The observation, that women are included in epidemiologic studies at a low rate if occupational cancer is concerned has been depicted in a recent article from Hohenadel, et al. [1]. That review addressed the issue by comparing two-time periods of the pertinent literature from1991-2009. Women became considered with proportions increasing from 39% in publications appearing 1991-1995 to 62% in 2006-2009 in articles that assessed risk estimates including likelihood requirements such as dose-response relationships. The fact that also after that second period articles appeared such as the astonishing publication of the experience of the Italian National Cancer Institute at Naples by Capasso, et al. [2] wondering about the risk of breast cancer affected by the ‘metabolic syndrome’ (MS) in postmenopausal women without adequate studies of the environmental and occupational hazards of cases and controls seems to throw light on the fact how diverse the approaches still are. Ignoring the well documented toxic exposures in the Naples area by waste incineration at Calvi Risorta the authors suggested that several evidences indicate an association between MS and breast cancer, primarily owing to insulin resistance and low serum HDL-C. Lacking was any scientific attempt to relate the metabolic disturbance to toxic effects from most probable dioxin exposure as a cause of endocrine disturbances from nearby massive particle emanations in the most affected region of Naples.
To show the need of follow-up studies into environmental research the following report is related to epidemiologic controlled studies addressing occupational risk factors indicating the increased breast cancer risk in women exposed to Hexachlorocyclohexane (HCH) and PCDDs in the chemical plant producing Lindane (gamma-HCH) until the closure of the site in 1984 [3].
The report is based on an occupational cluster of lethal malignancies related to epidemiologic controlled studies addressing occupational risk factors. Epidemiology is an observational science reconstructing unplanned natural experiments among defined human populations exposed towards environmental hazards. Various reports on cancer risk among men who were occupationally exposed to polychlorinated hydrocarbons containing PCDD and PCDF, in particular the dioxin TCDD have associated their cancer promoting effects given primary carcinogenic exposures. Mostly male workers - having been employed in chemical factories producing chlorinated pesticides - have contributed to the understanding of the role of polychlorinated dioxins as promoting factors under the condition that relevant initiating carcinogenic risk factors were present as necessary precursors. Nevertheless, the overall risk and especially mortality as a fatal clinical outcome are determined by the additional effect of promotors like the halogenated compounds such as PCDDs.
Epidemiological mortality studies showing elevated numbers of cancer deaths with confirmed diagnoses of their causes of death began in the 1960ies. A cohort study carried out after an accident in November 1953 at the BASF plant in Ludwigshafen, Germany, by the follow-up involving about 180 directly affected men, of whom only the core group was enlisted, because of re compensation claims [4]. The next follow-up after 40 years [5] of the same cohort was included in the European study on cancer mortality in workers exposed to chlorophenoxy herbicides and chlorophenols, which reported by 1991 an increased risk for male breast cancer (SMR 345, [6]).
The finding of multiple cancer localizations with excess observed deaths compared with expected rates without specific sites as target was one reason to postulate the promotor function of polychlorinated hydrocarbons such as TCDD, considered as contaminants rather than intended products in chemical industry. Risk ratios varied between 2- und 3-fold for cancer of most localizations for men for several cancers, thus amounting to probabilities far from chance events.
Women have been rarely exposed in chemical industrial processes, therefore the systematic follow-up of the entire crew of a chemical production site of chlorinated pesticides such as Lindane in Hamburg, Germany. After closure of the plant in 1984 circumstances provided the opportunity to study the 398 women ever employed. The significantly increased mortality of 19 women from breast cancer indicated a specific and highly probable modification of cancer risk for workers after this occupational and environmental exposure, because housings of employees were close to the plant and exposure was proven by analyses of whippings of the house dust.
This factory started production of chlorinated pesticides before the World War II and continued a bigger scale after 1950. Since 1952 documented 398 employed women formed a cohort followed up for survival until 2007 [3]. The male cohort of the same plant comprised more than 1800 workers.
Analytical epidemiology as an observational science reconstructing unplanned natural experiments among defined human populations exposed towards environmental hazards has been applied to explore cancer risks in countless occupational cohort studies. Various reports on cancer risk among men who were occupationally exposed to polychlorinated hydrocarbons containing PCDD and PCDF, the dioxin TCDD in particular, have associated their promoting effects of lethal cancer in cases with previous primary carcinogenic exposures.
In pertinent studies mostly male workers, having been employed in chemical factories producing chlorinated pesticides, have contributed to the understanding of the role of polychlorinated dioxins as promoting factors under the condition that relevant initiating carcinogenic risk factors were present as necessary precursors (overview in Manuwald, et al. [3]).
To use the evidence of environmental factors as causal for cancer from findings of occupational risk studies, the overall risk and especially fatal clinical outcome of chronic carcinogenic exposure at low levels being determined by the additional effect of promotors, confounding by concurrent risk factors has always to be considered.
In Germany epidemiological mortality studies resulted mostly in showing elevated numbers of cancer deaths with confirmed diagnoses of their causes of death. The obvious promotor function of polychlorinated hydrocarbons such as TCDD, considered as unexpected, unwarranted and undesired contaminants in chemical industry, became indicator of further damage including the central nervous system. Risk ratios for cancer by organ type in the accident cohort with one unique peak exposure varied between 2- und 3-fold for cancer for several male cancers. A somewhat different type of exposure was studied in production workers exposed for extended periods of time during the years 1952-1984 in the Hamburg plant manufacturing pesticides. In this plant women were exposed as a subcohort. The risk estimates of male mortality were published first by Manuwald, et al. [3] and showed increased risk ratios beginning already at low levels of chronic dioxin exposures, suggesting the promoting role of the chlorinated carbohydrates, including PCDDs (TCDD-OCDD). Women have been less frequently exposed in chemical industrial production lines. The systematic follow-up of the entire crew of the chemical plant in Hamburg, Germany, after closure of the plant in 1984 was an opportunity to study the subcohort of 398 women ever employed in more detail than the publication of Manuwald, et al. [3] by addressing the issue of the metabolic syndrome (MS).
The significantly increased mortality of 19 women from breast cancer pointed at a specific and highly probable modification of cancer risk for workers having been employed in this occupation and exposed to quasi environmental exposure against chlorinated hydrocarbons, because many of them were dwelling nearby the plant in enterprise-owned houses.
This factory started production of chlorinated pesticides before the World War II and continued at a bigger scale after 1950. Since 1952 documented 398 employed women formed a cohort followed up for survival until 2007 [3]. The male cohort of the same plant comprised more than 1800 workers over a time span of over 30 years.
The 19 deaths from breast cancer as a certified cause of death contrasted with 10 deaths expected on the official Hamburg female population mortality statistics and calculated on the person-years of observation for the entire cohort for the period until 2007.
This risk estimate indicated a 1.86-fold risk for breast cancer (SMR 1.86; 95% CI 1.12-2.91).
The figures relating to the intensity of exposure to TCDD show an increased risk already with low doses, which is evident from the comparison of the rates by four quartiles of increasing exposure estimates (measured as ppt TCDD) (Table 1).
Table 1. Breast cancer mortality in women exposed to TCDD (ppt) in quartiles by increasing intensity
Quartile |
I |
II |
III |
IV |
Trend |
N |
102 |
91 |
99 |
97 |
|
TCDD
ppt based on blood fat |
0 |
1-19.5 |
19.5 – 78.3 |
78.3 - 97 |
|
numbers observed./expected |
2/0/2.38 |
6/2.14 |
2/2.87 |
9/2.79 |
|
SMR* |
0.84 |
2.8 |
0.7 |
3.22 |
p = 0 |
95 % CI |
0.09-3.03 |
1.02-6.10 |
0.08- 2.52 |
1.47-6.10 |
|
*SMR: Standardized mortality rate, the expected number based on the mortality of the Hamburg female population was calculated on the person-years principle to be 10,2 for the entire female cohort
The mortality risk in the first quartile without any TCDD in blood fat was below expectation, indicating a selection by health of women in this cohort if no detectable TCDD-exposure incurred, who developed breast cancer according to a reduced rate (but with the upper confidence limit 3.03).
More quantitative evidence for the promotor-effect results from the internal comparison of women in the quartiles with increasing exposure indices during their occupational life, in that the risk ratio rose to almost threefold in quartile II with low exposure up to 19,5 ppt. in the subcohort with higher exposure (III) fewer than expected deaths remind of the fact that treatment and prevention of death may interact in that the incident cancer cases (not shown here) survived and are missing in the statistics due to their survivor status as the number of total breast cancers was higher. The upper confidence limit of 2.52, alas, indicates a possible risk in this type of study. The promotor effect is evident from the low-level risk increment, which is reason to dispute any requirement of a dose-effect. The highest risk in quartile IV results from a marked increase of deceased women above 3-fold compared to the expected figure. This is in strong accordance with a promotor function modifying the lethal outcome of the malignant disease and corresponds to a shorter survival without medical cure.
Mortality from all causes in women contrasted markedly in that observed deaths were mostly below expectation (Table 2).
Table 2. Mortality of women by all causes exposed to TCDD (ppt)
Quartile |
I |
II |
III |
IV |
Trend* |
N |
102 |
91 |
99 |
97 |
|
TCDD
ppt based on blood fat |
0 |
1-19.5 |
19.5 – 78.3 |
78.3 - 97 |
|
numbers observed./expected |
38/41.1 |
45/38.0 |
49/57.6 |
48/61.2 |
|
SMR |
0.92 |
1.18 |
0.85 |
0.78 |
p = 0.17 |
95 % CI |
0.65-1.27 |
0.86-1.58 |
0.63-1.12 |
0.58-1.04 |
|
*Cochran-Armitage trend test
The numbers per quartile were still too small for any estimation of representative figures, but total mortality was not significantly elevated above expectation, and not significantly lower (p for trend=0.17).
The lethal breast cancer occurred in two subgroups among the 19 cases, divided at age 60.
One group was far over 60 years of age at diagnosis and showed comparatively low levels of specific chlorinated toxic exposure values (β-HCH, TCDD, PCBBs) as well as a long survival after first diagnosis of their tumors.
The group of young-for-age breast cancer cases below age 60 showed the highest excess of deaths from mammary cancer compared with the expected numbers which is consistent with findings of TCDD and higher chlorinated species (up to OCDD) - dioxin measurements far exceeding the reference values in non-exposed collectives or factory workers with low values. Receptor status was determined only in 6 cases (30%). Considering their ages at deaths varying from 45-60 they did not all belong to the premenopausal ages, as defined by age below 55 years of age.
Diabetes as concurring risk but missing in mortality statistics
As part of the program of testing the diabetes precursors were determined in the same cohort, although not for the complete cohorts.
When looking at the incidence of other toxic effects involving dysfunctional endocrinological regulation, such as diabetes, there was also indication of an effect on the endocrine levels. The predictor of diabetes HbA 1 c was found in 17,2% of the cohort with 8,6% positive glucose tests in the urine. Significant was the finding of antibodies against the pancreatic insular cells correlated to the TCDD-burden. This was first interpreted as an immunotoxic effect [7].
Whether this observation reflects a specific causal association between the established neurotoxic effects of chlorinated compounds (Tetra-, Penta-, Hexa-, Hepta- and Octa-CCD) was of interest for clarification of the causal promoting role of these metabolic failures. This relates to the finding of an epidemic in a young Saudi-Arabian population sample of the increased risk of diabetes type 2 related to chlorinated carbohydrates in blood samples [8]. The dioxins, however, specifically affecting the endocrine active glandular structures such as thyreoid and breast tissue, directly or via autonomous nervous system, are suggestive as causing the early for age incidences in those women with premenopausal cancers of the glandular mammary tissues with fatal outcome.
Neurotoxic effects on the autonomous nervous system show effects on the hormonal level. The female breast is one endocrinologically stimulated tissue sensitive to toxic disturbances. Other glandular organs are the pancreas as well as the thyreoid gland, the latter having a fair survival probability and, therefore, missing in mortality analyses.
The direct neurotoxic effects of dioxins on the nervous system appears to cause - next to the strong neuropsychological disturbances such as toxic encephalopathy - also paralyzing and often undetected damage of the autonomous nervous system. Such effects have been well established by neuropsychological evidence since many decades.
Diabetes does not appear as a cause of death in mortality studies since the documentation of this kind of clinical entity is not so well defined as a cancer diagnosis and does not appear on death certificates with sufficient reliability and validity. However, in the morbidity files of the total cohort the clinically confirmed pancreatic diseases including the indicator of disturbed glucose metabolism HbA c1 provided some evidence of at least three cases of early diabetes type 2 during the ongoing dioxin exposures at the workplace.
Occurrences compared with the expected numbers consisted of cases with TCDD and OCDD-values far exceeding the reference values from non-exposed collectives or factory workers with far lower values (data not given).
Diabetes Type 2 as a dysfunction of the neurotoxicologically affected pancreas regulation was associated with pesticides in the literature. Spanish researchers associated ß-hexachlorocyclohexane and type 2 diabetes risk. According to Arrebola, et al. the insecticide lindane (gamma HCH) is contaminated by this metabolic byproduct ß-HCH with a prolonged half-time like dioxins – and Lindane is still used for medicinal treatment of lice and mites (scabies) by application on the often-irritated skin, which enhances resorption into the organisms [9].
This finding is of significance because of the reports focussing on the role of metabolic interim products published by Knecht, et al. [7].
In Germany, regional distribution of diabetes is pointing to a gradient falling from northeast to south for still unknown reasons (Figure 1). Descriptive statistics of diabetes in Germany show that Type 2 diabetes is caused by failures of insulin provision. The causes can be acquired by factors inhibiting usually normal functions of regulating organs. The recently described trend of the prevalence from northeast falling to south as a distribution pattern of diabetes in Germany points to a regional gradient for reasons still unknown [16]. Chemical applications in agricultural sites were more prevalent in the formerly collective eastern farming (1948-1998) but quantitative epidemiologic research such as in Spain is still missing.
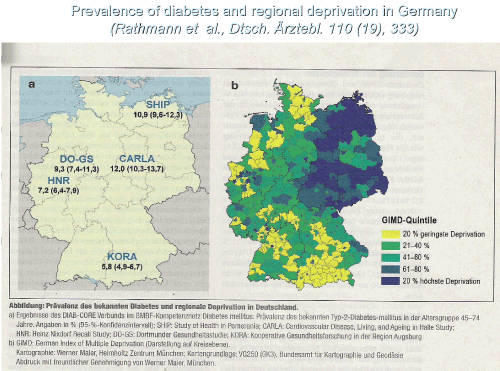
Figure 1. Prevalence of diabetes and regional deprivation in Germany.
Prenatal exposure to the oestrogenic endocrine-disrupting chemical DDE may contribute to the obesity epidemic in women, commonly called metabolic syndrome. Karmaus, et al. [10] have found this effect in daughters whose mothers have been exposed in Michigan before their pregnancy. Prenatal exposure to PCBs and DDE was estimated by extrapolating maternal measurements to the time that the women gave birth. A follow-up for cancer incidence in the cohort would be of special interest.
Furthermore, the metabolic syndrome is very likely associated with postmenopausal breast cancer risk [11], if applying different index categories, but the question remains still open and speculative at best, as long as the real causes of initiating exposures remain unidentified
As mentioned above, Capasso, et al. [2] suggested that “Several evidences indicate an association between Metabolic Syndrome and breast cancer, primarily owing to insulin resistance and low serum HDL-C “referring to an American study by Kabat, et al. [12]. In that large American study, the issue of metabolic syndrome and breast cancer risk was also addressed.
The conclusion of Capasso et al. was: “In time-dependent covariate analyses, however, certain scenarios indicated a positive association between the metabolic syndrome and breast cancer, due primarily to positive associations with serum glucose, serum triglycerides, and diastolic blood pressure.
Based on these speculative considerations the Italian authors suggested quite planely:
The team of the National Cancer Institute of Naples points out the association of the metabolic syndrome (MS) and breast cancer risk in postmenopausal women.
The incomplete data base includes premenopausal women not being included in the study group, and likewise missing female industrially exposed workers.
It was concluded that MS features before cancer incurrence present an indicator of breast cancer risk;
the unsettlement of the hormonal arrangement – in post-menopausal women – along with an increased obesity probably favour the hormone-dependent cell proliferation for tumorigenesis without giving hints as to the origin of the MS.
2021 Copyright OAT. All rights reserv
The given recommendation in conclusion, to “adjust their lifestyle” was out of place, since the numerous reports of environmental exposures to pyrolysis products in the region of Naples have reached a level, which means that there have been dioxin exposures through uncontrolled fires.
Several obvious weaknesses are apparent. The lack of data for the speculative conclusion concerns the omission of occupationally exposed women (why?) although in Naples notorious garbage disposal problems have been related to the “toxic waste inferno of Calvi Risorta” near the metropole, as well as the lack of any data on obesity or “unsettlement of the hormonal arrangement”. The questionable recommendation to reduce weight by adjustment of lifestyle is obsolete in view of the meanwhile well-known effects of xenobiotic effects of environmental intoxication by chlorinated compounds synthetized de novo in pyrolytic processes going on day and night causing the inferno in the region. The intention of the authors is very limited, although the motto “….although further studies are required to more precisely assess the relationship between MS and breast cancer, our study paves the way for the development of new primary prevention strategies against sporadic breast cancer” appears to be at least anachronistic in view of the environmental toxic threats known by almost daily complaints and media reports on the environmental risks to be the goal of true primary prevention.
Insulin resistance determines the increase of IGF-1 bioavailability that inhibits the hepatic synthesis of SHBG and stimulates cell proliferation through specific receptors [14].
HDL-C can be considered as an independent predictor of increased levels of several cancer-promoting hormones (insulin, estrogens and androgens), which regulate its levels through the modulation of liver hepatic lipase activity. According to our above-mentioned outcomes the keystone of this metabolic arrangement is weight gain and the consequent visceral adiposity is typical of the postmenopausal period [14].
This may have a role in MS development and may contribute to breast carcinogenesis through the activation of different pathways involving increased levels of aromatase and IGF-1, reduction of SHBG, pro-inflammatory and anti-apoptotic cytokines. Our results suggest the possibility of applying primary prevention of breast cancer, through lifestyle changes, to postmenopausal women who show MS features. Such preventive strategy will likely not protect from hereditary breast cancer, which represents around 10% of all breast tumours. Currently, for hereditary breast cancer only strict surveillance screenings, prophylactic surgery or chemoprevention support the physician in breast cancer detection and cure. However, for sporadic cancers, environment adjustments, physical activity intensification and healthy food intake could reduce body metabolic alterations and breast cancer risk.
In conclusion, environmental exposure to dioxins near incinerator plants such as reported with analytical and plausible measurements from China (and ever since from Taiwan) require further studies to more precisely assess the relationship between MS and breast cancer, for the development of new primary prevention strategies for sporadic breast cancer outside the industrial complex with hazards at the workplace. Hopes are that the REACH-program by the European Community/Union may be effective in preventing environmental and occupational hazards in the future by mindful recommendations unlike the ones given in Italy of a national institution. Women in Europe for a Common Future (WECF) [15] have compiled the present scientific evidence for environmental and occupational hazards in a publication available in English, Dutch and French. Remarkably the dioxins are included in their listing of hazardous substances and avoidable exposures by prevention.
- Hohenadel K, Raj P, Demers PA, Zahm SH, Blair A (2015) The inclusion of women in studies of occupational cancer: A review of the epidemiologic literature from 1991-2009. Am J Ind Med 58: 276-281. [Crossref]
- Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, et al. (2010) Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther 10: 1240-1243. [Crossref]
- Manuwald U, Velasco-Garrido M, Berger J, Manz A, Baur X (2012) Mortality study of chemical workers exposed to dioxins: follow-up 23 years after chemical plant closure. Occup Environ Med 69: 636-642. [Crossref]
- Thiess AM, Frentzel-Beyme R, Link R (1982) Mortality study of persons exposed to dioxin in a trichlorophenol-process accident that occurred in the BASF AG on November 17, 1953. Am J Ind Med 3: 179-189. [Crossref]
- Zober A, Messerer P, Huber P (1990) Thirty-four-year mortality follow-up of BASF employees exposed to 2,3,7,8-TCDD after the 1953 accident. Int Arch Occup Environ Health 62: 139-157. [Crossref]
- Saracci R, Kogevinas M, Bertazzi PA, Bueno de Mesquita BH, Coggon D, et al. (1991) Cancer mortality in workers exposed to chlorophenoxy herbicies and chlorophenols. Lancet 338: 1027-1932. [Crossref]
- Knecht U, Manz A, Walter D, Woitowitz HJ (1999) Arbeitsbedingte Gefährdung durch halogenierte Dioxine und Furane. Untersuchungen zur Belastungssituation in der Arbeitswelt und zur Mortalität und Morbidität exponierter Chemiearbeiter. Wirtschaftsverlag NW, Bremerhaven.
- Al-Othman A, Yakout S, Abd-Alrahman SH, Al-Daghri NM (2014) Strong associations between the pesticide hexachlorocyclohexane and type 2 diabetes in Saudi adults. Int J Environ Res Public Health 11: 8984-8995. [Crossref]
- Arrebola JP, Pumarega J, Gastull M, Fernandez MF, Martin-Olmedo P, et al. (2013) Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ Res 122: 31-37. [Crossref]
- Karmaus W, Osuch JR, Eneli I, Mudd LM, Zhang J, et al. (2009) Maternal levels of dichlordiphenyl-dichloroethylen (DDT) may increase weight und body mass index in adult female offspring. Occup Environ Med 66: 143-149. [Crossref]
- Rosato V, Bosetti C, Talamini R, Levi F, Negri E, et al. (2011) Metabolic syndrome and the risk of breast cancer. Recenti Prog Med 102: 476-478. [Crossref]
- Kabat G, Kim M, Caan B, Chlebowski RT, Gunter MJ, et al. (2009) Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer 15: 2704-2710. [Crossref]
- Kabat G, Chlebowski RT, Khandekar J, Ko MG, McTirnan A, et al. (2009) A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 18: 2046-2053.
- Remillard RB, Bunce NJ (2002) Linking dioxins to diabetes: Epidemiology and biologic plausibility. Environ Health Perspect 100: 853-858. [Crossref]
- Women in Europe for a Common Future (WECCF): Dioxins as risk factor of breast cancer. www.bcaction.de/pdf/ukult/umwelt_brustkrebs.pdf.
- Müller G, Kluttig A, Greiser KH, Moebus S, Slomiany U, et al. (2013) Regional and neighborhood disparities in the odds of type 2 diabetes: results from 5 population-based studies in Germany (DIAB-CORE consortium). Am J Epidemiol 178: 221-230.

