirrhosis and HCC [82,83]. One of the major downfalls of cadaveric liver transplantation is that patients must wait for a liver [84,85]. Current selection criteria in cadaveric donor programs are based on a retrospective analysis of tumor characteristics and allocate transplant only to those patients who satisfy Milan criteria [86]. Because LDLT has been a successful and fully accepted treatment for adult patients with end-stage liver disease, interest in this modality as the treatment for HCC has risen. More liberal criteria has been suggested based on the premise that the outcomes of these expanded criteria are similar to those of the more conservative criteria in terms of post-transplantation survival [87-89]. Based on these studies, LDLT was proposed for expanded criteria with little adverse effect on outcome. The pilot study on LDLT for HCC was started in February 1999 in Kyoto University with an approval from the institutional ethical committee with inclusion criteria consisting of otherwise untreatable HCC with complete exclusion of extra-hepatic lesion or macroscopic vascular invasion, irrespective of tumor size and number [89]. The study demonstrated favorable results in the patients fulfilling these selection criteria and concluded that Milan criteria do not seem to be suitable for selecting HCC patients for LDLT [89,90]. Similar results were reported by Yao, who concluded that the Milan criteria may be expanded with excellent survival in LDLT [91]. Although our results showed lower recurrence and better survival rates among patients with HCC within the Milan criteria, yet, they clearly demonstrate that patients with HCC outside the Milan criteria and excluded from cadaveric donor transplantation could survive nearly the same as patients with HCC within Milan criteria in LDLT programs. Therefore, the application of the Milan criteria for all patients with HCC would have denied many patients who can survive after transplantation. From the present study, we can conclude that transplantation is by far the best treatment option for patients with HCC, if a careful search reveals no extra-hepatic disease. In LDLT programs, where the patient has his special living donor, the UNOS and Milan criteria are not necessarily relevant.
Various anatomic variations encountered during LDLT have been detailed through careful dissection of cadaveric livers and examination of hepatic corrosion casts [92-94]. In spite of that, many technical complications are still reported in different centers and may be serious enough to lead to both graft failure and death. HAT is the most common and the most critical vascular complication [95-97]. It occurs in 12.0% of adult and more than 40.0% of pediatric recipients [97,98]. Early diagnosis with prompt intervention is essential because urgent retransplantation is required in most cases. HAS can be observed in 11.0% of liver recipients. It is usually localized at the site of anastomosis. PVT is one of the life threatening complications of liver transplantation, especially when occurs in the immediate postoperative period [99,100]. Thrombosis or stenosis of the portal venous trunk may be observed in 1.0% to 12.5% liver recipients [97,101,102]. Hepatic venous outflow obstruction may occur due to stenosis and/or thrombosis mainly at the anastomotic site or sites. Hepatic venous outflow obstruction may lead to cirrhosis of the graft if such obstruction continues to be present for a long time. The recent introduction of microsurgical techniques for arterial anastomosis in LDLT has greatly reduced the incidence of HAT compared with previous reports [103,104] The present study showed that, vascular thrombosis occurs mostly during hospital stay and may be responsible for early graft failure, while vascular stenosis appeared late in increasing frequency as the period of follow up increases and may be responsible for of late graft failure.
Biliary complications in the form of biliary leaks, bilomas and strictures were reported to occur with an incidence of 10.0% to 30.0% [105-108]. These complications were mostly attributed to ischemia and technical failures [109]. Preventive measures were suggested to decrease the rate of these complications. Greater precaution should be taken to preserve the peribiliary plexus around the resected bile duct in the donor.
Despite recent improvements in immunosuppressive therapy, hepatic allograft rejection remains a major cause of morbidity and graft loss in patients undergoing liver transplantation [110-113]. Our results denoted that HR is a rare complication that occurs early after transplantation and is usually fatal. There is no specific treatment for HR and the only way to save the life of the patient is urgent retransplantation. Therefore, prevention of the condition is essential and may be attained through the selection of ABO-identical or ABO-compatible donors, if possible. CR is usually irreversible and eventually results in the failure of most vascularized solid organ allografts. CR can occur within 3 weeks after liver transplantation and was given the name of acute vanishing bile duct syndrome [114], but commonly occurs after 2 months and usually within 1 year [115,116]. Our results showed that CR of a liver allograft may be reversible to some extent and similarly reported in world literature [117-120]. This reversibility usually occurs before the duct loss or obliterative arteriopathy have become severe. The results also showed that (50.0%) of the patients in the present study who developed CR have experienced one or more episodes of ACR. This may evolve directly from inadequately controlled ACR episodes as reported in other literatures [110,118,121]. GVHD is a rare complication that occurs after liver transplantation. Smith reported 12 cases of GVHD among 1082 liver transplantations done between 1991 and 1998 at Baylor University Medical Center [122]. Our results showed that, GVHD is usually a fatal disease and future approaches should focus on its prevention. In the light of the present study, this can be achieved by HLA matching before LDLT because the donors of all cases of GVHD were of HLA homozygous.
Currently infection is a major cause of death after liver transplantation [123]. The incidence differs considerably among transplantation centers and ranges between 35.0% and 68.0% [124-128]. The lower incidence of bacterial infections in the present study can be attributed to the proper timing of LDLT and the technical expertise of the center in executing the operation in short time with minimal blood loss. The high occurrence of bacterial infections in the early postoperative period was also reported in different centers of liver transplantation [126,128]. This may be explained by the intense immunosuppressive therapy given during this period to prevent rejection and the presence of bacteremia induced by intra-tracheal tubes, urinary catheters and intravenous lines. Additionally, ischemic and biliary complications of the graft occur more during this period. The danger concerning bacterial infections in liver transplantation lies in the difficulty of diagnosis. The usual signs and symptoms of infection may be masked or absent as a result of the patient’s immunosuppressed condition [129]. In addition, clinical manifestations of graft ischemia or graft rejection can mimic those of infection. The incidence of invasive fungal infection was reported to be lower than in other centers which reported a range between 4.0% to 48.0% [130-132]. Similar incidence was reported by other authors [131,133]. Our results denoted that the appearance of fungal infection carries a bad prognosis. Other authors reported a mortality rate of 50.0% to 80.0% in the presence of fungal infection [128,130,133]. They stated that prolonged operative time, increased intra-operative transfusion requirements, choledochojejunostomy, prolonged hospitalization, graft failure and retransplantation, vascular and gastrointestinal complications, recurrent bacterial infections and extended use of antibiotics beyond the first week after transplantation were risk factors for the development of fungal infection. They recommended the prophylactic use of intravenous amphotericin B to prevent postoperative fungal infection in these patients. CMV infection was reported to be 16.3% in the present study. This incidence represent the lower limit recorded in other series which ranged between 18.0% to 40.0% of patients [134,135]. This may be explained by the prophylactic protocol adopted in the present series to anticipate and prevent CMV infection. In the present study, CMV infection constituted >65.0% of all specific viral infections and most of them occurred during hospital stay (91.8%). This is in accord with other authors who found that most of CMV infections occur between 3 and 8 weeks after transplantation [136-138]. The early occurrence of CMV infection may be related to the intense immunosuppressive therapy during this period to prevent or treat episodes of rejection. The real problem in EBV infection is that it is a B cell lymphotropic virus capable of inducing proliferative changes leading to post-transplantation lymphoproliferative disorder [PTLD] and frank lymphoma. This complication occurred in [6.1%] of cases of EBV infection in the current study. Over-immunosuppressive therapy was considered as a risk factor in the development of PTLD, therefore cases of PTLD in the present study responded well to cessation of immunosuppression together with large doses of intravenous acyclovir. Accurate diagnosis and early treatment of EBV infection remains the most important to guard against development of EBV-associated PTLD. Sometimes this management is not sufficient and the disease may result in patient death [139].
In the current study of the present work, recurrence of disease was reported in 48/518 patients (9.3%) which is in accord with that reported by other authors, who found that recurrent disease after liver transplantation occurred in 10.0% of long-term survivors [122,140]. The rate of disease recurrence increases as the period of follow up increases. HCV recurs in all patients after liver transplantation. Certain factors have been reported to result in increased rate of HCV recurrence such as high viral load, increased donor age and in the setting of LDLT rather than cadaveric liver transplantation [141]. Recurrence of HBV was reported to occur in 40.0% of patients who undergo liver transplantation and the virus develops resistance to lamivudine therapy [122]. There is now increasing evidence that with the appropriate therapy, recurrence rates may be significantly reduced in HBV recurrence. In the current study, no case of HBV recurrence was reported. HCC recurrence was reported in 9/96 patients (9.4%) in the present study. Elsewhere, the recurrence rate was reported to be 12.7% [142]. All the patients who developed HCC recurrence in the graft died. Recurrent PSC occurred in 3/19 patients (15.8%) which is nearly similar to the rates reported elsewhere, which amounted to 20.0% [143,144]. All the cases of PSC recurrence were detected after 3 months from discharge. The clinical significance of recurrent PSC is that patients developed biliary strictures in all cases and it can mimic ductopenic rejection in presentation. Recurrent PSC has resulted in 2 patients of graft failure with subsequent death of both patients. Recurrent PBC was reported in 1/31 patients (3.2%) and was detected after 3 months from discharge. This rate is substantially lower than those reported by other authors who found it to occur in 12.0% [145]. Recurrent PBC does not represent any risk to graft failure during the 5-year follow-up. Longer follow-up may be required to determine the clinical significance of recurrent PBC. Recurrent AIH was reported in 2/3 patients (66.7%). Elsewhere, it was reported to occur between 25.0-33.0% [146]. Both patients are under medical treatment and did not develop graft failure during the period of follow-up. Therefore, recurrence of AIH did not represent a risk factor in graft failure during the period of follow up.
Graft failure after transplantation remains an important problem as it leads to patient death or retransplantation. It was reported in 48/518 patients (9.3%) in the current study. This represents the lowest of the recorded graft failure in world literature which ranged between 9.0%-27.0% [147-150]. The Cause of early graft failure in the present study were the same as those reported in cadaveric liver transplantation [65,97,151,152]. Primary non-function (PNF] which was reported to be the commonest cause of early graft failure in cadaveric liver transplantation [150]. did not occur in the present series due to the short cold ischemic time characteristic of LDLT. Factors responsible for the low rate of early graft failure in the present series include; optimum donor selection as regards age, sex, BMI and ABO-compatibility; computer-assisted planning and decision making in donor segmental hepatectomy and optimum GRWR; short cold ischemic time; high level of expertise in the center which performs more than 100 operation per year; and timely detection of vascular, biliary and immunological complications responsible for early graft failure together with early and efficient management which attained more than 90.0% success rate and substantially limited the number of early graft failure. Late graft failure occurred in 38/48 patients [79.7%]. These results are in accord with those reported in world literature [114,153-155]. Most of the underlying causes of late graft failure include patients with CR which were not responding to treatment and patients with disease recurrence which is unavoidable. Therefore, both these complications constitute real problems in liver transplantation.
Retransplantation may be needed to deal with patients for both early and late graft failure. In the present series, retransplantation was resorted to in 37 procedures which constituted [7.1%] performed in 36 patients. The current study concluded that retransplantation is a significant risk factor for patient mortality and a marginal risk factor for graft failure than primary transplantation. In 2003, Rosen found that, the age, serum bilirubin, creatinine, interval following primary transplantation as well as the UNOS status were predictive factors of outcome in patients with retransplantation [140]. In 2004, Postma studied 55 adult patients with retransplantation [156]. He found that, significant pre-transplant risk factors for unfavorable outcome include indications for transplantation other than HAT (especially CR), high creatinine level, high bilirubin level and low prothrombin level (high INR). He also found that, the era of transplantation affected the survival rate; survival at 1-year and 5-years improved from 56.0% and 48.0%, respectively before 1996 to 89.0% and 81.0%, respectively, after 1996. This is obviously related to the experience gained in overcoming the technical difficulties in dissection of the failing graft and its blood vessels. He concluded that survival rate after retransplantation is improving through the years and is presently quite high approaching the results obtained in elective cases of primary transplantation. Further improvement might be achieved by improvement of renal function before the actual retransplantation. Efforts are needed to reduce the risk factors before retransplantation in order to obtain better patient and graft survival. However, this aim may be difficult to obtain because after primary LDLT, donor candidates among the recipient’s family will be limited, forcing the selection of marginal donors (older donors, ABO-incompatible donors, small-for-size or steatotic grafts). This situation is undoubtly responsible for the significant graft failure and patient mortality obtained in cases of retransplantation in the present study.
In the current study, 110 deaths occurred from 518 patients, giving a long-term survival rate over 5 years follow-up to be (78.2%). This is considered acceptable in patients suffering from end-stage fatal liver disease. The two major factors that affects patient’s survival were found to be bacterial infection and graft failure. Most of the mortalities occurred during hospital stay which may be related to the preoperative status of the patient, the operative factors (the duration of the operation, blood loss, blood transfusion, CIT and WIT) and the intense immunosuppression therapy in the immediate postoperative period. Other factor that affects survival of the patients in the present study were the age of the recipient. The inferior results in adult patients compared to pediatric patients may be related to the common complications associated with the right lobe grafts usually performed in adult patients as well as the problems of small-for-size grafts. UNOS status 2 A was found to be significant factor that affects patients survival. Retransplantation also significantly affected the patient survival. This may be related to poor preoperative status, difficult surgery and exposure to the complications of intense immunosuppressive therapy. Right lobe graft was a significant factor in patient mortality. The number of deaths among recipients who received right lobe grafts were 26.3%. This may be due to the high rate of vascular and biliary variations in the right lobe grafts, technical difficulties in the process of transplantation and the high incidence of postoperative complications.
It is important to remember that successful liver transplantation does not return a patient to normal. Rather a new disease "a transplanted liver" replaces the former disease. However, this new state allows patients a chance of both long-term survival and a more normal life style than were possible during the late stages of their liver disease. After liver transplantation patients must take immunosuppressive medications for the remainder of their lives and to be on continuous follow up with their transplant center.
References
- Ahmed A, Keeffe EB (2007) Current indications and contraindications for liver transplantation. Clin Liver Dis 11: 227-247. [Crossref]
- Brown RS Jr (2008) Live donors in liver transplantation. Gastroenterology 134: 1802-1813. [Crossref]
- Coelho JC, de Freitas AC, Matias JE, de Godoy JL, Zeni Neto C, et al. (2007) Donor complications including the report of one death in right-lobe living-donor liver transplantation. Digestive surgery 24(3):191-6.
- Fisher RA, Cotterell AH, Maluf DG, Stravitz RT, Ashworth A, et al. Adult living donor versus deceased donor liver transplantation: a 10-year prospective single center experience. Annals of hepatology 8(4): 298-307.
- Coelho JC, Parolin MB, Baretta GA, Pimentel SK, de Freitas AC, et al. (2005) Donor quality of life after living donor liver transplantation. Arq Gastroenterol 42: 83-88. [Crossref]
- Coelho JC, Trubian PS, Freitas AC, Parolin MB, Schulz GJ, et al. (1992) Cost comparison of cadaveric liver transplantation with living-donor transplantation. Revista da Associacao Medica Brasileira 51(3):158-63.
- Yoshida R, Iwamoto T, Yagi T, Sato D, Umeda Y, et al. (2008) Preoperative assessment of the risk factors that help to predict the prognosis after living donor liver transplantation. World J Surg 32: 2419-2424. [Crossref]
- Ikegami T, Shimada M, Imura S, Arakawa Y, Nii A, et al. (2008) Current concept of small-for-size grafts in living donor liver transplantation. Surg Today 38: 971-982. [Crossref]
- Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, et al. (1999) Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 67: 321-327. [Crossref]
- Uchida Y, Kasahara M, Egawa H, Takada Y, Ogawa K, Ogura Y, et al. Long-term outcome of adult-to-adult living donor liver transplantation for post-Kasai biliary atresia. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(10):2443-8.
- Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K (2003) Living-donor liver transplantation: 12 years of experience in Asia. Transplantation 75: S6-11. [Crossref]
- Yamaoka Y, Morimoto T, Inamoto T, Tanaka A, Honda K, et al. (1995) Safety of the donor in living-related liver transplantation--an analysis of 100 parental donors. Transplantation 59(2): 224-226.
- Hayashi M, Sakamoto S, Kiuchi T, Tanaka K (2002) Current strategies for the use of sub-optimal grafts in living donor liver transplantation. Transplant Proc 34(7): 2567-8.
- Tanaka K, Kiuchi T, Kaihara S (2004) Living related liver donor transplantation: techniques and caution. Surg Clin North Am 84: 481-493. [Crossref]
- Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, et al. (1993) Surgical techniques and innovations in living related liver transplantation. Ann Surg 217: 82-91. [Crossref]
- Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, et al. (2006) Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 43: 345-351. [Crossref]
- Gruttadauria S, di Francesco F, Vizzini GB, Luca A, Spada M, et al. (2009) Early graft dysfunction following adult-to-adult living-related liver transplantation: predictive factors and outcomes. World journal of gastroenterology 15(36): 4556-4560.
- Castellvi JM, Xiol X, Guardiola J, Sabate I, Roca M, et al. (2004) Pretransplantation risk factors for graft loss after liver transplantation in cirrhotic patients; effect of cytomegalovirus serologic status. Transpl Int 17(3): 131-137.
- Ishigami M, Honda T, Okumura A, Ishikawa T, Kobayashi M, et al. (2008) Use of the Model for End-Stage Liver Disease (MELD) score to predict 1-year survival of Japanese patients with cirrhosis and to determine who will benefit from living donor liver transplantation. J Gastroenterol 43(5): 363-368.
- Habib S, Berk B, Chang CC, Demetris AJ, Fontes P, et al. (2006) MELD and prediction of post-liver transplantation survival. Liver Transpl 12: 440-447. [Crossref]
- Brandão A, Fuchs SC, Gleisner AL, Marroni C, Zanotelli ML, et al. (2009) MELD and other predictors of survival after liver transplantation. Clin Transplant 23: 220-227. [Crossref]
- Guo Z, He X, Wu L, Ju W, Hu A, et al. (2010) Model for end-stage liver disease versus the Child-Pugh score in predicting the post-transplant 3-month and 1-year mortality in a cohort of Chinese recipients. Surgery today 40(1): 38-45.
- Tsunematsu I, Ogura Y, Inoue K, Koizumi A, Tanigawa N, et al. (2006) Quantitative survival model for short-term survival after adult-to-adult living donor liver transplantation. Liver Transpl 12(6): 904-911.
- Mor E, Jennings L, Gonwa TA, Holman MJ, Gibbs J, et al. (1993) The impact of operative bleeding on outcome in transplantation of the liver. Surg Gynecol Obstet 176: 219-227. [Crossref]
- Mueller AR, Platz KP, Krause P, Kahl A, Rayes N, Glanemann M, et al. (2000) Perioperative factors influencing patient outcome after liver transplantation. Transpl Int 13 Suppl 1: S158-161.
- Dahm F, Georgiev P, Clavien PA (2005) Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. American journal of transplantation 5(11): 2605-2610.
- Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, et al. (2003) Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl 9: S29-35. [Crossref]
- Man K, Fan ST, Lo CM, Liu CL, Fung PC, et al. (2003) Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg 237: 256-264. [Crossref]
- Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, et al. (2003) Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl 9: 581-586. [Crossref]
- Jeon GS, Won JH, Wang HJ, Kim BW, Lee BM (2008) Endovascular treatment of acute arterial complications after living-donor liver transplantation. Clin Radiol 63: 1099-1105. [Crossref]
- Azzam AZ, Tanaka K. Management of vascular complications after living donor liver transplantation. Hepatogastroenterology. 2012;59(113):182-6.
- Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, et al. (2000) Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg 232: 490-500. [Crossref]
- Marcos A (2000) Right-lobe living donor liver transplantation. Liver Transpl 6: S59-63. [Crossref]
- Lo CM, Fan ST, Liu CL, Yong BH, Wong Y, et al. (2004) Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg 240: 151-158. [Crossref]
- Inomata Y, Kiuchi T, Kim I, Uemoto S, Egawa H, et al. (1999) Auxiliary partial orthotopic living donor liver transplantation as an aid for small-for-size grafts in larger recipients. Transplantation 67: 1314-1319. [Crossref]
- Lee SG, Park KM, Hwang S, Lee YJ, Kim KH, et al. (2002) Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg 25: 277-284. [Crossref]
- Kaihara S, Ogura Y, Kasahara M, Oike F, You Y, et al. (2002) A case of adult-to-adult living donor liver transplantation using right and left lateral lobe grafts from 2 donors. Surgery 131(6): 682-684.
- Lo CM, Fan ST, Liu CL, Wei WI, Lo RJ, et al. (1997) Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 226: 261-269. [Crossref]
- Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, et al. (2003) Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation 75: 1313-1317. [Crossref]
- Lo CM, Liu CL, Fan ST (2003) Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl 9(6): 626-628.
- Boillot O, Delafosse B, Méchet I, Boucaud C, Pouyet M (2002) Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet 359: 406-407. [Crossref]
- Takada Y, Ueda M, Ishikawa Y, Fujimoto Y, Miyauchi H, et al. (2004) End-to-side portocaval shunting for a small-for-size graft in living donor liver transplantation. Liver Transpl 10(6): 807-810.
- Adam R, Reynes M, Johann M, Morino M, Astarcioglu I, et al. (1991) The outcome of steatotic grafts in liver transplantation. Transplant Proc 23: 1538-1540. [Crossref]
- Loinaz C, González EM (2000) Marginal donors in liver transplantation. Hepatogastroenterology 47: 256-263. [Crossref]
- Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, et al. (1993) Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation 55: 807-813. [Crossref]
- Fishbein TM, Fiel MI, Emre S, Cubukc2021 Copyright OAT. All rights reservers with microvesicular fat safely expands the donor pool. Transplantation 64: 248-251. [Crossref]
- Ureña MA, Ruiz-Delgado FC, González EM, Segurola CL, Romero CJ, et al. (1998) Assessing risk of the use of livers with macro and microsteatosis in a liver transplant program. Transplant Proc 30: 3288-3291. [Crossref]
- Briceño J, Solórzano G, Pera C (2000) A proposal for scoring marginal liver grafts. Transpl Int 13 Suppl 1: S249-252. [Crossref]
- Rossi M, De Simone P, Peritore D, Iappelli M, Pretagostini R, et al. (2001) Liver transplantation: expanding the donor pool. Transplant Proc 33: 1307-1309. [Crossref]
- Hayashi M, Fujii K, Kiuchi T, Uryuhara K, Kasahara M, et al. (1999) Effects of fatty infiltration of the graft on the outcome of living-related liver transplantation. Transplant Proc 31(1-2): 403.
- Soejima Y, Shimada M, Suehiro T, Kishikawa K, Yoshizumi T, et al. (2003) Use of steatotic graft in living-donor liver transplantation. Transplantation 76: 344-348. [Crossref]
- Hegab B, Abdelfattah MR, Azzam A, Mohamed H, Al Hamoudi W, et al. (2012) Day-of-surgery rejection of donors in living donor liver transplantation. World J Hepatol 4(11): 299-304.
- Lo CM, Shaked A, Busuttil RW (1994) Risk factors for liver transplantation across the ABO barrier. Transplantation 58: 543-547. [Crossref]
- Yandza T, Lambert T, Alvarez F, Gauthier F, Jacolot D, et al. (1994) Outcome of ABO-incompatible liver transplantation in children with no specific alloantibodies at the time of transplantation. Transplantation 58: 46-50. [Crossref]
- Gugenheim J, Samuel D, Reynes M, Bismuth H (1990) Liver transplantation across ABO blood group barriers. Lancet 336: 519-523. [Crossref]
- Hanto DW, Fecteau AH, Alonso MH, Valente JF, Whiting JF (2003) ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: evidence for accommodation. Liver Transpl 9(1): 22-30.
- Farges O, Kalil AN, Samuel D, Saliba F, Arulnaden JL, et al. (1995) The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplantation 59(8): 1124-1133.
- Belle SH, Beringer KC, Detre KM (1995) An update on liver transplantation in the United States: recipient characteristics and outcome. Clin Transpl [Crossref]
- Varela-Fascinetto G, Treacy SJ, Lillehei CW, Jonas MM, Lund DP, et al. (1999) Long-term results in pediatric ABO-incompatible liver transplantation. Transplant Proc 31(1-2): 467-468.
- Ferriani VP, Barbosa JE, de Carvalho IF (1990) Serum haemolytic classical and alternative pathways of complement in infancy: age-related changes. Acta Paediatr Scand 79(3):322-7.
- Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, et al. (2008) Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology 47: 143-152. [Crossref]
- Kasahara M, Kiuchi T, Takakura K, Uryuhara K, Egawa H, et al. (1999) Postoperative flow cytometry crossmatch in living donor liver transplantation: clinical significance of humoral immunity in acute rejection. Transplantation 67: 568-575. [Crossref]
- Egawa H, Ohmori K, Haga H, Tsuji H, Yurugi K, et al. (2007) B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl 13(4):579-88.
- Kozaki K, Egawa H, Kasahara M, Oike F, Yoshizawa A, Fukatsu A, et al. Therapeutic strategy and the role of apheresis therapy for ABO incompatible living donor liver transplantation. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2005;9(4):285-91.
- Marino IR, Doyle HR, Aldrighetti L, Doria C, McMichael J, Gayowski T, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22(6):1754-62.
- Ikegami T, Nishizaki T, Yanaga K, Shimada M, Kishikawa K, et al. (2000) The impact of donor age on living donor liver transplantation. Transplantation 70: 1703-1707. [Crossref]
- Trey C. The fulminant hepatic failure surveillance study. Brief review of the effects of presumed etiology and age of survival. Can Med Assoc J. 1972;106:Suppl:525-7.
- Tanaka K, Uemoto S, Inomata Y, Tokunaga Y, Ueda M, Tokka A, et al. Living-related liver transplantation for fulminant hepatic failure in children. Transpl Int. 1994;7 Suppl 1:S108-10.
- Hattori H, Higuchi Y, Tsuji M, Inomata Y, Uemoto S, et al. (1998) Living-related liver transplantation and neurological outcome in children with fulminant hepatic failure. Transplantation 65: 686-692. [Crossref]
- Miwa S, Hashikura Y, Mita A, Kubota T, Chisuwa H, Nakazawa Y, et al. Living-related liver transplantation for patients with fulminant and subfulminant hepatic failure. Hepatology. 1999;30(6):1521-6.
- Emre S, Schwartz ME, Shneider B, Hojsak J, Kim-Schluger L, Fishbein TM, et al. Living related liver transplantation for acute liver failure in children. Liver Transpl Surg. 1999;5(3):161-5.
- Mack CL, Ferrario M, Abecassis M, Whitington PF, Superina RA, Alonso EM. Living donor liver transplantation for children with liver failure and concurrent multiple organ system failure. Liver Transpl. 2001;7(10):890-5.
- Testa G, Malagó M, Nadalin S, Hertl M, Lang H, et al. (2002) Right-liver living donor transplantation for decompensated end-stage liver disease. Liver Transpl 8: 340-346. [Crossref]
- Freeman RB. The impact of the model for end-stage liver disease on recipient selection for adult living liver donation. Liver Transpl. 2003;9(10 Suppl 2):S54-9.
- Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, et al. (1991) Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology 13: 619-626. [Crossref]
- Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, et al. (1998) Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology 28: 585-589. [Crossref]
- Ghobrial RM, Amersi F, Farmer DG, Chen P, Anselmo DM, Baquerizo A, et al. Rapid and severe early HCV recurrence following adult living donor liver transplantation [abstract]. Am J Transplant. 2002;2:316A.
- Gaglio PJ, Malireddy S, Russo M, Lapointe-Rudow D, Emond JC, Brown RS. Hepatitis C recurrence in recipients of grafts from living vs cadaveric liver donors [abstract]. Hepatology. 2002;36:265A.
- Forman LM, Lucey MR. Orthotopic liver transplantation for hepatitis C: Analysis of allograft survival using UNOS database [abstract 82]. Am J Transplant. 2001;1(suppl 1):156.
- Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, et al. (2000) HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol 32: 673-684. [Crossref]
- Everson GT, Trotter J (2003) Role of adult living donor liver transplantation in patients with hepatitis C. Liver Transpl 9: S64-68. [Crossref]
- Llovet JM, Bustamante J, Castells A, et al. Liver transplantation for hepatocellular carcinoma. Results of a restrictive policy [abstract]. Hepatology. 1996;24:350A.
- Azzam AZ (2015) Liver transplantation as a management of hepatocellular carcinoma. World J Hepatol 7: 1347-1354. [Crossref]
- Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, et al. (1985) Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 56: 918-928. [Crossref]
- Calvet X, Bruix J, Brú C, Ginés P, Vilana R, et al. (1990) Natural history of hepatocellular carcinoma in Spain. Five year's experience in 249 cases. J Hepatol 10: 311-317. [Crossref]
- Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8(9):765-74.
- Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S (2000) Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer 88: 538-543. [Crossref]
- Fung J, Marsh W (2002) The quandary over liver transplantation for hepatocellular carcinoma: the greater sin? Liver Transpl 8: 775-777. [Crossref]
- Azzam AZ, Tanaka K. Adoption of new selection criteria on living donor liver transplantation for hepatocellular carcinoma and their impact on the outcome. Hepatogastroenterology. 2011;58(112):1873-6.
- Kaihara S, Kiuchi T, Ueda M, Oike F, Fujimoto Y, et al. (2003) Living-donor liver transplantation for hepatocellular carcinoma. Transplantation 75: S37-40. [Crossref]
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-403.
- Emond JC, Renz JF. Surgical anatomy of the liver and its application to hepatobiliary surgery and transplantation. Semin Liver Dis. 1994;14(2):158-68.
- Reichert PR, Renz JF, D'Albuquerque LA, Rosenthal P, Lim RC, et al. (2000) Surgical anatomy of the left lateral segment as applied to living-donor and split-liver transplantation: a clinicopathologic study. Ann Surg 232: 658-664. [Crossref]
- Renz JF, Reichert PR, Emond JC (2000) Hepatic arterial anatomy as applied to living-donor and split-liver transplantation. Liver Transpl 6: 367-369. [Crossref]
- Yamaoka Y, Ozawa K, Tanaka A, Mori K, Morimoto T, et al. (1991) New devices for harvesting a hepatic graft from a living donor. Transplantation 52: 157-160. [Crossref]
- Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212(3):368-75; discussion 75-7.
- Wozney P, Zajko AB, Bron KM, Point S, Starzl TE (1986) Vascular complications after liver transplantation: a 5-year experience. AJR Am J Roentgenol 147: 657-663. [Crossref]
- Pawlak J, Małkowski P, Michałowicz B, Zieniewicz K, Nyckowski P, et al. (1995) [Orthotopic liver transplantation in a patient with primary biliary cirrhosis]. Pol Tyg Lek 50: 53-55. [Crossref]
- Bakthavatsalam R, Marsh CL, Perkins JD, Levy AE, Healey PJ, Kuhr CS. Rescue of acute portal vein thrombosis after liver transplantation using a cavoportal shunt at re-transplantation. Am J Transplant. 2001;1(3):284-7.
- Hirata M, Harihara Y, Hisatomi S, Miura Y, Yoshino H, et al. (2000) A case of esophageal variceal rupture following acute portal vein thrombosis three days after living-related liver transplantation. Transplant Proc 32: 2266-2268. [Crossref]
- Dodd GD, 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994;192(3):657-61.
- Lee J, Ben-Ami T, Yousefzadeh D, Ramirez J, Funaki B, et al. (1996) Extrahepatic portal vein stenosis in recipients of living-donor allografts: Doppler sonography. AJR Am J Roentgenol 167: 85-90. [Crossref]
- Inomoto T, Nishizawa F, Sasaki H, Terajima H, Shirakata Y, et al. (1996) Experiences of 120 microsurgical reconstructions of hepatic artery in living related liver transplantation. Surgery 119: 20-26. [Crossref]
- Hatano E, Terajima H, Yabe S, Asonuma K, Egawa H, et al. (1997) Hepatic artery thrombosis in living related liver transplantation. Transplantation 64: 1443-1446. [Crossref]
- Lerut J, Gordon RD, Iwatsuki S, Esquivel CO, Todo S, et al. (1987) Biliary tract complications in human orthotopic liver transplantation. Transplantation 43: 47-51. [Crossref]
- Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, et al. (1992) Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology 16: 49-53. [Crossref]
- Stratta RJ, Wood RP, Langnas AN, Hollins RR, Bruder KJ, Donovan JP, et al. Diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Surgery. 1989;106(4):675-83; discussion 83-4.
- Heffron TG, Emond JC, Whitington PF, Thistlethwaite JR, Jr., Stevens L, Piper J, et al. Biliary complications in pediatric liver transplantation. A comparison of reduced-size and whole grafts. Transplantation. 1992;53(2):391-5.
- Cardot C, Candinas D, Miza D, Gunson B, Davison S, Murphy MS, et al. Biliary complications after liver transplantation: Birmingham's experience. Transpl Int. 1995;8:133-40.
- Wiesner RH, Ludwig J, van Hoek B, Krom RA. Current concepts in cell-mediated hepatic allograft rejection leading to ductopenia and liver failure. Hepatology. 1991;14(4 Pt 1):721-9.
- Krom RA, Wiesner RH, Rettke SR, Ludwig J, Southorn PA, et al. (1989) The first 100 liver transplantations at the Mayo Clinic. Mayo Clin Proc 64: 84-94. [Crossref]
- Ascher NL, Stock PG, Bumgardner GL, Payne WD, Najarian JS (1988) Infection and rejection of primary hepatic transplant in 93 consecutive patients treated with triple immunosuppressive therapy. Surg Gynecol Obstet 167: 474-484. [Crossref]
- Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9(2):204-9.
- Ludwig J, Wiesner RH, Batts KP, Perkins JD, Krom RA. The acute vanishing bile duct syndrome (acute irreversible rejection) after orthotopic liver transplantation. Hepatology. 1987;7(3):476-83.
- Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, et al. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31(3):792-9.
- Wiesner RH, Batts KP, Krom RA. Evolving concepts in the diagnosis, pathogenesis, and treatment of chronic hepatic allograft rejection. Liver Transpl Surg. 1999;5(5):388-400.
- Demetris AJ, Qian S, Sun H, Fung JJ, Yagihashi A, et al. (1991) Early events in liver allograft rejection. Delineation of sites of simultaneous intragraft and recipient lymphoid tissue sensitization. Am J Pathol 138: 609-618. [Crossref]
- Blakolmer K, Seaberg EC, Batts K, Ferrell L, Markin R, Wiesner R, et al. Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol. 1999;23(11):1328-39.
- Freese DK, Snover DC, Sharp HL, Gross CR, Savick SK, et al. (1991) Chronic rejection after liver transplantation: a study of clinical, histopathological and immunological features. Hepatology 13: 882-891. [Crossref]
- Demetris AJ, Fung JJ, Todo S, McCauley J, Jain A, et al. (1992) Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy--a clinicopathologic study of 96 patients. Transplantation 53: 1056-1062. [Crossref]
- Wiesner RH, Ludwig J, Van Hoek B, Krom R. Chronic Hepatic Allograft Rejection: a review of ductopenic rejection and the vanishing bile duct syndrome. In: Paul LC, Solez K, editors. Organ Transplantation: long-term results. New York: Marcel Dekker; 1992. p. 197-216.
- Yusoff IF, House AK, De Boer WB, Ferguson J, Garas G, Heath D, et al. Disease recurrence after liver transplantation in Western Australia. J Gastroenterol Hepatol. 2002;17(2):203-7.
- Markin RS, Langnas AN, Donovan JP, Zetterman RK, Stratta RJ (1991) Opportunistic viral hepatitis in liver transplant recipients. Transplant Proc 23: 1520-1521. [Crossref]
- Paya CV, Hermans PE, Washington JA 2nd, Smith TF, Anhalt JP, et al. (1989) Incidence, distribution, and outcome of episodes of infection in 100 orthotopic liver transplantations. Mayo Clin Proc 64: 555-564. [Crossref]
- Lumbreras C, Lizasoain M, Moreno E, Aguado JM, Gomez R, et al. (1992) Major bacterial infections following liver transplantation: a prospective study. Hepatogastroenterology 39: 362-365. [Crossref]
- Jacobs F, Van de Stadt J, Bourgeois N, Struelens MJ, De Prez C, Adler M, et al. Severe infections early after liver transplantation. Transplant Proc. 1989;21(1 Pt 2):2271-3.
- Kusne S, Dummer JS, Singh N, Iwatsuki S, Makowka L, Esquivel C, et al. Infections after liver transplantation. An analysis of 101 consecutive cases. Medicine (Baltimore). 1988;67(2):132-43.
- Colonna JO 2nd, Winston DJ, Brill JE, Goldstein LI, Hoff MP, et al. (1988) Infectious complications in liver transplantation. Arch Surg 123: 360-364. [Crossref]
- Kim JH, Perfect JR (1989) Infection and cyclosporine. Rev Infect Dis 11: 677-690. [Crossref]
- Wajszczuk CP, Dummer JS, Ho M, Van Thiel DH, Starzl TE, et al. (1985) Fungal infections in liver transplant recipients. Transplantation 40: 347-353. [Crossref]
- Viviani MA, Tortorano AM, Malaspina C, Colledan M, Paone G, Rossi G, et al. Surveillance and treatment of liver transplant recipients for candidiasis and aspergillosis. Eur J Epidemiol. 1992;8(3):433-6.
- Schröter GP, Hoelscher M, Putnam CW, Porter KA, Starzl TE (1977) Fungus infections after liver transplantation. Ann Surg 186: 115-122. [Crossref]
- Castaldo P, Stratta RJ, Wood RP, Markin RS, Patil KD, et al. (1991) Clinical spectrum of fungal infections after orthotopic liver transplantation. Arch Surg 126: 149-156. [Crossref]
- Wiens M, Schmidt CA, Lohmann R, Oettle H, Blumhardt G, et al. (1993) Cytomegalovirus disease after liver transplantation: diagnostics and therapy. Transplant Proc 25: 2673-2674. [Crossref]
- Stratta RJ, Shaefer MS, Markin RS, Wood RP, Kennedy EM, Langnas AN, et al. Clinical patterns of cytomegalovirus disease after liver transplantation. Arch Surg. 1989;124(12):1443-9; discussion 9-50.
- Sido B, Hofmann WJ, Otto G, Amann K, Arnold JC, et al. (1993) Cytomegalovirus infection of the liver graft early after transplantation: incidence and clinical relevance. Transplant Proc 25: 2671-2672. [Crossref]
- Bronsther O, Makowka L, Jaffe R, Demetris AJ, Breinig MK, et al. (1988) Occurrence of cytomegalovirus hepatitis in liver transplant patients. J Med Virol 24: 423-434. [Crossref]
- Alessiani M, Kusne S, Fung JJ, Torre-Cisneros J, Jain A, et al. (1991) CMV infection in liver transplantation under cyclosporine or FK 506 immunosuppression. Transplant Proc 23: 3035-3037. [Crossref]
- Egawa H, Ohishi T, Arai T, Inomata Y, Uemoto S, Asonuma K, et al. Application of in situ hybridization technique for quantitative assessment of ongoing symptomatic Epstein-Barr virus infection after living related liver transplantation. Clin Transplant. 1998;12(2):116-22.
- Rosen HR, Prieto M, Casanovas-Taltavull T, Cuervas-Mons V, Guckelberger O, et al. (2003) Validation and refinement of survival models for liver retransplantation. Hepatology 38: 460-469. [Crossref]
- Fukumoto T, Berg T, Ku Y, Bechstein WO, Knoop M, et al. (1996) Viral dynamics of hepatitis C early after orthotopic liver transplantation: evidence for rapid turnover of serum virions. Hepatology 24: 1351-1354. [Crossref]
- Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240(3):451-9; discussion 9-61.
- Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30(5):1121-7.
- Gow PJ, Chapman RW (2000) Liver transplantation for primary sclerosing cholangitis. Liver 20: 97-103. [Crossref]
- Balan V, Batts KP, Porayko MK, Krom RA, Ludwig J, et al. (1993) Histological evidence for recurrence of primary biliary cirrhosis after liver transplantation. Hepatology 18: 1392-1398. [Crossref]
- Jeffrey GP, Brind AM, Ormonde DG, Frazer CK, Ferguson J, et al. (1999) Management of biliary tract complications following liver transplantation. Aust N Z J Surg 69: 717-722. [Crossref]
- D'Alessandro AM, Ploeg RJ, Knechtle SJ, Pirsch JD, Stegall MD, et al. (1993) Retransplantation of the liver--a seven-year experience. Transplantation 55: 1083-1087. [Crossref]
- Knechtle S, D'Alessandro A, Reed A, Sollinger H, Pirsch J, et al. (1991) Liver retransplantation: the University of Wisconsin experience. Transplant Proc 23: 1955. [Crossref]
- Shaw BW, Jr., Gordon RD, Iwatsuki S, Starzl TE. Retransplantation of the liver. Semin Liver Dis. 1985;5(4):394-401.
- Fangmann J, Ringe B, Hauss J, Pichlmayr R (1993) Hepatic retransplantation: the Hannover experience of two decades. Transplant Proc 25: 1077-1078. [Crossref]
- Bäckman L, Gibbs J, Levy M, McMillan R, Holman M, et al. (1993) Causes of late graft loss after liver transplantation. Transplantation 55: 1078-1082. [Crossref]
- Quiroga J, Colina I, Demetris AJ, Starzl TE, Van Thiel DH (1991) Cause and timing of first allograft failure in orthotopic liver transplantation: a study of 177 consecutive patients. Hepatology 14: 1054-1062. [Crossref]
- Ludwig J, Batts KP, MacCarty RL (1992) Ischemic cholangitis in hepatic allografts. Mayo Clin Proc 67: 519-526. [Crossref]
- Demetris AJ (1992) Ischemic cholangitis. Mayo Clin Proc 67: 601-602. [Crossref]
- Demetris AJ, Jaffe R, Starzl TE (1987) A review of adult and pediatric post-transplant liver pathology. Pathol Annu 22 Pt 2: 347-386. [Crossref]
- Postma R, Haagsma EB, Peeters PM, van den Berg AP, Slooff MJ (2004) Retransplantation of the liver in adults: outcome and predictive factors for survival. Transpl Int 17: 234-240. [Crossref]


