Abstract
Acne is one of the most common inflammatory dermatoses among adolescents and adults, frequently leaving scars. Improving acne scar appearance represents an important challenge of cosmetic dermatology. This study sought to evaluate cosmetic outcomes of onion-extract-based Mederma®PM Intensive Overnight Scar Cream on acne scars.
Thirty subjects were included in this single-center, Investigator-blinded, randomized, intra-individual comparison versus control study. At screening, mean age of subjects was 29.7 years and 73.3% of subjects were skin phototype III. The Investigator reported overall appearance of targeted test-scar was significantly improved after the 12-week application period, starting from Day 15 ± 2 days. Each individual parameter, texture, softness and rednessof scar after cream was applied, was significantly improved at Days 15 ± 2 days, 57 ± 2 days and 85 ± 2 days respectively. Likewise, more than 57.0% of subjects found overall test-scar appearance slightly better or much better than control-scar from Day 57 ± 2 days and reported statistically significant improvements of individual appearance parameters and particularly scar visibility reduction and smoothness of the test-scar. The product’s cosmetic acceptability was well-appreciated by subjects and local tolerability was excellent. In conclusion, under the study conditions, Mederma®PM Intensive Overnight Scar Cream improved acne scar appearance after 2 weeks of once-nightly application and efficacy was optimal after 2 months of use.
Keywords
atrophic acne scarring, scar appearance, night application, onion extract, cosmetic outcomes
Introduction
Acne vulgaris is one of the most common inflammatory dermatoses affecting nearly all adolescents and a large proportion of adults [1,2], many of whom may experience facial scarring [3]. Some forms of facial scarring have been reported to occur in up to 95.0% of acne patients, 30.0% of whom may be affected by severe scarring [3]. Scarring results from an altered wound healing response to cutaneous inflammation, with inflammatory cell infiltrates found in 77.0% of atrophic scars [4]. Among the different categories of acne scars, atrophic scars are characterized by overall localized reduction in collagen content [5,6]. They are more common than other types of scars and can be further categorized into three subtypes, based on morphologic criteria such as size and depth: ice-pick, boxcar and rolling [5]. Acne scars are often a major source of aesthetic and psychological concerns [7] for the affected subjects and reducing/improving the appearance of these scars represents an important challenge of cosmetic dermatology. Such scars usually need cosmetic care and will not go away completely on their own. Many invasive treatment options are available for improving acne scar appearance, including dermabrasion, laser treatment, punch techniques, fat transplantation, other tissue augmenting agents, needling and combined therapy [8]. However, their high cost and problematic side effects (e.g. erythema, oedema, post-treatment scabbing…) restrict their applications. Topical botanical agents are commercially available and have visible cosmetic outcomes on scars, such as surgical scars [9-11]. Mederma®PM Intensive Overnight Scar Cream (Laboratoire HRA Pharma) is a concentrated onion extract-based cream with Tripeptol™ (a skin nourishing complex with peptides, collagen, and antioxidants), first developed and commercialized in USA. It has shown to reduce scar appearance [12] and it is marketed in many countries worldwide.
Materials and Methods
Study Design and subjects
This was a single-center, Investigator-blinded, randomized, intra-individual comparison versus control study. It was conducted in a single investigational center (Centre Pharmacologie Clinique Appliqué à la Dermatologie, CPCAD) located in the Public University Hospital of Nice, France.
Eligible subjects were aged 18 to 50 years old with skin type I to VI according to Fitzpatrick classification [13] and with a past history of mild to moderate acne, and at least one mild to moderate atrophic acne scar on each side of the face. Efforts were made at baseline by the Investigator to select comparable target scars on each side of the face. This was feasible in some cases but, for some subjects, their scars appearance on either side of the face was different; therefore, for some parameters (namely overall appearance, redness and texture) scar comparability at baseline was not possible. Subjects understood the full nature and purpose of the study and were willing to sign a written consent to participate in the study. Female subjects of childbearing potential had to use one of the reliable methods of contraception during the investigation and agreed not to change it during the study. Excluded from the study were, among others: subjects who were pregnant, breast-feeding or intending to get pregnant; subjects having used within 3 months before inclusion, Retin A or other Rx/OTC Retinyl A, or having planned to use these treatments during the study; having carried out within 6 months before inclusion cosmetic care of acne scarring, including chemical peeling, dermabrasion, laser treatment, punch techniques, fat transplantation, other tissue augmenting agents, needling, or combined therapy, or having planned to use these procedures during the study; subjects with known allergies or sensitivities to ingredients contained in the investigational product (IP); suffering from a serious or progressive disease that could compromise their participation in the study according to the Investigator (e.g., diabetes, cardiac pathologies, hepatic disorder, renal disorder, pulmonary disease, cancer, neurological or psychological disease, inflammatory/immunosuppressive disease).
The maximum study duration participation per subject was 14 weeks: a 2-week screening period followed by a 12-week product application period.
Investigational product
The IP was Mederma® PM Intensive Overnight Scar Cream, manufactured, formulated and provided to the Investigator under the responsibility of HRA Pharma. Mederma® PM Intensive Overnight Scar Cream formulation contains several ingredients like allium cepa, an onion extract, dimethicone and Tripeptol™, a skin nourishing complex with peptides, collagen, and antioxidants. These components work with the skin's night-time regenerative activity to visibly reduce the appearance of scars caused by acne. Onion extract-based formulations have previously been demonstrated to improve the appearance of scars, to reduce complaints about the scar appearance in the scar areas and to provide functional improvement of scarred areas [10-12,14]Mederma® PM Intensive Overnight Scar Cream was applied once nightly.
Ethical aspects
The study protocol was reviewed by the French Ethics Committee (EC) Sud Est VI prior to inclusion of subjects. According to the French Health Authorities and based on French legislation (article L1121-1 of the Public Health Code): as the cosmetic IP is already commercialised in the European Union, its standard use during the study guaranteed its safety of use; interventions were considered to have no risk to the subjects involved. EC’s approval was, therefore, not mandatory. The study was conducted in accordance with the protocol, the Declaration of Helsinki, and in compliance with the applicable local regulatory requirements.
Study procedures and Randomization
Subjects attended 6 visits at the study center: a screening visit (between Day-14 and Day 1), a baseline visit at Week 1/Day 1 ± 2 days during which the IP was dispensed to subjects and 4 tolerance and efficacy evaluation visits (Week 2/Day 15 ± 2 days; Week 4/Day 29 ± 2 days; Week 8/Day 57 ± 2 days and Week 12/Day 85 ± 2 days). Subjects were to apply the IP once nightly for 12 weeks.
At baseline visit, each subject who fulfilled all inclusion/non-inclusion criteria was assigned a randomization number. This randomization number was computer-generated and dispensed in thechronological order of his/her randomization in the trial and no number should be omitted or skipped. The date and time of randomization defined this number, independently of the Site Identification Number that was initially assigned at the screening visit. The randomization list allocated for each subject the side of the face where the IP was to be applied, only on the targeted scar. The control-scar did not receive any product. The study was single-blinded: the randomization list was kept out of the sight of the Investigator by the biomedical research assistant in charge of IP dispensation, therefore, the Investigator did not know on which target scar (left side or right side of face) the IP was applied.
All collected data (participant outcome reports and Investigator evaluations) were recorded in the electronic Case Report Form (e-CRF) by the Investigator or designated person.
Study outcomes
The main cosmetic outcome was to target scars’ overall appearance, as rated by the Investigator after 12 weeks (Day 85) using a 100-point Visual Analogue Scale (VAS) (0 being the worst imaginable case and 100 being the best imaginable case); and by subjects, at each evaluation visit, using a 5-point ordinal scale (appearance on left side: much better / slightly better / no difference / slightly worse / much worse than right side).
Other cosmetic outcomes were: Investigator’s ratings of scar’s overall appearance, at each evaluation visit, using the 100-point VAS; Investigator’s ratings of individual appearance parameters: softness, redness and texture (roughness) using a 11-point ordinal scale (0 = not soft at all/no redness at all/no roughness at all, 10 = maximum softness/redness/roughness); subjects’ ratings of softness, redness, texture and discomfort using a 11-point ordinal scale (0 = not soft at all/no redness at all/no roughness at all/no discomfort at all, 10 = maximum softness/redness/roughness/discomfort); subject reported outcomes (itching, reduction in scar visibility, improvement in scars thickness and smoothness); first visible effects of the tested product (subject’s assessment); subjects’ cosmetic acceptability (18 questions on subject’s perception/acceptability of the product).
Statistical analysis
All statistical analyses were performed using R software version 4.0.2. Data were presented as mean ± standard deviation (SD), number (n) and percentages (%), where appropriate. For each study endpoint, statistical comparisons between product application and non-application sites were performed using a paired Student t-test or a Wilcoxon matched-pairs signed-rank test, at all post-baseline visits and changes from baseline were calculated. Multiplicity was controlled using the Benjamini-Hochberg procedure. The answers to subjects’ overall appearance assessment were analysed and presented after consideration of randomisation results, i.e. which target scar (test or control) on which side of the face. The answers to the cosmetic acceptability questionnaire were summarized in a frequency histogram by category. AEs were presented descriptively. Statistical significance was identified where P value was less than 0.05 with a 2-tailed test.
Results
Subject’s disposition and baseline characteristics
From September 2021 to December 2021, 30 subjects were screened, randomised and completed the study as planned in the protocol. Most subjects were female (n=28; 93.3%) and mean age (± SD) was 29.7 (7.6) years. Among included subjects, 22 (73.3%) had type III skin (Table 1). Physical examination results of subjects by the Investigator at screening were within normal ranges.
Table 1. Subjects’ baseline characteristics
|
|
N=30 |
Age (years) |
|
29.7±7.6 |
Gender |
|
|
|
Female |
28 (93.3) |
|
Male |
2 (6.7) |
Skin type |
|
|
|
Type II |
3 (10.0) |
|
Type III |
22 (73.3) |
|
Type IV |
3 (10.0) |
|
Type V |
1 (3.3) |
|
Type VI |
1 (3.3) |
Values are presented as mean ± SD or numbers (%).
Main cosmetic outcome
The Investigator rated the target scar’s overall appearance at Day 85, after 12 weeks of IP nightly application, using a specific VAS (Figure 1). At Day 1 (baseline), mean score (± CI) of overall appearance of test-scar was lower than control-scar (49.73 ± 7.57 and 57.90 ± 7.85 respectively). At Day 85, overall appearance of the test-scar (58.60 ± 6.78) was statistically significantly (p<0.001) improved as compared to Day 1. No significant change in overall appearance was observed in the control-scar, between Day 1 and Day 85 (57.90 ± 7.85 vs 57.27 ± 6.76) (Figure 1). At Day 85, the improvement from baseline in the overall scar appearance was significantly greater with Mederma® PM Intensive Overnight Scar Cream vs control (mean ± SD change from baseline: 8.87 ± 12.03 vs -0.63 ± 16.56; p=0.001). The Investigator also rated the target scar’s overall appearance at each evaluation visit, from Day 15 to Day 57. Overall appearance of test-scar was statistically significantly improved from Day 15 (53.97 ± 7.27; p=0.035 ) to Day 57 (59.40 ± 6.38; p<0.001) as compared to Day 1. The improvement from baseline in the overall scar appearance was significantly greater with Mederma® PM Intensive Overnight Scar Cream vs control at Day 15 (4.23 ± 10.47 vs 0.80 ± 8.94; p=0.036), at Day 29 (8.00 ± 12.46 vs 2.00 ± 10.83; p=0.005) and at Day 57 (9.67 ± 12.17 vs 3.87 ± 12.51; p=0.003). The overall appearances of test-scars and control-scars were also rated by subjects at each evaluation visit. The number of subjects who reported scar’s overall appearance slightly better or much better with Mederma® PM Intensive Overnight Scar Cream compared to the control-scar, increased over time (Figure 2); from 40.0% at Day 15 to 48.0% at Day 29 and starting at Day 57, more than 57.0% of the subjects found that the overall appearance of the test-scar was slightly better or much better than the control-scar (Figure 2).
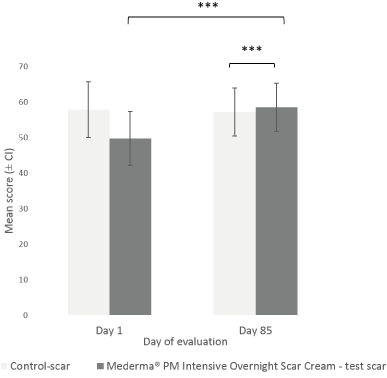
Figure 1. Investigator’s assessment of target scars overall appearance after week 12 (Day 85). Mean scores (± CI, confidence intervals) of overall target scar appearance as rated by the Investigator at Day 1 (baseline) and Day 85. Asterisksindicate statistically significant difference (***, p<0.001) between Mederma® PM Intensive Overnight Scar Cream (black graph) and control (grey graph) scars at Day 85 or test-scars between Day 1 and Day 85
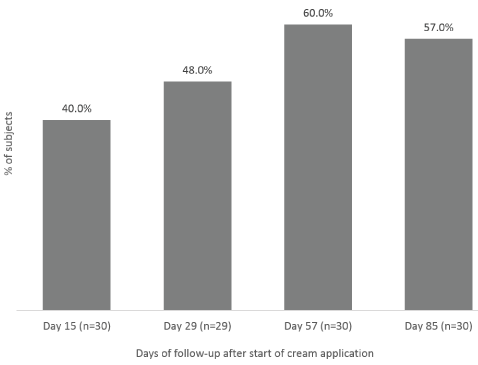
Figure 2. Subjects’ assessment of target scars overall appearance. Percentage of subjects assessing scar overall appearance as “slightly better” or “much better” with Mederma® PM Intensive Overnight Scar Cream compared to the control-scar
Other cosmetic outcomes
At each evaluation visit, the Investigator rated individual parameters of scar appearance; that are softness, redness and texture (roughness) of the target scars. Significant improvements in the test-scar softness were observed at Day 57 (6.03 ± 0.36; p=0.036) and Day 85 (6.67 ± 0.34; p<0.001) with Mederma® PM Intensive Overnight Scar Cream as compared to baseline (Day 1) (5.63 ± 0.35). No significant change in softness was observed for the control-scar. The improvement from baseline in scar softness was significantly greater with Mederma® PM Intensive Overnight Scar Cream compared to control at the end of the 12-week cream application period (1.03 ± 1.10 vs 0.13 ± 0.90; p<0.001). In addition, test-scar redness using Mederma® PM Intensive Overnight Scar Cream significantlydecreased at Day 85 (1.30 ± 0.43) as compared to Day 1 (2.27 ± 0.53; p<0.001). The improvement from baseline in scar redness was significantly greater with Mederma® PM Intensive Overnight Scar Cream than without the cream at Day 85 (-0.97 ± 1.33 vs -0.34 ± 1.04; p=0.045). Finally, statistically significant improvement in target test-scars’ texture (roughness) was observed from Day 57 compared to Day 1 (-1.27 ± 1.34 vs -0.47 ± 1.17; p=0.005). Moreover, at each visit subjects also rated softness, redness, texture and discomfort of the target scars (Figure 3). Significant improvements in test-scar softness from baseline (5.60 ± 0.74) were observed at Day 57 (6.70 ± 0.48; p=0.001) and Day 85 (7.03 ± 0.40; p<0.001). No significant change in softness was observed for the control-scar. The improvement from baseline in scar softness was significantly greater with Mederma® PM Intensive Overnight Scar Cream than without the cream, from Day 57 (1.10 ± 1.65 vs 0.20 ± 1.63; p=0.015) and Day 85 (1.43 ± 1.59 vs -0.17 ± 1.72; p<0.001) (Figure 3a). Likewise, statistically significant improvements in scar redness were observed at Days 15, 57 and 85 with Mederma® PM Intensive Overnight Scar Cream (3.03 ± 0.80 at Day 1; 2.47 ± 0.71 at Day 15 (p=0.022); 2.10 ± 0.66 at Day 57 (p=0.011); 1.90 ± 0.59 at Day 85 (p=0.001)) (Figure 3b). No significant change in redness was observed for the control-scar. The improvement from baseline in scar redness was significantly greater with Mederma® PM Intensive Overnight Scar Cream than without the cream at Day 15 (-0.57 ± 1.28 vs 0.37 ± 1.47; p=0.026) at Day 57 (-0.93 ± 1.89 vs 0.10 ± 1.40; p=0.038) and at Day 85 (-1.13 ± 1.76 vs 0.17 ± 1.44; p=0.006) (Figure 3b). Similarly, scar roughness (texture) decreased with the IP, as reported by subjects, from Day 15 (4.03 ± 0.99 at Day 1; 3.17 ± 0.71 at Day 15 (p=0.012); 3.03 ± 0.63 at Day 29 (p=0.005); 2.67 ± 0.44 at Day 57 (p=0.003); 2.53 ± 0.52 at Day 85 (p=0.001)) (Figure 3c). No significant change in scar texture was observed for the control-scar. The improvement from baseline in scar texture was significantly greater with Mederma® PM Intensive Overnight Scar Cream than without the cream at Day 15 (-0.87 ± 1.83 vs 0.07 ± 1.36; p=0.014) at Day 29 (-1.10 ± 1.93 vs 0.07 ± 1.75; p=0.014); at Day 57 (-1.37 ± 2.28 vs 0.17 ± 1.51; p=0.004) and at Day 85 (-1.50 ± 2.30 vs 0.50 ± 1.78; p<0.001) (Figure 3c). The discomfort of the scar was significantly reduced with Mederma® PM Intensive Overnight Scar Cream from Day 29 (4.60 ± 1.07 at Day 1; 3.66 ± 0.87 at Day 29; 3.07 ± 0.84 at Day 57; 2.90 ± 0.83 at Day 85; p<0.001) (Figure 3d).The discomfort slightly decreased for the control-scar however no statistically significant change was observed except at Day 29 (p=0.043). The improvement in the scar discomfort from baseline was significantly greater with Mederma® PM Intensive Overnight Scar Cream than without cream at Day 29 (-1.10 ± 1.45 vs -0.41 ± 1.05; p=0.046) at Day 57 (-1.53 ± 1.93 vs -0.50 ± 1.68; p=0.008) and at Day 85 (-1.70 ± 2.18 vs -0.30 ± 1.73; p=0.005 (Figure 3d).
Finally, at each evaluation visit, subjects assessed the itching sensation, reduction of scar visibility, improvement of scar’s thickness and smoothness. No itching was reported throughout the duration of the study. The reduction in scar visibility was significantly greater with the IP than without it from Day 15 (0.23 ± 0.18; p=0.017 at Day 15; 0.48 ± 0.23; p<0.001 at Day 29; 1.10 ± 0.27; p<0.001 at Day 57 and 1.33 ± 0.30; p<0.001 at Day 85) (Figure 4a). At the end of the 12-week application period, subjects reported improvement of target scar with Mederma® PM Intensive Overnight Scar Cream while no change was reported for control-scar.Target scar’s thickness improvement scores at all post-baseline visits were significantly greater with Mederma® PM Intensive Overnight Scar Creamthan without it (0.27 ± 0.21; p=0.018 at Day 15; 0.55 ± 0.23; p<0.001 at Day 29, 0.83± 0.28; p<0.001 at Day 57; 1.07 ± 0.32 at Day 85; p<0.001) (Figure 4b). The subjects did not notice any improvement from baseline in control-scar thickness. Likewise, test-scar smoothness improvement was significantly greater than control-scar at all post-baseline visits (0.53 ± 0.26; p<0.001 at Day 15; 0.66 ± 0.24; p<0.001 at Day 29; 1.07 ± 0.32; p<0.001 at Day 57; 1.43 ± 0.32; p<0.001 at Day 85) (Figure 4c) and no change in smoothness was reported for control-scar.
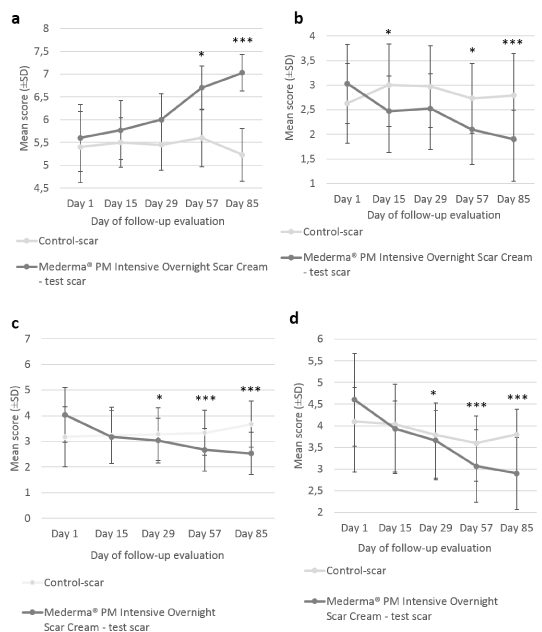
Figure 3. Subjects’ assessment of targets scar softness, redness, discomfort and texture. Mean scores (± CI, confidence intervals) of target scar (a) softness, (b) redness, (c) texture and (d)discomfort, as rated by the subject over time. Asterisk indicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between Mederma® PM Intensive Overnight Scar Cream (black graph) and control (grey graph)
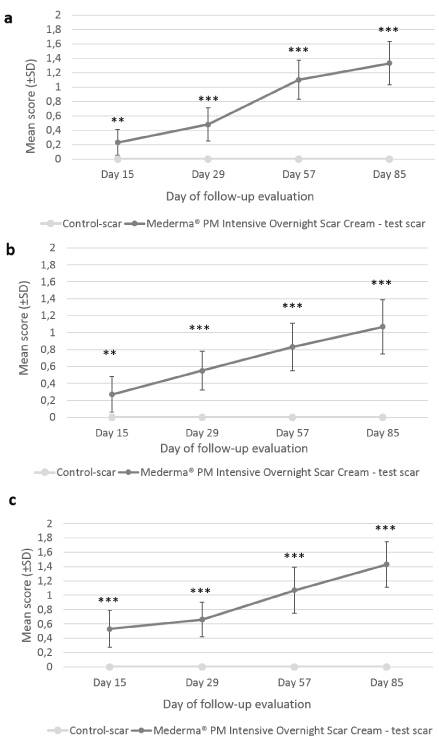
Figure 4. Subjects’ assessment of target scars visibility, thickness and smoothness. Mean scores (± CI, confidence intervals) of target scar (a) visibility, (b) thickness and (c) smoothness as rated by the subject over time. Asteriskindicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between Mederma® PM Intensive Overnight Scar Cream (black graph) and control (grey graph)
Figure 5. Subjects’ positive cosmetic acceptability of Mederma® PM Intensive Overnight Scar Cream. Percentage of subjects who totally and somewhat agreed with the proposed statements at Day 85 (end of study). Only positive answers given by more than 60.0% of subjects are presented
First Positive Visible Effects of Mederma® PM Intensive Overnight Scar Cream
The first subjects to observe and report the first visible effects of Mederma® PM Intensive Overnight Scar Cream on their target scar did so after the first few applications and quite early during the 12-week application period (Table 2). As soon as Day 1, the first subject reported the first visible effects on scar softness and overall appearance improvement. The first effect observed by a participant on scar discomfort improvement was at Day 3 and scar redness reduction and scar size reduction were reported at Day 4 and Day 8 respectively. The first subject reported the first visible effect on roughness reduction was noticed by the first subject at Day 9 and on thickness reduction at Day 20 (Table 2).
Table 2. First day of visible effect(s) reported by the first subjects
|
Mederma® PM Intensive Overnight Scar Cream scar (N=30) |
First day of first visible effect on: |
|
Scar softness |
Day 1 |
Overall appearance improvement |
Day 1 |
Discomfort improvement |
Day 3 |
Scar redness reduction |
Day 4 |
Scar size reduction |
Day 8 |
Roughness reduction |
Day 9 |
Scar thickness |
Day 20 |
Cosmetic acceptability
At the end of the study, participating subjects were asked 18 questions concerning their perception of Mederma® PM Intensive Overnight Scar Cream, to which they could totally or somewhat agree, neither agree nor disagree, somewhat disagree or disagree. Overall, on 11 out of 18 questions, 63.3% - 100.0% of subjects gave positive answers, i.e. totally or somewhat agreed with the proposed statements (Figure 5) and 80.0% of subjects disagreed with the statement that the product stained fabric, like clothes and towels. Precisely, 63.3% of subjects found that Mederma® PM Intensive Overnight Scar Cream made acne scar appear less pitted and more even with the surrounding skin. Moreover, 96.7% of subjects positively appreciated the sensory properties (colour and texture) of this cosmetic product. After 12 weeks of once-nightly application on skin, all subjects (100.0%) found the product easy to apply and more than 93.3% -100.0% appreciated its pleasant aspect and easiness to penetrate the skin without leaving oily effect and discreetness after application (Figure 5). In addition, 66.7% of subjects found their skin moisturized after product application and 70.0% would recommend it to anyone with bothersome acne scars. Finally, 90.0% of subjects would easily fit Mederma® PM Intensive Overnight Scar Cream into their daily skincare routine.
Safety and cutaneous tolerability
Safety and cutaneous tolerability of Mederma® PM Intensive Overnight Scar Cream were excellent throughout the study, for almost all participating subjects; only 1 (3.3%) out of 30 included subjects experienced 1 adverse event (AE) of mild intensity during the study. This AE was not related to the IP (sore throat) and it was resolved at the end of the study. Throughout the study, all 30 included subjects (100.0%) reported no signs of irritation.
Discussion
The blinded Investigator and subjects both assessed that overall appearance of target test-scars was significantly improved after a 12-week application period; an improvement statistically significantly greater compared to control-scar from Day 15. The responses in the subject’s questionnaires were consistent with the conclusion reached by the Investigators assessments. Overall, more than 57.0% of the subjects found overall scar appearance with the product was slightly better or much better than control-scar from Day 57. When considering individual appearance parameters, Investigator reported that scar redness and texture significantly improved over time, statistically significantly more on the test-scar than the control-scar. Subjects also reported statistically greater improvements in test-scar redness, texture, softness and discomfort. Of course, during the application period, a subjects’ perception may evolve, and acne scars may naturally change over time; this variability may be reflected by slight evolution of control-scar appearance evaluations.
Our results were in line with previously published articles that have shown that onion extract-based formulations significantly improved appearance of several types of scars [10,11,14], among which a study showing the cosmetic benefits of once-nightly application of Mederma® PM Intensive Overnight Scar Cream [12]. Moreover, previous publications support that the skin has a circadian cycle, and that skin permeability is higher in the evening and night than in the morning, strongly suggesting that this may have a clinical significance in topical product application [15,16]. In our study, randomisation of the scar on which the product was applied and comparison to a control-scar provided a rigorous tool to evaluate the cause-effect relationship between Mederma® PM Intensive Overnight Scar Cream application and cosmetic outcomes. Of course, objectivity of subjects’ self-rating assessments of appearance improvements may be challenged as subjects were not blinded to the test-scar. However, their assessments were very similar to those of the Investigator, who was blinded to the test-scar, supporting cosmetic outcomes in favour of Mederma® PM Intensive Overnight Scar Cream. Moreover, in cosmetic studies such as this one, subject self-rating of aesthetic responses provides useful “consumer-based” information on acceptability of cosmetic care products. Knowing how atrophic acne scars can have a substantial, negative impact on subjects’ self-esteem and social interactions [17], cosmetic care products that improve scars’ overall appearance, contribute in providing high subject satisfaction. In our study, this was clearly reflected in the well-appreciated cosmetic acceptability of Mederma® PM Intensive Overnight Scar Cream and excellent local tolerability. The IP was applied on only a small area of the skin (only one target acne scar one side of the face) and it is evident that a certain percentage of subjects may not feel better or more confident about their appearance after 12 weeks of cream application, since they may have more than one acne scar to “take care of”. Having said that, 90.0% of subjects found that the product could easily fit their skincare routine and 70.0% would recommend it to persons with bothersome acne scars. This globally reflects a very positive acceptance of Mederma® PM Intensive Overnight Scar Cream by users.
In conclusion, under these study conditions, Mederma® PM Intensive Overnight Scar Cream provided a benefit in the cosmetic appearance of acne scars; first cosmetic effects (overall appearance and softness) were visible 1 day after application, 6 out of 7 cosmetic outcomes (scar roughness, size and redness reduction, softness, overall appearance improvement, discomfort) were visible after only 9 days of once-nightly application. Efficacy in cosmetic appearance of acne scars was optimal after 2 months of use.
Acknowledgments
HRA Pharma funded this study. We are thankful to CPCAD and investigational site staff for their collaboration and work.
Conflicts of interest
JZB (Medical Operations Lead), PA (Scientific Affairs Assistant & Clinical Operations Assistant), TJ (Global Category Lead Wound Care) and CAA (Global Head of Medical Affairs) are employees at HRA Pharma. CQR (CPCAD Managing Director) is an employee at CPCAD (Centre Pharmacologie Clinique Appliqué à la Dermatologie), the organization that has conducted the study. MK (Medical writer) is an employee at ICTA, the organization to which the article writing was subcontracted.
References
- Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, et al. (2008) The prevalence of acne in adults 20 years and older. J Am AcadDermatol58:56-9.[Crossref]
- Connolly D, Vu HL, Mariwalla K, Saedi N (2017) AcneScarring-Pathogenesis, Evaluation, and Treatment Options. J Clin AesthetDermatol10:12-23.[Crossref]
- Layton AM, Henderson CA, Cunliffe WJ (1994) A clinicalevaluation of acnescarring and its incidence. Clin ExpDermatol19:303-8.[Crossref]
- Lee WJ, Jung HJ, Lim HJ, Jang YH, Lee SJ, et al. (2013) Serial sections of atrophicacnescars help in the interpretation of microscopicfindings and the selection of good therapeuticmodalities. J EurAcadDermatolVenereol27:643-6.[Crossref]
- Jacob CI, Dover JS, Kaminer MS (2001)Acnescarring:a classification system and review of treatment options. J Am AcadDermatol45:109-17.[Crossref]
- Rivera AE (2008) Acnescarring:areview and currenttreatmentmodalities. J Am AcadDermatol59:659-76.[Crossref]
- Koo J (1995) The psychosocial impact of acne: patients' perceptions. J Am AcadDermatol32:S26-30.[Crossref]
- Lanoue J, Goldenberg G (2015)Acnescarring:areview of cosmetictherapies. Cutis95:276-81.[Crossref]
- Augusti KT (1996)Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J Exp Biol34:634-40.[Crossref]
- Clarke LF, Baker B, Trahan C, Myers L, SE M (1999) A prospective double-blinded study of Mederma skin Care vs. Placebo for post-traumaticscarreduction. CosmeticDermatology.
- Zurada JM, Kriegel D, Davis IC (2006)Topicaltreatments for hypertrophicscars. J Am AcadDermatol55:1024-31.[Crossref]
- Plaum S, Fleischer A, Verma A, Olayinka B, Hardas B (2014) The ability of a new proprietaryonionextract gel to reduce the appearance of scars: A randomized, controlled study. AESTHETIC DERMATOLOGY70:5.
- Fitzpatrick TB, Pathak M, Parrish JA (1974) Protection of human skin against the effects of the sunburn ultraviolet (290-320 nm). In: Fitzpatrick TB, al. (Eds) Sunlight and Man, normal and abnormalphotobiologicalresponsespp. 751.
- Draelos ZD, Baumann L, Fleischer AB, Plaum S, Avakian EV, et al. (2012) A new proprietaryonionextract gel improves the appearance of new scars:arandomized, controlled, blinded-investigator study. J Clin AesthetDermatol5:18-24.[Crossref]
- Geyfman M, Andersen B (2009) How the skin can tell time. J Invest Dermatol129:1063-6.[Crossref]
- Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, et al. (1998) Time-dependent variations of the skin barrierfunction in humans:transepidermal water loss, stratum corneumhydration, skin surface pH, and skin temperature. J Invest Dermatol110:20-3.[Crossref]
- Tan J, Beissert S, Cook-Bolden F, Chavda R, Harper J,et al. (2022) Evaluation of psychologicalwell-being and social impact of atrophicacnescarring: A multinational, mixed-methods study. JAADInt6:43-50. [Crossref]




