Abstract
The main thymus function is restricted by thymic epithelial cells. The isolation, storage and evaluation of human thymic samples quality is critical for development of autologous transplantation strategy. In this study, thymic cell samples were prepared by enzymatic digestion of thymus tissue and stored in liquid nitrogen using a cryoprotective medium containing Me2SO and dextran-40. To evaluate the quality of stored thymic cell samples, immediate post-thaw cell viability, cryopreservation efficacy, confluence, cell composition, gene expression and specific aggregation and differentiation in co-culture were assessed. Confluency evaluation was more easily assessed on culture days 7 and 14. Long-term storage of thymic cell samples was unfavorable for CD326+ thymic epithelial cells expressing FOXN1 gene but had no sufficient impact on STRO-1+ mesenchymal cells and fibroblast (FSP+ cells). Frozen-thawed thymic cell samples produced stromal-epithelial monolayer formed by CD326+ thymic epithelial cells, STRO-1+ mesenchymal cells and FSP+ fibroblasts that was effective in co-culture with autologous CD45+ thymocytes in terms of their differentiation into CD4+ and CD8+ thymocytes. Summarizing the data, we conclude that such parameters as the immediate post-thaw cell viability, monolayer formation efficiency and functional activity are critical for quality evaluation of stored in liquid nitrogen thymic cell samples.
Key words
cell phenotype, cryopreservation, gene expression, human thymus, long-term storage, stromal-epithelial cells
Abbreviations
7-AAD: 7-aminoactinomycin D; Abs: Antibodies; CPM: Cryoprotective Medium; FBS: Fetal Bovine Serum; FSP: Fibroblast Surface Protein; FACS: Fluorescein Activated Cell Sorter; TEC: Thymic Epithelial Cells; cTEC: cortical Thymic Epithelial Cells; mTEC: medullary Thymic Epithelial Cells; TESC: Thymic Epithelial Stem Cells; TCS: Thymic Cell Samples
Introduction
Thymus is the major site of T lymphocyte generation and regulation of adaptive immune system. Since, thymectomy is a component of neonatal surgery for congenital heart diseases, it may have multiple adverse consequences for patients such as infections, oncological and autoimmune diseases related to impaired and changed immunological function [1-7]. On the other hand, it provides a great possibility for collection and storage of thymic tissue for autologous transplantation [8]. However, some problems still exist. First of all, how thymic tissue may be effectively stored, what parameters for quality evaluation are optimal and how thymic tissue may be effectively used for transplantation. In general, the main thymus function is restricted by CD326+ thymic epithelial cells (TEC), which are presented by thymic epithelial stem cells (TESC) expressing FOXN1 gene, cortical TEC (cTEC) expressing CD205 gene and medullary TEC (mTEC) expressing AIRE gene [9-14]. Thus, isolation, storage and quality evaluation of thymic cell samples (TCS) is critical for development of TEC-based thymus reconstitution in thymectomized patients. In our previous study, we tested nine cryoprotective media (CPM) compositions, which included penetrating (Me2SO, glycerol) and non-penetrating (dextran-40, sucrose, hydroxyethyl starch) components on the viability and growth efficacy of frozen-thawed human thymic samples to select an optimal CPM suitable for long-term storage of thymic tissue and stromal-epithelial population enriched by TEC [15]. Our primary focus was on thymic tissue obtained from newborns and infants with inherent heart diseases under the age of three years because these patients are the most exposed to immune system alterations following total or partial thymectomy [1-3,7,16,17].
In the present study, our main objective was the further optimization of quality indicators to prepare the ground for future clinical trials resulting from development and optimization of reproducible protocols for isolation, cryopreservation and quality evaluation of human TCS. Immediate survival, cryopreservation efficacy, confluency value, cell composition, mainly for CD326+ TEC, STRO-1+ mesenchymal cells [18,19], FSP+ (fibroblast surface protein) cells [20,21] and CD45+ thymocytes [22] as well as FOXN1 gene expression for TESC [11,12], CD205 gene expression for cTEC [23], AIRE gene expression for mTEC [9,14] and specific aggregation and differentiation in co-culture in vitro, were assessed.
Materials and methods
Thymus tissue preparation and storage
Ninety-three thymuses were received from the Pediatric Department of the Institute of Heart of the Ministry of Health of Ukraine (Kyiv, Ukraine) from patients with congenital heart disease in age from two weeks to three years. Thymuses were removed as a part of standard surgical procedure on heart according to the regulations of the Ministry of Health of Ukraine with informed parental consent for thymic tissue collection and use for research within ThymiStem project. The research was approved by Bioethics Committees of the Institute of Heart and the University “Ukraine”. Thymic samples were prepared and stored in liquid nitrogen as described in our paper [15]. For this study, we selected TCS that were prepared by enzymatic digestion of thymus tissue with collagenase IV (0.125% w/v; Gibco, USA) and DNase I (0.1% w/v; Gibco, USA) [24,25] with our modifications [15] and frozen in a CPM containing 5% dextran-40 solution (95%) + 5% Me2SO (both compounds from Sigma-Aldrich, USA) named CPM-7 [15]. In brief, thymic cell suspensions received by enzymatic digestion were placed into 2 ml round bottom cryovials (Greiner Bio-One, Germany) with 1ml of CPM-7. The cryovials were then moved into a freezer in a styrofoam box to provide gradual temperature drop (from a room temperature to -80°C), and the next day, were moved into Dewars with liquid nitrogen for long-term storage at -196°C. The enzymatically prepared cell suspensions that contained about 90% of thymocytes and about 10% of stromal-epithelial cells were frozen in concentration 1-5 × 107 cells/ml to obtain finally about 1-5 × 106 of stromal-epithelial cells per sample.
Cryopreservation efficacy was evaluated in percentage as the ratio of output viable cell number after thawing to input viable cell number before freezing and multiplied by 100. Number of thymuses that was used for each experiment is specified in legends to figures.
Thymic cell samples thawing
Cryovials with TCS were taken out from liquid nitrogen and put into a water bath at 37°C for 2-3 min. After that, the thawed cells were transferred into 15 ml tube, and 10 ml of Ca++/Mg++- free DPBS with 5% of fetal bovine serum (FBS) and 0.1% DNase I (FACS buffer) were added slowly into the tube. The suspension was washed 3 times by centrifugation at 300 g for 5 min. After washing, pelleted cells were resuspended in FACS buffer or a culture medium for further cell analysis or in vitro culturing. All procedures were carried out at the room temperature under sterile conditions.
Cell viability evaluation by microscopy and flow cytometry
Immediate post-preparation cell viability was measured with trypan blue and 7-aminoactinomycin D (7-AAD) staining by light microscopy and flow cytometry, respectively. For trypan blue staining, cell suspension was prepared in concentration of 1 × 106 cells/ml, and 20 µl of cell suspension was mixed with equivalent value of 0.4% trypan blue solution (Sigma, USA). After staining for 3-5 min, 10 µl of cell suspension was applied to hemocytometer chamber and the percentage of viable cells was counted with light microscopy according to the manufacture instruction.
For cell viability evaluation with 7-AAD staining, the cells were aliquoted up to 1 × 105 cells per 100 μl in FACS tubes, washed 2 times by adding 2 ml of FACS buffer and centrifugation at 300 g for 5 min at the room temperature. The cells were resuspended in 500 µl of FACS buffer, and 5 µl of 7-AAD (BD Pharmingen, USA) were added to each sample. The samples were incubated for 5-10 min in the dark prior to data acquisition, and cell viability was analyzed with flow cytometer FACSCalibur (Becton Dickinson, USA).
Cell phenotype evaluation by flow cytometry
In general, cell phenotype was analized by staining with antibodies (Abs) to CD326 (TEC) and to CD45 (thymocytes) antigenes (PerCP/Cy5.5-labeled mouse IgG2bκ and IgG1κ, respectively) and to STRO-1 (mesenchymal cells) antigen (FITC-labeled mouse IgMλ) (Biolegend, USA), and to FSP (fibroblasts) (PE-labeled recombinant human IgG1) (Miltenyi Biotec, Germany) using flow cytometer FACSCalibur (Becton Dickinson, USA). In some experiments, where it was nessasary, we used an additional set of Abs to CD326 antigen (Alexa Fluor 488-labeled mouse IgG1κ) (Thermo scientific, USA), to CD45 antigen (PE-labeled mouse IgG1κ) (BD Pharmigen, USA), to STRO-1 antigen (PE-labeled mouse IgM) (Abcam, UK), and to CD205 (cTEC) antigen (PE-labeled mouse IgG1κ), to TCRαβ (T cells) antigen (APC-labeled mouse IgG1κ), to CD4 (helper T cells) antigen (PE-labeled rat IgG2bκ) and to CD8 (cytotoxic T cells) antigen (Alexa Fluor 488-labeled mouse IgG1κ) (Biolegend, USA). Cell staining was conducted in accordance with the manufacturer's recommendations. In brief, 105 cells in 100 µl of FACS buffer and 5 µl of Abs were added into each FACS tube to each tested sample. All procedures were performed on ice. Samples were incubated for 30 min at 4-8°�, washed with 1 ml of FACS buffer by centrifugation at 300 g for 5 min at the room temperature. Supernatants were removed, and 500 µl of FACS buffer were added into each tube. The samples were kept at 4-8°� in the dark until analysis. To exclude cell autofluorescence, control non-stained TCS were evaluated before assessment of TCS stained by conjugated Abs.
Thymic cell samples mRNA evaluation by real-time PCR
Total RNA was extracted from TCS using Direct-zol RNA MiniPrep Plus w/ TRI Reagent kit (Zymo Research, USA) according to the manufacturer’s protocol to evaluate mRNA expression of FOXN1, CD205, AIRE and BAX genes by real-time PCR [9,11,23,24]. The quantity of total RNA was measured by NanoDrop 1000 (Thermo Scientific, USA). All samples had an absorption ratio A260/A280 more than 1.8.
For cDNA synthesis, 11.5 µl of DEPC-treated water (ThermoFisher Scientific, USA), 1 µl (200 U) of RevertAid H Minus Reverse Transcriptase (ThermoFisher Scientific, USA), 4 µl of 5x Reaction Buffer (ThermoFisher Scientific, USA), 0.5 µl (20 U) of RiboLock™ RNase Inhibitor (ThermoFisher Scientific, USA), 2 µl of 10 mM dNTP Mix (1 mM final concentration) (ThermoFisher Scientific, USA) and 1 µl (0.5 µg) of Oligo (dT) 18 (ThermoFisher Scientific, USA) were mixed in 0.2 ml Mastercycler Personal tube (Eppendorf, Germany). Finally, 100 ng of the total RNA were added to the tube with reagents for cDNA synthesis. The reverse transcription was initiated by incubation at 25°C for 10 min, and the next stage was performed at 42°C for 90 min. The reaction was terminated by heating to 70°C within 10 min. The cDNA amount was measured by NanoDrop 1000, and the cDNA samples were stored at -20°C for further real-time quantitative PCR analysis.
Quantification of the gene expression was performed by quantitative real-time PCR in triplicate. For this, 12.5 µl of 2x Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, USA), 2 µl (10 pM) of forward and reverse primers each (Metabion, Germany) and 7.5 µl of DNA/RNA-free sterile water (Thermo Scientific, USA) were added in 0.2 ml optical tube. To this mixture, 1 µl of the cDNA obtained from 100 ng of the total RNA was added. The final volume of the reaction mixture should not exceed 25 µl. Standard curves using serial dilution of FOXN1 , CD205 , AIRE and BAX PCR products (1000, 100, 10, 1, 0.1, 0.01, 0.001 aM) were generated and the amount of gene copies from the standard curves in each sample were assessed. The reaction was performed during preliminary denaturation (95°C for 10 min), 40 cycles of real-time PCR (95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec), final elongation (72°C for 80 sec) and melting (55°C for 0.05 sec, 95°C for 0.5 sec) using CFX 96 Real-Time PCR System (Biorad, USA). Next primer’s nucleotide sequences to genes BAX, AIRE, CD205 and FOXN1 were used to evaluate their mRNA expression levels in human thymic samples: for FOXN1 – GAGAGTGGTGCTGGGATGTT (forward) and ATGGGTTTTGGGAAGAGAGG (reverse); for CD205 – TGCGTGGCTATGTCTACTGG (forward) and CTTGCGGGGAAACTCTGC (reverse); for AIRE – GCGGGAGAGGAGGTAAGAG (forward) and AGGACCCACACACAGTAGGG (reverse); for BAX – CTCACCGCCTCACTCACC (forward) and ACCCCCTCAAGACCACTC (reverse). We designed the primers using IDT's PrimerQuest Software (Integrated DNA Technologies, USA) (http://eu.idtdna.com/Primerquest/Home/Index) and checked their specificity with Primer-BLAST program (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Standard curves were generated for each candidate gene using the Ct value that resulted from duplicate serial dilutions of the cDNA obtained from thymic samples. The calculation of mRNA copies number was fulfilled using the formula: X = (υamplified PCR product / mcDNA) × NA, where X – amount of analyzed mRNA copies in total RNA (copy number/ng total RNA); υamplified PCR product – substance amount of amplified cDNA after real-time PCR analysis (aM); mcDNA – cDNA amount in 1 µl put in for real-time PCR analysis (ng); NA – Avogadro’s constant (6.02 × 1023 mol-1).
Stromal-epithelial cell cultures preparation and confluency/cell composition evaluation
A procedure for generation of mouse thymic stromal-epithelial cell cultures was described [26] and modified for human thymus [15]. In brief, with some further modifications, 4 × 107 thymic cells were placed into a 25-cm2 tissue culture flask (T25) (Sarstedt, Germany) with 4 ml of RPMI-1640 (Sigma, USA) culture medium containing 5% FBS (Gibco, USA), 25 mM HEPES (Sigma, USA), 2 mM L-glutamine (ThermoFisher Scientific, USA), 50 U/ml penicillin (ThermoFisher Scientific, USA), and 50 µg/ml streptomycin (ThermoFisher Scientific, USA). Culture flasks were transferred into CO2-incubator with 37oC and 5% CO2 atmosphere. On the next day, the culture medium containing thymocytes was gently aspirated to remove as many non-adherent cells as possible and keep the attached cells, and fresh portion of the culture medium was added. This procedure was repeated also on culture day 3. The cultural medium was changed further in value of 3/4 as soon as became acidic (usually it takes 5-7 days).
The cell growth was evaluated on culture days 3, 7 and 14 by confluency value as described [15] with further modifications. Confluence is commonly used to estimate the adherent cell number in a culture, referring to the proportion of a culture flask surface covered by cells [27]. Visual identification, confluency evaluation and imaging of cell growth in cell cultures were performed with an inverted microscope (Carl Zeiss Axio Vert. A1). Image processing was performed using ZEN light edition 2011 program. The confluency percentage was calculated using free software ImageJ2 program (http://imagej.net/ImageJ2). Value in “area fraction” field was accepted as confluency value of cell monolayer (see the “Summary” file in ImageJ2) [27].
For analysis of cell composition in the formed monolayer culture, supernatant was fully removed from the culture flask (T25) and 2 ml of 0.5% trypsin with 0.2% EDTA (Sigma, USA) warmed up to 37°C were added into the culture flask for 3 min. To inactivate trypsin-EDTA, 5 ml of FACS buffer were added to the culture flask, and cells were gently suspended. Cell suspensions were transferred into 15 ml tubes and centrifuged at 300 g for 5 min. The supernatant was aspirated, and the cells were analyzed by flow cytometry with Abs to CD326, STRO-1, FSP and CD45 surface antigens as described above.
CD45+ cell enrichment by magnetic selection
To receive CD45+ enriched fraction of autologous thymic cells intended for functional testing of stromal-epithelial fraction, thymic tissue was prepared by enzymatic digestion and cell suspensions were frozen in CPM-7 in concentration of 2 × 107 cells/ml as described above. The TCS were stored in liquid nitrogen for 7 days. Before using, the cell samples were thawed as described above, and non-adhesive CD45+ cells were collected using QuadroMACS (Miltenyi Biotec, Germany) following the manufacture’s instruction and as was described previously [10,24] with some modifications. In brief, after thawing and washing, the cells were resuspended in concentration of 1 × 107 cells per 100 µl in FACS buffer, and 5 µl of magnetic beads conjugated with anti-CD45 Abs (Miltenyi Biotec, Germany) were added to every tube. The mixture was incubated for 15 min at 4-8°C, and then washed with FACS buffer by centrifugation at 300 g for 5 min. The samples were resuspended in FACS buffer to have 1 x 107 cells per 100 µl and transferred onto the column. After the solution has passed through the column, 5 ml of FACS buffer were added per column to wash out any remaining unbound cells. The columns were removed from the magnet, and 10 ml of FACS buffer were added into the columns. With the plunger provided, cells were released out from the columns, and all the flow solution was thoroughly collected. The cells were centrifuged at 300 g for 5 min, and the supernatant was aspirated. The viability of the collected cells was evaluated by trypan blue staining, and cell composition was evaluated by flow cytometry with Abs to CD45, CD4, CD8, TCRα/β, CD326, STRO-1 and FSP surface antigens as described above.
Thymic stromal-epithelial cells functionality evaluation in co-culture in vitro
Some co-culture models for functionality evaluation of monolayer cultures were described [11,14,28]. In our experiments, to prepare thymic stromal-epithelial monolayer cultures, TCS stored for 3 days in liquid nitrogen were seeded in 75-cm2 culture flasks (T75) (1 × 107 cells/ml) and cultured as described above for 3 days. Attached cells were collected by trypsin-EDTA treatment (3 ml per flask) as described above. Collected cells were washed twice in 2 ml of FACS buffer by centrifugation at 300 g for 5 min at the room temperature, and cell phenotype was analyzed by flow cytometry with Abs to CD326, STRO-1, FSP and CD45 surface antigens as described above.
For further functional evaluation, 4 × 105 collected adherent cells were seeded into T25 culture flasks with 4 ml of the culture medium and cultured for 1 day for cell attachment. On the next day, autologous CD45+ thymic cell fraction enriched by thymocytes (up to 98%) as described above was added into the culture flasks with adherent thymic stromal-epithelial cell cultures in concentration of 5 × 106 cells/ml (ratio 50:1). The flasks with thymocytes without adherent cells and with adherent cells without thymocytes were used as controls.
Visual identification and imaging of cell interaction in the cell co-cultures were fulfilled on day 2 with the inverted light microscope as described above. The percentage of the specifically interacting cells in co-cultures was calculated as the number of adherent cells that were aggregated at least with five non-adherent cells multiplied by 100 and divided by a total number of adherent stromal-epithelial cells. Cell compositions of non- adherent CD45+ fraction and stromal-epithelial fraction were analyzed separately on co-culture day 2 with Abs to CD326, STRO-1, FSP and CD45 surface antigens (stromal-epithelial fraction) and with Abs to CD45, CD4, CD8, TCRα/β, CD326, STRO-1 and FSP surface antigens (non-adherent CD45+ fraction) by flow cytometry as described above.
Statistics
Experimental data were processed by means of variation statistics with STATISTICA 10 program. Mean (�) and standard error (SE) were used. To compare the parametric data in two groups, t-test was used. When � < 0.05 data were considered as statistically significant.
Results
Effect of thymic cell samples long-term storage on cryopreservation efficacy
In previous study, we tested nine compositions of CPM that contained penetrating (Me2SO, glycerol) and non-penetrating (sucrose, dextran-40, hydroxyethyl starch) cryoprotectants with adding or excluding FBS. The criterions for evaluation of the most appropriate CPM were the percentage of viable cells immediately after thawing as well as their ability to form monolayer of stromal-epithelial cells and to establish long-term cultures of these cells. From these data, the most suitable CPM for freezing of thymic tissue was the combination of dextran-40 and Me2SO (CPM-7) [15]. This combination was preferable because did not include FBS, and presence of penetrative cryoprotectant Me2SO (5%) in low concentration significantly increased the cell viability rate in frozen TCS [15]. Following these arguments, CPM-7 was selected and used for further cryopreservation efficacy evaluation of enzymatically prepared TCS during long-term storage in liquid nitrogen that was calculated on days 3, 7, 14, 30, 180 and more than 1 year (up to 2 years) (Figure 1).
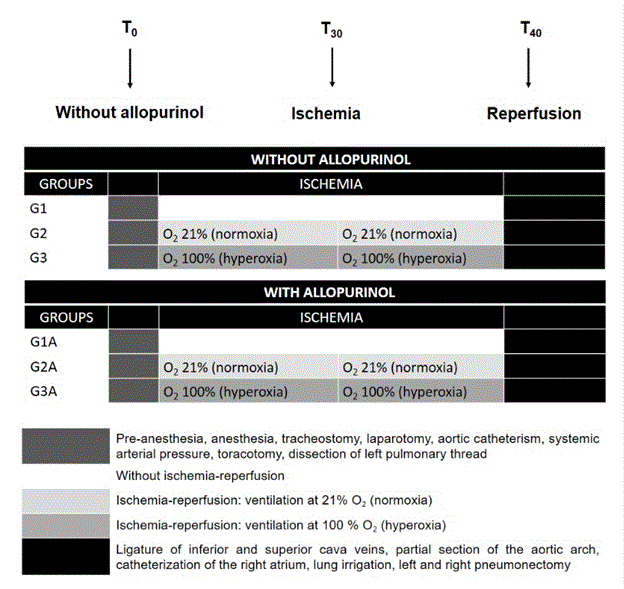
Figure 1. Cryopreservation efficacy of thymic cell samples long-term storage. Thymic cell samples were prepared and stored as described in Materials and methods. Cryopreservation efficacy (A) was calculated as the ratio of output viable cell number after thawing to input viable cell number before freezing (stained by trypan blue) and multiplied by 100%. Cell number (B) and cell viability (C) were analyzed with trypan blue staining by light microscopy directly after thawing and with 7-AAD staining by flow cytometry (D). Data show Mean + SE for n = 10 thymuses (days 3 and 7), n = 5 thymuses (day 14), n = 3 thymuses (days 30 and 90), n = 6 thymuses (day 180) and n = 4 thymuses (>365 days) (one thymic cell sample for each thymus). *p < 0.05 compared to corresponding cell number before freezing.
In this experiment, immediate post-thaw total cell viability was stable during the whole storage time and reached 70-75% as evaluated by trypan blue staining (Figure 1C) and 85-90% as evaluated by 7-AAD staining (Figure 1D) that is comparable with cell viability enzymatically prepared TCS before freezing [15]. Cryopreservation efficacy (Figure 1A) evaluated in percentage as the ratio of output viable cell number after thawing to input viable cell number before freezing and multiplied by 100 (Figure 1B) was also relatively stable for all storage days and ranged from 40% to 60% (Figure 1A) for primarily frozen TCS in concentrations from 20 to 50 × 106 cells per sample (Figure 1B). At this, the absolute losses of cells in process of preparation after thawing could consist about 50% (Figure 1B).
Effect of thymic cell samples long-term storage on cryopreservation efficacy with focus on cell composition
Since, the thymus compoused by different cell types that play a key role in functionality of the organ, we analized the main cell populations dynamics during of TCS long-term storage in CPM-7 next after thawing. In these experiments, cryopreservation efficacy of TEC (CD326+ cells), mesenchymal cells (STRO-1+ cells), fibroblasts (FSP+ cells) and lymphoid cells (CD45+ cells) in common thymic cell suspension was analyzed (Figure 2).
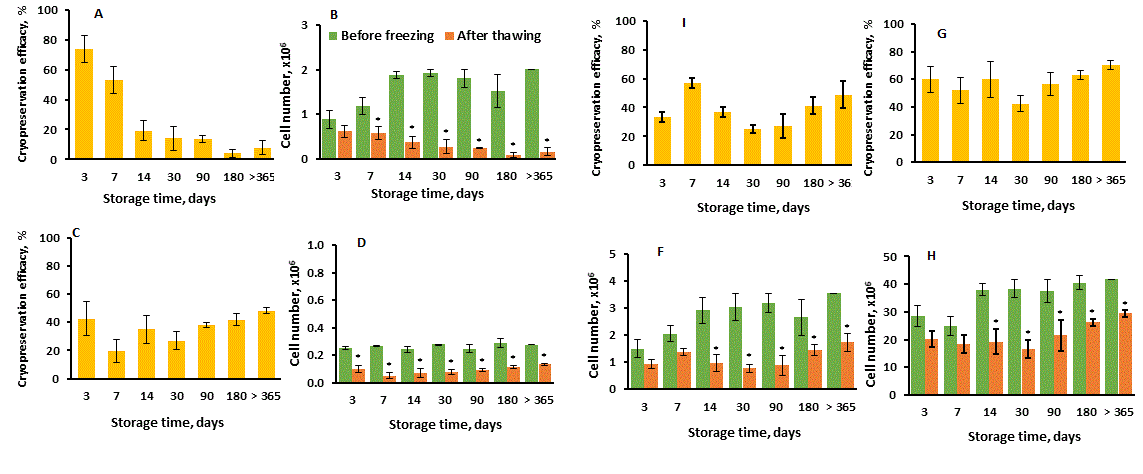
Figure 2. Cryopreservation efficacy of thymic cell samples long-term storage with focus on different thymic cell types. Thymic cell samples were prepared and stored as described in Materials and methods. Cryopreservation efficacy (A, C, I, G) was calculated as in Figure 1. Cell numbers (B, D, F, H) were calculated as the total cell number counted in hemocytometer (Figure 1B) multiplied by a corresponding cell type percentage assessed by flow cytometry and divided by 100%. (A, B) CD326+ epithelial cells; (C, D) STRO-1+ mesenchymal cells; (I, F) FSP+ fibroblasts; (G, H) CD45+ lymphoid cells. Data show Mean + SE for n = 6 thymuses (day 3), n = 7 thymuses (day 7), n = 3 thymuses (days 14, 30, 90, 180 and >365) (one thymic cell samples for each thymus). *p < 0.05 compared to corresponding cell number before freezing.
Previously, we presented some data concerning survival dynamics of CD326+ TEC during shot-term (up to 30 days) and long-term (up to 180 days) TCS storage in CPM-7 [15]. Data presented here show the number of CD326+ TEC as well as cryopreservation efficacy decreased progressively and significantly up to 180 day of cryopreservation and was relatively stable with further prolongation of storage time up to more one year (Figure 2A,B). At the same time, the percentage of CD326+ cells in the general population of prepared thymic cells primarily was only about 8% and decreased finally to about 1.5% (data not showed). Other cell populations (STRO-1+ mesenchymal cells, FSP+ fibroblasts and CD45+ lymphoid cells) showed the relatively stable cryopreservation efficacy on each analyzing day during the all storage time in contrast to CD326+ TEC (Figure 2C,I,G), although the absolute numbers of the survived cells were also significantly decreased (Figure 2D,F,H). These data shows that CD326+ TEC are more sensitive to long-term storage than other types of thymic cells in the same cryoconditions, and they probably require any special conditions for more effective cryopreservation that should be investigated.
Effect of thymic cell samples long-term storage on monolayer formation efficacy
Monolayer formation efficacy by TCS after different terms of storage in liquid nitrogen was evaluated on culture days 3, 7 and 14 (Figure 3).
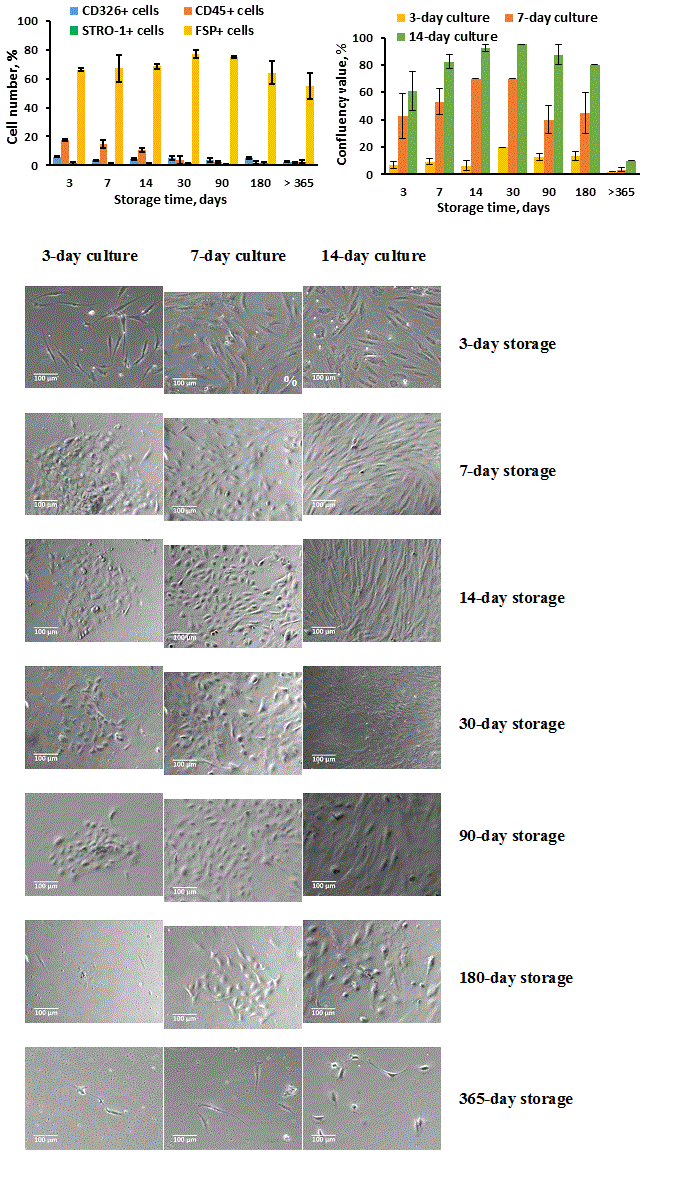
Figure 3. Stromal-epithelial cell monolayer formation efficacy by thymic cell samples stored in liquid nitrogen for different terms. Thymic cell samples were prepared and stored as described in Materials and methods. After freezing-thawing procedure, the cells were seeded into T25 culture flasks in primary concentration of 2 ? 107 cells/ml in 4 ml of the culture medium. The cell growth (B, C) was evaluated by confluency value on culture days 3, 7 and 14, and cell composition (A) was evaluated by flow cytometry on culture day 14. (A, B) Data show Mean + SE for n = 6 thymuses (3-day storage), n = 7 thymuses (7-day storage), n = 3 thymuses (14-, 30-, 90-, 180- and >365-day storage) (one thymic cell sample for each thymus). (C) Images show cell growth efficacy of frozen-thawed thymic cell samples received from one thymus for one representative experiment (x100 magnification).
Data presented here show that prolonged storage (more 180 days) of TCS may lead to significant loss of growth ability. In contrast to this, the TCS storage in liquid nitrogen up to 180 days significantly did not affect confluency efficiency (Figure 3B, C), though the cell losses may consist about 50% immediately after thawing of cryopreserved samples as showed in Figures 1 and 2. The all analyzed samples seeded in culture showed high ability to produce monolayer of stromal-epithelial cells (except 365-day storage) with prevailing growth of FSP+ fibroblast-like cells, specially in 14-day cultures (Figure 3A, C).
Effect of thymic cell samples long-term storage on mRNA expression by epithelial cell population
Since, in common population of thymic stromal-epithelial cells, epithelial cells play specific role in differentiation and positive/negative selection of thymocytes, our focus was on TCS long-term storage effect on the TEC populations activity that was evaluated in terms of mRNA expression by FOXN1 (TESC), CD205 (cTEC) and AIRE (mTEC) genes [9,11,13,23,24]. In these experiments, mRNA expression of BAX gene (cell apoptosis marker) was also measured to evaluate cell survival. Measurements were fulfilled on storage days 180 and 365 for non-cultured TCS and cultured for 14 days after thawing (Figure 4).
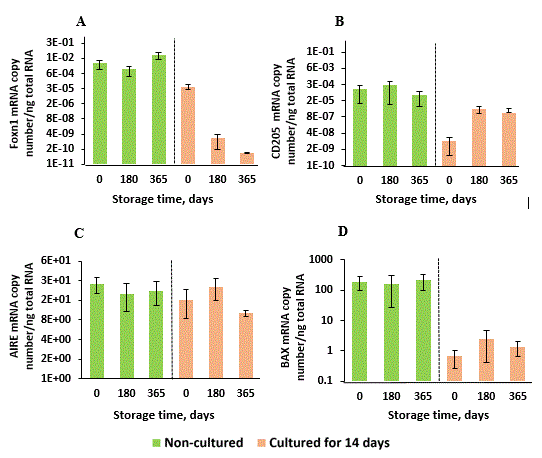
Figure 4. FOXN1, CD205, AIRE and BAX genes mRNA expression by thymic cell samples long-term stored in liquid nitrogen. Thymic cell samples were prepared and stored as described in Materials and methods. After freezing-thawing procedure, the cells were cultured for 14 days as described in Material and methods and in Figure 3. mRNA expression levels were measured before and after culturing of thymic cell suspensions on storage days 0 (non-frozen control), 180 and 365. Data show Mean + SE for n = 3 thymuses (0-day storage), n = 5 thymuses (180-day storage) and n = 3 thymuses (365-day storage) (one thymic cell sample for each thymus).
In these experiments, we did not find any significant changes of mRNA expression in cryopreserved non-cultured samples for all selected genes compared to control non-frozen samples (Day 0). However, long-term storage and frozen-thawing procedure were critically negative for TESC expansion in culture (Figure 4A), and it was not significant for cTEC and mTEC (Figure 4B, C). Relating to BAX gene expression in non-cultured TCS as well as in cultured for 14 days after thawing, long-term storage did not influence cell apoptosis significantly (Figure 4D). Low BAX gene expression in 14-day cell culture samples compared to non-cultured samples can be explained by stromal-epithelial monolayer cleaning from CD45+ non-adhesive thymocytes that consist about 90-95% of cells in primary TCS, and these cells were removed in process of the culture medium changing during 14-day culture after thawing.
Effect of thymic stromal-epithelial cultures on thymocytes differentiation in autologous co-cultures (preliminary data)
To evaluate the specific functionality of thymic stromal-epithelial cells, 1-day monolayer cultures with low percentage of confluency enriched by stromal-epithelial compartment, were prepared from frozen-thawed TCS and co-cultured with autologous CD45+ thymocyte fraction for two additional days to explore the effect of the thymic stromal-epithelial monolayer fraction on thymocyte differentiation. Cell aggregates that included one attached cell and at least five thymocytes, we considered as specific interaction (Figure 5).
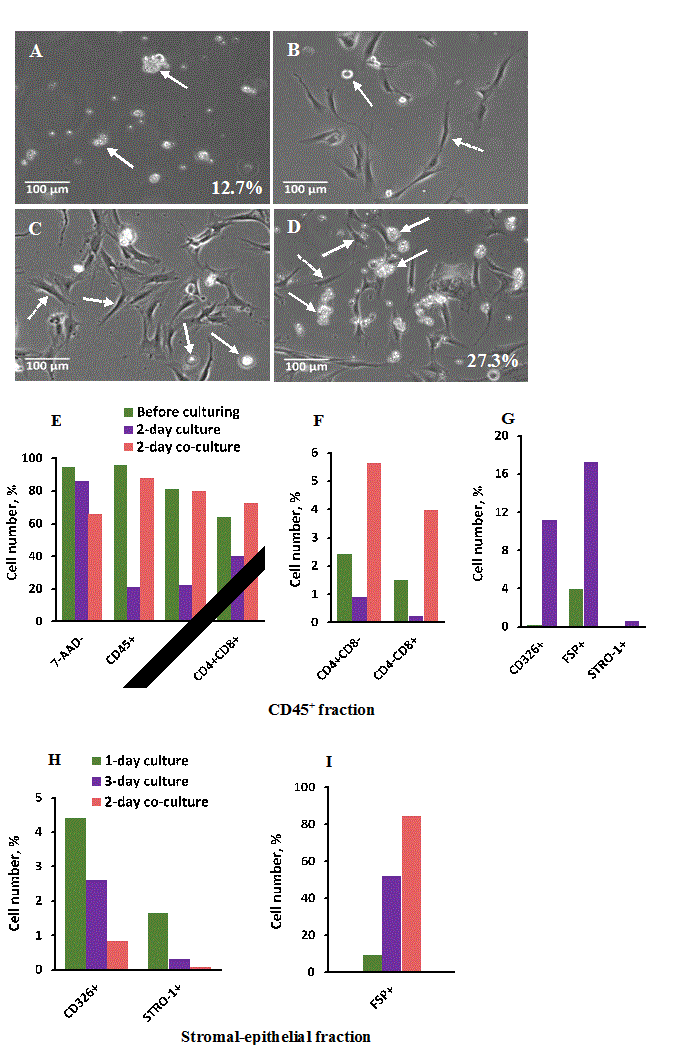
Figure 5. Cell interaction of thymic stromal-epithelial monolayer with autologous thymic CD45+ cells in 2-day co-cultures and changes of their cell compositions during co-culturing. Stromal-epithelial monolayer cultures were obtained from thymic cell samples prepared by enzymatic digestion, stored in liquid nitrogen for 3 days, pre-cultured in vitro for 3 days after thawing to remove non-adherent CD45+ cells and re-cultured for 1 day to establish monolayer culture. Autologous thymic CD45+ cells were obtained by CD45+ positive selection of thymic cell samples on QuadroMACs after 7-day storage in liquid nitrogen. Cell viability and cell composition were evaluated by flow cytometry as described in Materials and methods. (A) 2-day culture of CD45+ enriched non-adherent cell fraction. (B) 1-day and (C) 3-day stromal-epithelial enriched monolayer cultures without addition of autologous thymic CD45+ cells. (D) Stromal-epithelial monolayer culture after 1-day pre-culture without and next 2-day co-culture with autologous thymic CD45+ cells. Solid arrows show single epithelial-like cells in the control cultures (B, C) and specific interaction between epithelial-like cells and autologous thymic CD45+ cells in the control culture (A) and experimental co-culture (D) expressed in percentage. Dashed arrows show fibroblast-like cells (x100 magnification). (E, F, G) Cell composition of CD45+ enriched non-adherent fraction before culture and after 2-day culture without (from A) and with 1-day stromal-epithelial monolayer culture (E, F from D). (H, I) Cell composition of thymic stromal-epithelial monolayer cultures before (from B and C) and after 2-day co-culture with CD45+ enriched fraction (from D). One human thymus was used for the single experiment.
The image demonstrates that the large thymocyte aggregates were formed around epithelial-like cells (showed by solid arrows) in contrast to fibroblast-like cells that did not formed aggregates with thymocytes or included only single thymocytes (showed by dashed arrows). Control images (Fig 5B, C) demonstrate the absence of cell aggregates and viable CD45+ cells in analogous stromal-epithelial monolayer cultures and the presence of single epithelial-like cells (solid arrows) and fibroblast-like cells (dashed arrows). However, 2-day control culture of CD45+ enriched thymic cell suspension included about 12.7% of cell aggregates (Figure 4A) that were formed by contamination with autologous stromal-epithelial cells as defined by flow cytometry (Fig 5G), and the percentage of aggregates correlated well with the percentage of CD326+ TEC in this culture. The percentage of the specifically aggregated cells in the co-cultures was significantly higher and reached up to 27.3% (Figure 5D). However, it poorly correlated with low number (about 1%) of CD326+ TEC in this 2-day monolayer co-culture (Figure 5H) where the majority of monolayer cells (about 80%) were represented by fibroblast-like cells (Figure 5I). It means that not only TEC population specifically interacts with autologous thymocytes and forms aggregates with them. Interestingly that in 2-day co-cultures the percentage of CD326+ TEC as well as STRO-1+ mesenchymal cells significantly decreased compared to 1-day and 3-day monolayer cultures without autologous thymocytes (Figure 5H). In contrast, the percentage of FSP+ fibroblasts increased in co-culture compared to analogous 1-day and 2-day thymocyte-free monolayer cultures (Figure 5I) that demonstrates the autologous thymocytes also support the fibroblast growth.
Critically important changes were found in the CD45+ non-adhesive thymic cell population after 2-day co-culturing with the 1-day autologous monolayer culture compared to 2-day culture of CD45+ cell fraction in the absence of the monolayer culture (Figure 5E,F). Thus, autologous monolayer culture stimulated the percentage increase of the total CD45+ cell fraction as well as its non-mature (CD4+CD8+) and mature (TCRα/β+, CD4+CD8-, CD4-CD8+) sub-fractions. Together, the presented data demonstrate the established monolayer cell cultures are functional in terms of differentiation and probably proliferation and survival supporting of the CD45+ thymocytes.
Discussion
In our previous study [15] we showed that CPM consisting of non-penetrating compound dextran-40 in combination with penetrating compound Me2SO (CPM-7) was the most suitable according to the parameters of immediate post-thaw cell viability and functionality of frozen thymic cell suspensions among eight other combinations including commercial GMP manufactured Stem-CellBanker CPM (Nippon Zenyaku Kogyo, Japan) that was developed specifically for stem cells and keratinocytes storage and also contains Me2SO [29]. Although presence of Me2SO is a negative factor for the quality of stored clinical samples, it is sufficient component of any CPM. This conclusion correlates with data of other authors where CPM with 5-10% Me2SO was considered as an optimal for mesenchymal cells cryoprotection and lower concentrations were not effective as well as some other cryoprotectants [30]. Taking into account possible clinical use of the stored thymic samples, the absence of serum in CPM-7 and low concentration of Me2SO (5%) makes this combination preferable for farther investigation.
Next important parameter for thymic stromal-epithelial cell samples quality is the use of enzymatic digestion before or after freezing of thymic tissue. Because our previous data showed significantly decreased cell growth efficacy after enzymatic digestion of frozen-thawed thymic fragments [15], and these data correlated with other observations for other human tissues [31], we finally used enzymatic digestion procedure only before freezing of thymic cell samples.
Some authors noted the cell viability decreasing during long-term storage of different tissues in liquid nitrogen [32-34]. It could lead to some deceleration of cell proliferation and increase of monolayer formation time by stromal-mesenchymal cells. However, it was not critical for the cell function compared to non-frozen samples [32-36]. Moreover, some investigations showed the high stability of mesenchymal cells viability from cord blood and adipose tissue during long-term storage in liquid nitrogen [30,31,37]. Although, we did not find any investigations of long-term storage effect on thymic stromal-epithelial cells, our data for thymic samples correlate with these common observations at least for STRO-1+ mesenchymal cells. However, as our results showed, the influence of long-term storage on TEC is more complicated. CD326+ TEC had a high viability rate after freezing-thawing procedure, but survived much worse during the long-term storage, especially if it exceeded 30-day and especially 180-day storage. Significant decrease of CD326+ cell cryopreservation efficacy was observed also in a period up to 14-day storage that may be explained by necrosis of frozen TEC, which were damaged by freezing procedure.
Despite of CD326+ TEC progressive destruction during long-term storage, these cells preserve growth activity and together with STRO-1+ mesenchymal cells and FSP+ fibroblasts can successfully form stromal-epithelial monolayer culture even after 6-month storage of TCS in liquid nitrogen. In general, it depends on concentration of viable cells after thawing that can be seeded in primary culture but it should be no lesser than 20-25% as our previous observations showed (data not showed). However, if mesenchymal cells retained their viability, growth and functional potential (differentiation into specific tissues) quite well even after 2-5-year storage [30,31,37], thymic stromal-epithelial cells showed very poor immediate survival and growth activity after 365-day storage; especially it was critical for TESC expressing FOXN1 gene. As our data show, the decline of FOXN1 gene expression may be the result of the long-term storage direct effect on TCS, but it also may be the result of TESC differentiation in non-functional epithelial cells that do not express FOXN1 gene, as described for thymus age-related involution [13].
The immediate survival (cell viability) and growth activity are very important quality parameters of stored TCS but more significant is the specific functionality of their stromal-epithelial compound, which can be evaluated by supporting of non-mature lymphoid compound differentiation into mature cells in co-cultures in vitro . Since, some authors declared very fast loss by TEC of their functional properties in vitro [13], we evaluated both the effect of freezing-thawing procedure and thymic monolayer co-culture preparation on differentiation of autologous frozen-thawed CD45+ thymocytes. This required at least four days for monolayer culture establishment and additionally three days for co-culturing. Our primary data showed this model is correct and clearly demonstrates the differentiation of CD45+ thymocytes into mature single positive CD4+ and CD8+ thymocytes in co-cultures. Similar models were used for functionality evaluation of reprogramed mouse fibroblasts (12-day co-culture) [11] and human thymic mesenchymal stromal cells (1-day autologous co-culture) [28]. Taking into account lower percentage of CD326+ TEC in monolayer culture compared to number of the specific aggregates in co-culture, we can suggest that not only epithelial cells form aggregates with autologous thymocytes but stromal mesenchymal cells [28] and some part of fibroblasts [38,39] may probably participate in this association with autologous CD45+ thymocytes and support their differentiation. Moreover, some FSP+ cells probably are not fibroblasts and could be related to TEC [20] as well as some STRO-1+ cells could be related to thymic endothelial cells [18], which also may contribute to the specific intercellular cooperation in thymic co-cultures.
Summarizing the data presented in this study, we can conclude that monolayer confluency evaluation is optimal on days 7 - 14. Long-term storage of TCS is unfavorable for CD326+ TEC expressing FOXN1 gene but has no significant impact on STRO-1+ mesenchymal cells, FSP+ fibroblasts and CD45+ thymocytes. Frozen-thawed TCS produce stromal-epithelial monolayer that is functionally effective in co-culture with autologous CD45+ thymocytes in terms of their differentiation into single-positive CD4+ and CD8+ thymocytes. Such parameters as the immediate post-thaw cell viability, monolayer formation efficiency and functional activity are critical for quality evaluation of stored TCS.
Acknowledgments
We gratefully acknowledge Dr. Irina Aksyonova and Prof. Boris Todurov from the Institute of Heart, Ministry of Health of Ukraine (Kyiv, Ukraine) for providing of human thymuses and Dr. Natalia Khranovska from the National Cancer Institute, Ministry of Health of Ukraine (Kyiv, Ukraine) for providing of access to Flow cytometer.
Statement of funding
This work has received funding from the European Union’s Seventh Programme for research, technological development and demonstration, within the THYMISTEM project under grant agreement No [602587].
Conflict of interest
The authors declare no conflict of interest.
References
- Afifia A, Rajaa S, Penningtonb D, Tsanga V (2010) For neonates undergoing cardiac surgery does thymectomy as opposed to thymic preservation have any adverse immunological consequences? Interactive Cardio Vascular and Thoracic Surgery 11: 287-291. [Crossref]
- van den Broek T, Delemarre EM, Janssen WJM, Nievelstein RAJ, Broen JC, et al. (2016) Neonatal thymectomy reveals differentiation and plasticity within human naive T cells. J ClinInvest 126: 1126-1136. [Crossref]
- Eysteinsdottir JH, Freysdottir J, Haraldsson A, Stefansdottir J, Skaftadottir I, et al. (2004) The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life. Clin Exp Immunol 136: 349-355. [Crossref]
- Halnon N, Jamienson B, Plunkett M., Kitchen CMR, Pham T, Krogstad P (2005) Thymic function and impaired maintenance of peripheral T cell populations in children with congenital heart disease and surgical thymectomy. Pediatr Res 57: 42-84. [Crossref]
- Mancebo E, Clemente J, Sanchez J, Ruiz-Contreras J, De Pablos P, et al. (2008) Longitudinal analysis of immune function in the first 3 years of life in thymectomized neonates during cardiac surgery. Clin Exp Immunol 154: 375-383. [Crossref]
- Prelog M, Keller M, Geiger R, Brandstätter A, Würzner R, et al. (2009) Thymectomy in early childhood: Significant alterations of the CD4(+)CD45RA(+)CD62L(+) T cell compartment in later life. Clin Immunol 130: 123-132. [Crossref]
- Sauce D, Appay V (2011) Alterted thymic activity in early life: how does it affect the immune system in young adults? Curr Opin Immunol 23: 543-548. [Crossref]
- Cacheiro LH, Glover PL, Perkins EH (1985) Restoration of immune competence with cryopreserved thymus. Transplantation 40: 2-110. [Crossref]
- Baik S, Jenkinson EJ, Lane PJL, Anderson G, Jenkinson WE (2013) Generation of both cortical and Aire+ medullary thymic epithelial compartments from CD205+ progenitors. Eur J Immunol 43: 589-594. [Crossref]
- Bredenkamp N, Jin X, Liu D, O’Neill KE, Manley NR, Blackburn CC (2015) Construction of a functional thymic microenvironment from pluripotent stem cells for the induction of central tolerance. Regen Med 10: 317-329. [Crossref]
- Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC (2014) An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat Cell Biol 16: 902-908. [Crossref]
- Garfin PM, Min D, Bryson JL, Serwold T, Edris B, et al. (2013) Inactivation of the RB family prevents thymus involution and promotes thymic function by direct control of Foxn1 expression. J Exp Med 210: 1087-1097. [Crossref]
- O'Neill KE, Bredenkamp N, Tischner C, Vaidya HJ, Stenhouse FH, et al. 2016) Foxn1 is dynamically regulated in thymic epithelial cells during embryogenesis and at the onset of thymic involution. PLOS One 11: 1-14. [Crossref]
- Passos GA, Mendes-da-Cruz DA, Oliveira EH (2015) Editorial: The Role of Aire, microRNAs and Cell-Cell Interactions on Thymic Architecture and Induction of Tolerance. Front Immunol 6: 615. [Crossref]
- Shichkin VP, Gorbach OI, Zuieva OA, Grabchenko NI, Aksyonova IA, et al. (2017) Effect of cryopreservation on viability and growth efficiency of stromal-epithelial cells derived from neonatal human thymus. Cryobiology 78: 70-79. [Crossref]
- Halnon NJ, Cooper P, Chen DY, Boechat MI, Uittenbogaart CH (2011) Immune dysregulation after cardiothoracic surgery and incidental thymectomy: Maintenance of regulatory T cells despite impaired thymopoiesis. Clin Dev Immunol 2011: 1-11. [Crossref]
- Kurobe H, Tominaga T, Sugano M, Hayabuchi MY, Egawa Y, et al. (2013) Complete but not partial thymectomy in early infancy reduces T-cell-mediated immune response: three-year tracing study after pediatric cardiac surgery. J Thorac Cardiovasc Surg 145: 62-656. [Crossref]
- Ning H, Lin G, Lue TF, Lin CS (2011) Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen. Biochem Biophys Res Commun 413: 353-357. [Crossref]
- Simmons PJ, Torok-Storb B (1991) Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 78: 55-62. [Crossref]
- Kahounová Z, Kurfürstová D, Bouchal J, Kharaishvili G, Navrátil J, et al. (2017) The fibroblast surface markers FAP, anti-fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial-to-mesenchymal transition. Cytometry A. [Crossref]
- Singer KH, Scearce RM, Tuck DT, Whichard LP, Denning SM, et al. (1989) Removal of fibroblasts from human epithelial cell cultures with use of a complement fixing monoclonal antibody reactive with human fibroblasts and monocytes/macrophages. J Invest Dermatol 92: 166-170. [Crossref]
- Lai JC, Wlodarska M, Liu DJ, Abraham N, Johnson P (2010) CD45 regulates migration, proliferation, and progression of double negative 1 thymocytes. J Immunol 185: 2059-2070. [Crossref]
- Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G (2009) Checkpoints in the development of thymic cortical epithelial cells. J Immunol 182: 130-137. [Crossref]
- Gray DHD, Fletcher AL, Hammett M, Seach N, Ueno T et al. (2008) Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Meth 329: 56-66. [Crossref]
- Stoeckle C, Rota IA, Tolosa E, Haller C, Melms A, Adamopoulou E (2013) Isolation of myeloid dendritic cells and epithelial cells from human thymus. J Vis Exp 19: 1-11. [Crossref]
- Shichkin VP, Spivak NY (2006) Cytokine-deficient mice as a model for generation of autologous anti-cytokine monoclonal antibodies. Immunology Letters 102: 148-157. [Crossref]
- Busschots S, O'Toole S, O'Leary JJ, Stordal B (2014) Non-invasive and non-destructive measurements of confluence in cultured adherent cell lines. MethodsX 2: 8-13. [Crossref]
- Azghadi SMR, Suciu M, Gruia AT, Barbu-Tudoran L, Cristea MI et al. (2016) Mesenchymal stromal cells support the viability and differentiation of thymocytes through direct contact in autologous co-cultures. Histochem Cell Biol 146: 153-165. [Crossref]
- Holm F, StroEm S, Inzunza J, Baker D, StroEmberg A-M et al. (2010) An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells. Hum Reprod 25: 1271-1279. [Crossref]
- Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW (2015) Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. J Cryobiol 71: 181-197. [Crossref]
- Badowski M, Muise A, Harris DT (2014) Mixed effects of long-term frozen storage on cord tissue stem cells. Cytotherapy 16: 1313-1321. [Crossref]
- Carvalho KAT, Cury CC, Oliveira L, Cattaned RII, Malvezzi M, et al. (2008) Evaluation of bone marrow mesenchymal stem cell standard cryopreservation procedure efficiency. Transplant Proc 40: 839-841. [Crossref]
- Spurr EE, Wiggins NE, Marsden KA, Lowenthal RM, Ragg SJ (2002) Cryopreserved human haematopoietic stem cells retain engraftment potential after extended (5 – 14 years) cryostorage. Cryobiology 44: 210-217. [Crossref]
- Yang H, Acker JP, Cabuhat M, Letcher B, Larratt L, McGann LE (2005) Association of post-thaw viable CD34+ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Transplant 35: 881-887. [Crossref]
- Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM (2007) Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med 1: 322-324. [Crossref]
- Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama K, et al. (2011) Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. Int J Hematol 93: 99-105. [Crossref]
- Harris DT (2016) Long-term frozen storage of stem cells: challenges and solutions. Journal of Biorepository Science for Applied Medicine 4: 9-20.
- Lilic M, Santori FR, Neilson EG, Frey AB, Vukmanovic S (2002) The role of fibroblasts in thymocyte-positive selection. J Immunol 169: 4945-4950. [Crossref]
- Mohtashami M, Shah DK, Kianizad K, Awong G, Zúñiga-Pflücker JC (2013) Induction of T-cell development by Delta-like 4-expressing fibroblasts. Int Immunol 25: 601-611. [Crossref]





