Objective: A cluster schedule of subcutaneous allergen immunotherapy (SCIT) comprises the administration of several doses of an allergenic extract on the same day during the build-up phase in weekly intervals to achieve the maintenance dose.
The objective was to determine whether the cluster SCIT schedules starting the administration with the highest available concentration vial (vial B) provided an adequate safety profile.
Methods: A real-world observational study was designed. Patients between 5-65 years old with a diagnosis of rhinitis and/or bronchial asthma due to hypersensitivity to house dust mites, treated with SCIT, in cluster schedules starting with vial B (10,000 Therapeutic Units/ml), were included.
Results: A total of 258 patients with a mean age of 20.3 years (95% CI 18.8-21.9), 134 females (51.9%), were included from 11 allergy units in Spanish hospitals. Eight cluster SCIT schedules were analyzed adding up 1193 doses. Forty-six patients (17.8%) suffered 89 adverse reactions (7.5% of doses): 79 local (6.6% of doses) and 10 systemic (0.8% of doses). The systemic reactions observed in seven patients were grade 1 (6) and grade 2 (4). Three patients (1.2%) withdrew the immunotherapy due to adverse reactions: systemic reaction grade 1, systemic reaction grade 2 and one local reaction. No significant safety differences between cluster schedules were observed.
Conclusions: Cluster schedules of SCIT at high doses were well tolerated and reduced the build-up phase to 1-3 weeks, instead of the conventional eight weeks, thereby contributing to the improvement of patient comfort and treatment compliance.
Allergen immunotherapy; cluster schedule; allergy; house dust mites.
Allergen-specific immunotherapy (AIT) has proven efficacy for the treatment of patients with allergic rhinitis and asthma [1,2]. The AIT consists in the administration of increasing doses of specific allergens to the patients to reach a clinical desensitization to the allergen. The AIT can be administered by different ways, sublingual, intranasal and subcutaneous (SCIT), being the last one the most frequently used in our setting. SCIT comprises a slow increase in the doses (build-up phase) until the maintenance dose is reached. Then monthly SCIT doses are administered for at least three years. In the build-up phase the patient must visit the medical center weekly and wait for 30 minutes after the injection for safety control. This fact could lead to a lack of adherence of the patients due to the inconveniences of the multiple periodic visits required. As composition and manufacturing procedures differs between different AIT products, it is necessary to analyze the efficacy and safety in specific studies for each one. Acaroid® (Allergopharma, Spain), the SCIT product analyzed in this study for patients sensitized to house dust mites (HDM), the conventional schedule comprises the use of two vials: vial A with 1,000 Therapeutic Units/ml (TU/ml) and vial B with 10,000 TU/ml [3]. The major allergen content of the high-dose HDM allergoid Dermatophagoides pteronyssinus (DP) is 12μg/ml Der p 1 and 10 μg/ml Der p 2, and for the high-dose HDM allergoid Dermatophagoides farinae (DF) it is 20 μg/ml Der f 1 and 15 μg/ml Der f 2 [4]. The conventional schedule recommends four weekly increasing doses with vial A, and four with vial B, to reach the maintenance dose of 0.6 ml of vial B (6000 TU), so a total of eight injections for eight weeks are needed [3]. To reduce the duration of the build-up phase, the cluster schedules for SCIT have been introduced, with the administration of several doses on the same day, at a 30-minute interval, in weekly visits. The cluster schedule recommended by the manufacturer in the Acaroid® Summary of Product Characteristics (SPC) is completed in three visits, with a total of 6 injections, where: on the first day, two doses of 0.3 ml from vial A; on day 8, two doses of 0.1 ml and 0.2 ml from vial B; and on day 15 two doses of 0.3 ml from vial B are administered [3]. So, only 0.6 ml of vial A are used, and this vial is then discarded. To date, there is no consensus about which cluster schedule is most effective and safe", and each physician follow their experience in the decision of the best scheme for each patient [5]. Specifically, no information has been published on the build-up immunotherapy using the highest allergen doses contained in the so-called vial B for AIT for HDM, although good safety results were reported for seasonal allergens [6].
The objective of this study was to determine whether the cluster schedules of SCIT, starting administration of the therapeutic extract with the highest available concentration vial (vial B) of Acaroid®, has adequate safety in real-world patients [3].
This real-world observational cohort study was approved by the Ethics Committee of the Hospital Universitari Vall d'Hebron (Barcelona, Spain on 23 November 2018; ID-RTF021), conducted in line with national regulations and the Declaration of Helsinki (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/), and completed in 11 immunotherapy units in Spanish public hospitals in October 2018-June 2019.
The primary objective was to evaluate the safety, during the build-up phase, of cluster schedules of SCIT starting with high doses (vial B) of Acaroid® in patients sensitized to HDM [3].
The eligibility criteria were: 1) Patients between 5 and 65 years of age; 2) Diagnosis of allergic rhinitis (ARIA) [7] and/or bronchial asthma (GINA) [8]; 3) HDM hypersensitivity established by diagnostic skin prick testing and positive allergen-specific IgE; 4) Immunotherapy started with high doses (vial B); 5) Patients with a history of treatment with some other AIT either simultaneously or in the previous three years were not included; 6) Patients were excluded in the event of any contraindication to SCIT described in the Acaroid® SPC3; 7) Patients, and their parents or legal representatives in the case of minors, have signed the written informed consent form.
Patients were treated with subcutaneous Acaroid®, starting with high doses (vial B) in cluster schedules. Each physician followed different cluster schedules, starting with high doses (vial B) as per their experience and being the best schedule for each patient.
Information was recorded regarding patient age, gender, and socioeconomic level, medical history, concomitant medication, history of the allergic disease and the symptoms.
The composition of the SCIT prescribed could be DF 100%, DP 100%, or mixed (50% of each allergen).
The patient’s adverse reactions (AR) to SCIT observed during the build-up phase of treatment were recorded, specifying the start and end date of each reaction, whether the reaction was immediate (occurring in the first 30 minutes after vaccination) or delayed (30 minutes or more after injection), the treatment measures adopted, the classification of the AR (local or systemic), and its outcome. The definition and severity of the AR was evaluated based on the criteria and recommendations of the World Allergy Organization (WAO). Systemic adverse reactions were classified into 5 grades (1-5) according to severity [8,9].
Based on the results of the meta-analyses of local and systemic adverse reaction of Feng et al for-cluster schedules of SCIT for mites showed an incidence of 25 local reactions in 2989 doses (0.84%) and 19 systemic AR in 2989 injections (0.64%) [5], we estimated a sample size of 258 patients with a power of 99% for the evaluation of the percentage of AR to SCIT with a precision of 0.07 (Sample Power, SPSS Chicago, United States).
A descriptive analysis was made by calculating frequencies and percentages for the qualitative variables and mean, standard deviation, minimum and maximum, and 95% confidence interval for quantitative variables. Comparisons between qualitative variables were completed using Fisher’s test or the Chi2 test, and the student’s t-test was used for the comparison of independent groups in the case of quantitative variables. Multivariate logistic regression was applied to analyze the factors related to the appearance of AR (age, duration of the allergic disease, gender, presence of asthma or composition of the SCIT). Statistical significance was established at 0.05. (IBM-SPSS 25.0-United States).
A total of 258 patients satisfying all the selection criteria were included. Their sociodemographic and clinical history are described in Table 1.
Table 1: Sociodemographic and clinical history of the patients included in the study.
| |
n (%)
or mean (95% Confidence Interval)
n=258 |
Age |
20.3 (18.8-21.9) |
|
<12 years |
67 (26) |
|
12-17 years |
89 (34.5) |
|
≥ 18 years |
102 (39.5) |
Gender |
Male |
124 (48.1) |
| |
Female |
134 (51.9) |
Socioeconomic level |
Low |
35 (13.6) |
| |
Middle |
202 (78.3) |
| |
High |
21 (8.1) |
System affected receiving treatment |
Respiratory |
133 (51.6) |
Cardiovascular |
9 (3.5) |
|
Gastrointestinal |
25 (9.7) |
|
Genitourinary |
11 (4.3) |
|
Musculoskeletal |
15 (5.8) |
| |
Neurological |
13 (5) |
|
Endocrine |
17 (6.6) |
|
Hematological |
7 (2.7) |
|
Dermatological |
57 (22.1) |
|
Psychiatric |
14 (5.4) |
|
Oncological |
8 (3.1) |
All patients were sensitized to DP, and 80.2% (207) were also sensitized to DF. The allergic symptoms at study entry are described in Table 2.
Table 2: Description of allergic symptoms before allergen immunotherapy.
Allergic symptoms description |
N (%) |
Allergic disease classification
N=258 |
Conjunctivitis only |
0 (0) |
Rhinitis only |
39 (15.1) |
Asthma only |
7 (2.7) |
Conjunctivitis and rhinitis |
68 (26.4) |
Conjunctivitis and asthma |
1 (0.41) |
Rhinitis and asthma |
51 (19.8) |
Conjunctivitis and rhinitis and asthma |
92 (35.7) |
Patients with conjunctivitis (62.4%)
N=161 |
Infrequent 2 days/week or less |
55 (34.2) |
Frequent 2-5 days/week |
84 (52.2) |
Very frequent more than 5 days/week |
22 (13.7) |
Patients with rhinitis (96.9%)
N= 250 |
Intermittent |
31 (12.4) |
Persistent |
219 (87.6) |
Infrequent 2 days/week or less |
16 (6.4) |
Frequent 2-5 days/week |
139 (55.6) |
Very frequent more than 5 days/week |
95 (38) |
Patients with asthma (58.5%)
N= 151 |
Intermittent |
86 (56.9) |
Persistent |
65 (43.1) |
Infrequent 2 days/week or less |
82 (54.3) |
Frequent 2-5 days/week |
62 (41.1) |
Very frequent more than 5 days/week |
7 (4.6) |
Allergen immunotherapy
The combination of DP 50% and DF 50% was the most frequent composition including 207 patients (80.2%), followed by 51 (19.8%) subjects with a 100% DP.
The cluster schedules of SCIT administration are detailed in Table 3. A total of 1193 SCIT injections were administered. No significant differences were observed between patients in different cluster schedules in their baseline demographic or clinical characteristics.
Table 3: Description of cluster schedules for allergen immunotherapy build-up phase analyzed in the study.
Build-up phase cluster schedule |
Doses of vial B of Acaroid® (ml) |
Weeks to achieve maintenance dose |
Nº of patients (%) |
Nº of injections (%)* |
1 |
0.1+0.2; 0.3+0.3; 0.6 |
2 |
139 (53.9) |
702 (58.8) |
2 |
0.1+0.1; 0.3+0.3; 0.6 |
2 |
19 (7.4) |
97 (8.1) |
3 |
0.1+0.2; 0.4; 0.6 |
2 |
8 (3.1) |
30 (2.5) |
4 |
0.3+0.3; 0.6 |
1 |
49 (19) |
154 (12.9) |
5 |
0.1; 0.2; 0.3; 0.3+0.3; 0.6 |
4 |
2 (0.8) |
12 (1) |
6 |
0.1; 0.2; 0.2+0.2; 0.6 |
3 |
3 (1.2) |
12 (1) |
7 |
0.2+0.4; 0.6 |
1 |
21 (8.1) |
67 (5.6) |
8 |
0.05+0.1; 0.1+0.2; 0.3+0.3; 0.6 |
3 |
17 (6.6) |
119 (10) |
Total |
|
|
258 (100) |
1193 (100) |
*Number of injections administered to the patients in each schedule, includes any repeated doses.
Number and description of adverse reactions
A total of 89 AR was observed during the study, 79 local and 10 systemic. The systemic reactions were described as urticaria (3), discomfort (2), cough (1), dyspnea (2), asthma (3), bronchospasm (4) and hypertension (1), and classified as grade 1 (6) and grade 2 (4). One grade 2 systemic reaction was declared as clinically significant and manifested as immediate bronchospasm requiring reduction of the AIT dose in cluster schedule 7 (Table 3), this patient was lost to follow-up.
Regarding the onset of the AR, 13/89 (14.6%) were immediate, and 76/89 (85.4%) delayed, with 10 (12.7%) of the local reactions being immediate, and 69 (87.3%) delayed. A total of 3 (30%) of the systemic reactions were immediate, and 7 (70%) were delayed.
A large part of the local and systemic AR 37/89 (41.6%) appeared with the first dose of AIT, 32/79 local and 5/10 if systemic. All the AR were resolved without sequelae neither complication. The mean duration of the local AR was 2.9 days (95% CI 2.3-3.6), and 2.4 days (95% CI 1-3.8) for systemic AR.
As consequence of the AR, in 64/89 (71.9%) of the AR no actions on AIT were taken; the actions are unknown for one AR; and the AIT dose was reduced in 17/89 (19.1%), delayed in 2/89 (2.2%), and suspended in 5 (5.6%) AR, observed in three patients: one suspended due to local AR and two due to systemic grade 1 and grade 2 AR.
A total of 75/89 (84.3%) AR did not require medication for their control and 14 (15.7%) required medical treatment.
Number of patients with adverse reactions
In the whole sample, 46/258 patients (17.8%,95% CI 13.4-23.1) presented at least one AR to SCIT, with a mean of 1.9 AR by patient (95% CI 1.6-2.3). In 39 patients, only local AR were observed, six patients manifested systemic AR and one patient presented both local and systemic AR. The rate of patients with local AR was 40/258 (15.5%, 95% CI 11.3-20.5), and 7/258 (2.7%, 95% CI 1.1-5.5) with systemic AR.
The distribution of patients with AR by cluster schedule administered is shown in Figure 1A. A higher rate of patients with AR was observed in cluster schedule 7 than in cluster schedule 1 (47.6% vs 12.9%, p=0.01). No other significant differences were observed between cluster schedules. In Figure 2A, the percentage of patients reporting at least one treatment-related AR is described by age group and local or systemic classification.
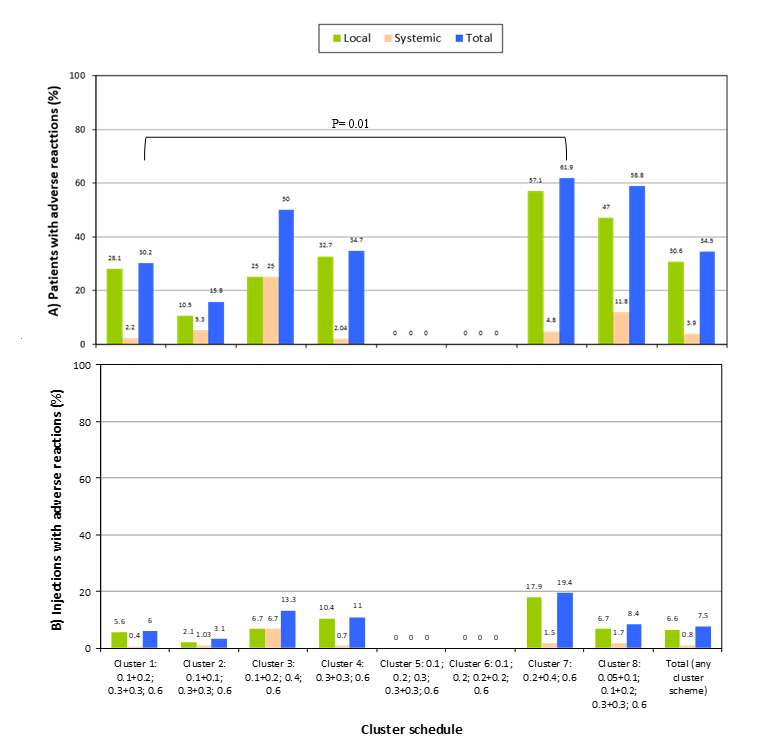
Figure 1. Rate of adverse reactions to the allergen immunotherapy with Acaroid® by number of patients (A) and by number of injections (B), in eight cluster schedules and the total group.
The number of patients with AR observed in the study were compared to other references (Table 4).
Table 4: Safety analysis of cluster subcutaneous immunotherapy schedules during the build-up phase for different allergen sources.
Reference |
|
|
|
Allergen immunotherapy cluster schedule |
Build-up phase
Nº Adverse reactions |
Adverse reactions
by number of patients at risk
% |
Adverse reactions
per number of AIT injections*
% |
Local |
Systemic |
Local |
Systemic |
Local |
Systemic |
n |
Study design |
Allergen |
Nº injections per visit |
Weeks |
I |
D |
Total |
I |
D |
Total |
Garriga-Baraut, 2020 |
258 |
O |
Dermatophagoides pteronyssinus (19.8%)
Dermatophagoides pteronyssinus + Dermatophagoides farinae (80.2%) |
8 different schedules |
1 to 4 |
10 |
69 |
79 |
3 |
7 |
10 |
15.5 (95%CI 11.3-20.5) |
2.7 (95%CI 1.1-5.5) |
6.6 (95%CI 5.3-8.2) |
0.8 (95%CI 0.4-1.5) |
Tabar, 200511 |
120 |
RCT |
Dermatophagoides pteronyssinus |
4/3/2/2/2/2/1 |
6 |
6 |
0 |
6 |
1 |
3 |
4 |
5 |
3.3 |
0.4 |
0.2 |
Zhang, 200912 |
45 |
RCT |
Dermatophagoides pteronyssinus |
3/2/2/2/2/2/1 |
6 |
11 |
0 |
11 |
5 |
0 |
5 |
24.4 |
11.1 |
1.8 |
0.8 |
Nieto, 201313 |
434 |
O |
Dermatophagoides pteronyssinus (65.9%)
Dermatophagoides pteronyssinus + Dermatophagoides farinae (34.1%) |
2/2/2 |
2 |
50 |
29 |
79 |
6 |
3 |
9 |
18.2 |
2.1 |
2.4 |
0.3 |
Walker, 200114 |
22 |
RCT |
Grass pollen |
3/2/2/1/1/1/1 |
4 |
0 |
0 |
0 |
0 |
4 |
4 |
0 |
18.2 |
0 |
1.7 |
Crimi, 200415 |
15 |
RCT |
Parietaria |
2/2/2/2/1/1/1/1 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Nanda, 200416 |
20 |
RCT |
Cat hair and dander |
2/2/2/2/1 |
5 |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
5 |
0 |
0.6 |
Colás, 200617 |
41 |
RCT |
Salsola Kali |
3/3/1 |
2 |
16 |
0 |
16 |
4 |
12 |
16 |
39 |
39 |
5.6 |
5.6 |
Subiza, 200818 |
22 |
RCT |
Dactylis Glomerata, Trisetum Paniceum |
2/2/1 |
2 |
0 |
7 |
7 |
0 |
0 |
0 |
31.8 |
0 |
6.4 |
0 |
González-Gutiérrez, 201219 |
127 |
O |
Grass pollen |
2/2/2/1 |
4 |
8 |
24 |
32 |
3 |
11 |
14 |
25.2 |
11 |
3.6 |
1.6 |
|
177 |
O |
Grass pollen |
2/2/1 |
4 |
4 |
22 |
26 |
5 |
15 |
20 |
14.7 |
11.3 |
2.9 |
2.3 |
Klimek, 201420 |
61 |
RCT |
Grass and rye pollen |
2/2/1 |
5 |
7 |
0 |
7 |
2 |
3 |
5 |
11.5 |
8.2 |
2.3 |
1.6 |
Solá, 20186 |
110 |
O |
Grass pollen and/or Olea europaea |
2/2/2 |
2 |
3 |
50 |
53 |
2 |
6 |
8 |
48.2 |
7.2 |
8 |
1.2 |
O: Observational; RCT: randomized clinical trial; n: number of patients in active treatment group; I: Immediate reaction, appears less than 30 minutes from immunotherapy injection.; D: Delayed reaction, appears 30 minutes or more after immunotherapy injection; 95%CI: 95% Confidence Interval.
*When not described in the reference, the number of AIT injections was calculated as: number of patients multiplied by number of injections in build-up phase.
Number of adverse reactions per allergen immunotherapy injection
In the whole sample, 89/1193 injections (7.5%, 95% CI 6-9.1) were observed to have at least one AR to SCIT. The rate of local AR per SCIT injection was 79/1193 (6.6%, 95% CI 5.3-8.2), and the rate of systemic reactions per SCIT injection 10/1193 was 0.8% (95% CI 0.4-1.5).
In Figure 1B, the percentage of SCIT injections with AR is shown by cluster schedule administered. No significant differences between cluster schedules were observed.
The number of AR by injection observed in the study were compared to other references and shown in Table 4.
No relationship was observed between the presence of AR and clinic-demographic risk factors for the development of AR (age, duration of the allergic disease, gender, presence of asthma or composition of the SCIT) in the multivariate analysis.
The objective of this study was to determine if cluster schedules starting SCIT with the highest available concentration vial (vial B) for a specific subcutaneous AIT product (Acaroid®), provide an adequate safety profile in real-world clinical practice [3]. This way of administration shortens the SCIT build-up phase, reaching the optimum maintenance dose in 1-4 weeks, instead of 8 weeks required with the conventional schedule, which reduces patient and healthcare system costs related to the visits that can improve the adherence to the treatment. Additionally, the use of vial B from the beginning of the treatment favored a more efficient use of the product.
In the study, 258 patients received 1193 SCIT doses of Acaroid®, administered in cluster schedules. The cluster schedule most frequently used were 0.1+0.2/0.3+0.3/0.6, in 53.9%, and 0.3+0.3/0.6, in 19%. The schedule nº1 (Table 3) was the most prescribed and was similar to the recommended by the manufacturer without the two doses of vial A. In general, no significant safety differences between cluster schedules were observed, though schedule 7 had a higher rate of patients with adverse reactions (Figure 1A), compared to cluster schedule 1 (p=0.01).
The safety summary is that 17.8% patients suffered 89 AR (7.5% of doses), 79 local (6.6% of doses) and 10 systemic (0.8% of doses). A large part of the local and systemic reactions appeared with the first dose of SCIT (43%) in concordance with data described previously [10]. It must be noted that most local and systemic AR were delayed (85.4%), appearing after the first 30 minutes post-injection when the patient leaves the clinic. As such, it is especially important to inform the patients about this fact in be able to recognize the delayed systemic AR (70%) and for them to receive appropriate treatment on time. The systemic reactions, observed in seven patients, were grade 1 (6) and grade 2 (4) and only three patients (1.2%) withdrew the immunotherapy due to adverse reactions.
As a main reference, in a study including 12895 patients, the systemic reaction rates were of 0.5% per dose in adults, most were immediate and occurred during the build-up phase [10]. This data was within the confidence interval of the results of our study for the cluster schedules initiated with vial B (0.8, 95% CI 0.4-1.5). Since the introduction of cluster SCIT, the results of many clinical trials, observational studies and meta-analyses assessing their efficacy and safety have been published, concluding that the safety profile was comparable to that observed with the conventional treatment regimens, although further well-designed randomized controlled trials are still needed [5,11-20].
We compared the safety results of our study with studies with cluster schedules (Table 4). Studies from line 2 to 4 were completed with SCIT products containing the same allergen source (HDM), and the Nieto et al study [13] used the same product (Acaroid®) with the cluster schedule recommended in the SPC [3]. The Solá et al study was the only one initiating the SCIT with the vial B higher doses as we did, but their study was for other allergens [6]. But the studies could differ in many immunotherapy factors related to the incidence of adverse reactions, or patient characteristics [21-25]. For these reasons, from the data shown in Table 4, the results of our study could only directly be compared to Nieto et al, [13] observing a similar percentage of AR by number of patients, but a higher rate of AR per SCIT injection (p<0.05). These differences could be explained by the percentage of patients with mixed mites in our study (80.2%, versus 34.1%) who have been described as having a higher risk of AR, although in the multivariate analysis we have not found any relationship between SCIT composition and the presence of AR. In Figure 2, we showed the results of the pooled data of 279 patients included in six randomized controlled trials in patients treated with Acaroid® under the conventional schedule, completing the maintenance phase of up to three years, by age group [26]. Although in our study only the build-up phase is analyzed, it is known that most adverse reactions appear during this period, so a comparable maintenance safety profile can be anticipated from the observation of our results.
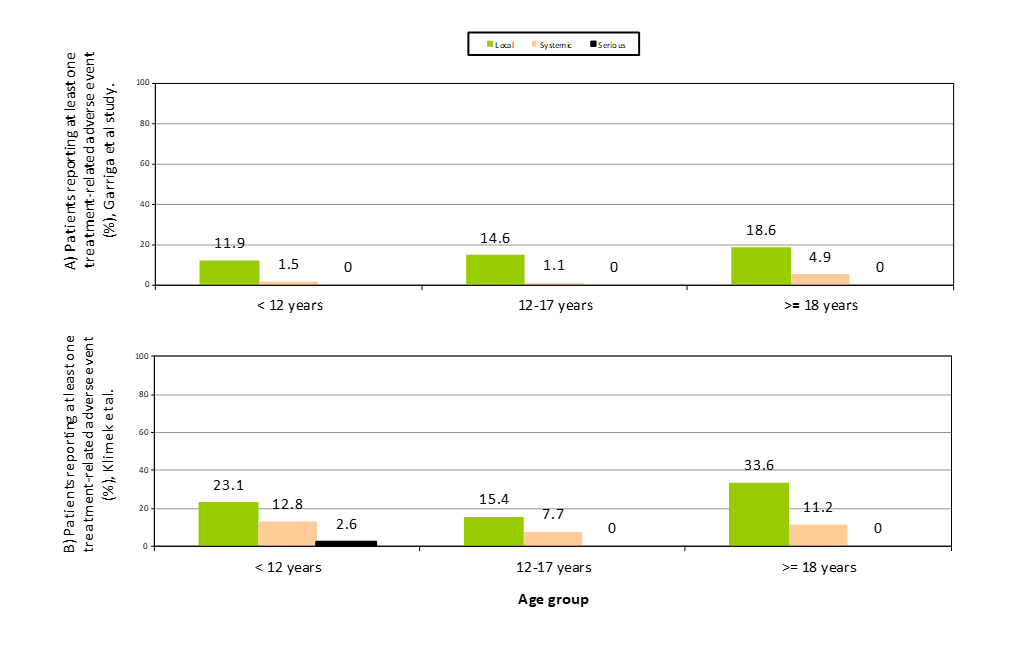
Figure 2. Summary of patients reporting adverse reactions related to subcutaneous allergen immunotherapy with Acaroid® by age group: comparison of Garriga-Baraut et al (cluster, vial B, build-up phase: A) versus Klimek et al [26] results (conventional schedule, up to three years of treatment: B).
The study had the limitations inherent to observational studies where there exists the possibility of bias related to the distribution of characteristics between comparative groups. To control this problem, we analyzed the homogeneity of the patients in the different cluster groups in the main variables, evidencing no differences but limited to the low sample in some groups. We analyzed eight different cluster schedules by physicians used in clinical practice, but some schedules could be underrepresented, and mixed results could result in an under- or overestimate of the global safety evaluation. We did not include a control group with the conventional scheme nor with the cluster scheme recommended by the manufacturer, so our results could only be compared with other published with comparable populations. Although there is no consensus about which cluster schedule is best, we found two schedules most frequently used in real-world setting that could be used for future clinical trial evaluations.
As conclusion, cluster schedules of SCIT at high allergen doses of Acaroid® used in regular clinical practice were well tolerated and shortened the build-up phase to 1-3 weeks, instead of the conventional eight weeks. This could benefit the patient in terms of comfort and treatment compliance and reduce the costs related to AIT while maintaining patient safety. Considering the previous, SCIT schedules should be adapted to the patient's needs.
This study was sponsored by Merck S.L.U. Allergopharma Spain, Spain. The sponsor participated in the design, interpretation of data and the decision to submit the manuscript for publication.
Begoña Soler López was contracted by Merck S.L.U. Allergopharma Spain, for the drafting of the publication and management; Nataly Cancelliere was an employee of the study sponsor. Teresa Garriga-Baraut perceived a fee for the design and coordination of the study. The other authors declare no conflict of interest.
Data obtained in the study will be available upon reasonable request.
- Robert G, Pfaar O, Akdi CA, Ansotegui IJ, Durham SR, et al. (2018) EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy 73: 76-98. [Crossref]
- Agache I, Lau S, Akdi CA, Smolinska S, Bonini M, et al. (2019) EAACI Guidelines on Allergen Immunotherapy: House dust mite-driven allergic asthma. Allergy 74: 855-873. [Crossref]
- Acaroid® summary of product characteristics, 2021.
- Brehler R, Kahlert H, Thum-Oltmer S (2010) Hypoallergene Präparate in der SCIT. Allergo J Int 19: 477-84.
- Feng S, Xu Y, Ma R, Sun Y, Luo X, et al. (2014) Cluster subcutaneous Allergen Specific Immunotherapy for the treatment of Allergic Rhinitis: A Systematic Review and Meta-Analysis. PLoS One 9: e86529. [Crossref]
- Solá Martínez FJ, de Luque Piñana V, González-Mancebo E, Sánchez-Guerrero I, García-González F, et al. (2018) Observational study on the tolerability of cluster subcutaneous immunotherapy in patients whit rhinoconjunctivitis with or without asthma sensitized to pollen: the SIMO study. Safety in Health.
- Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, et al. (2010) Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 126: 466-476. [Crossref]
- Global initiative for asthma. Global strategy for asthma management and prevention. 2018 GINA report.
- Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G (2010) Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 125: 569-574. [Crossref]
- Weber CM, Calabria CW (2010) Assessing the safety of subcutaneous immunotherapy dose adjustments. Ann Allergy Asthma Immunol 105: 369-375. [Crossref]
- Tabar AI, Echechipia S, García BE, Olaguibel JM, Lizaso MT, et al. (2005) Double-blind comparative study of cluster and conventional immunotherapy schedules with dermatophagoides pteronyssinus. J Allergy Clin Immunol 118: 109-118. [Crossref]
- Zhang L, Wang C, Han D, Wang X, Zhao Y, et al. (2009) Comparative study of cluster and conventional immunotherapy schedules with dermatophagoides pteronyssinus in the treatment of persistent allergic rhinitis. Int Arch Allergy Immunol 148: 161–169. [Crossref]
- Nieto García A, Nevot Falcó S, Carrillo Díaz T, Cumplido Bonny JA, Izquierdo Calderón JP, et al. (2013) Safety of cluster specific immunotherapy with a modified high-dose house dust mite extract. Eur Ann Allergy Clin Immunol 45: 78-83. [Crossref]
- Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR (2001) Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol 107: 87–93. [Crossref]
- Crimi N, Li Gotti F, Mangano G, Paolino G, Mastruzzo C, et al. (2004) A randomized, controlled study of specific immunotherapy in monosensitized subjects with seasonal rhinitis: effect on bronchial hyperresponsiveness, sputum inflammatory markers and development of asthma symptoms. Ann Ital Med Int 19: 98–108. [Crossref]
- Nanda A, O’connor M, Anand M, Dreskin SC, Zhang L, et al. (2004) Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol 114:1339–44. [Crossref]
- Colás C, Monzón S, Venturini M, Lezaun A (2006) Double-blind, placebo-controlled study with a modified therapeutic vaccine of Salsola Kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol 117: 810-816. [Crossref]
- Subiza J, Feliu A, Subiza JL, Fernández-Caldas E, Uhlig J (2008) Short term pre-seasonal cluster immunotherapy with a modified mixture of grasses results in significant improvement in nasal challenge test after 2 months of treatment. J Allergy Clin Immunol 121: S126.
- González-Gutiérrez ML, Domínguez-Ortega J, Torres-Hernández JA, De-Luque-Piñana V, Izquierdo-Calderón JP, et al. (2013) Safety of 2 build-up cluster immunotherapy schedules with a high-dose hypoallergenic pollen therapeutic extract. J Investig Allergol Clin Immunol 23: 197-211. [Crossref]
- Klimek L, Uhlig J, Mösges R, Retting K, Pfaar O (2014) A high polymerized grass pollen extract is efficacious and safe in a randomized double-blind, placebo-controlled study using a novel up-dosing cluster-protocol. Allergy 69: 1629-1638. [Crossref]
- Bernstein DI, Wanner M, Borish L, Liss GM (2004) Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-201. J Allergy Clin Immunol 113: 1129-1136. [Crossref]
- Justicia JL, Barasona MJ, Serrano P, Moreno C, Guerra F (2007) Predicting patients at high-risk of systemic reactions to cluster allergen immunotherapy: A pilot prospective observational study. J Investig Allergol Clin Immunol 17: 386-392. [Crossref]
- Epstein TG, Liss GM, Murphy-Berents K, Bernstein DI (2014) AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008-2012: an update of fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract 2: 161-167. [Crossref]
- Caminati M, Dama AR, Djuric I, Montagni M, Schiappoli M, et al. (2015) Incidence and risk factors for subcutaneous immunotherapy anaphylaxis: the optimization of safety. Expert Rev Clin Immunol 11: 233-245. [Crossref]
- Bernstein DI, Epstein T (2011) Systemic reactions to subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am 31: 241-249. [Crossref]
- Klimek L, Fox GC, Thum-Oltmer S (2018) SCIT with a high-dose house dust mite allergoid is well tolerated: safety data from pooled clinical trials and more than 10 years of daily practice analyzed in different subgroups. Allergo J Int 27: 131-139. [Crossref]
Editorial Information
Editor-in-Chief
Ying-Fu Chen
Kaohsiung Medical University, Taiwan
Article Type
Research Article
Publication history
Received date: December 14, 2021
Accepted date: December 22, 2021
Published date: December 27, 2021
Copyright
©2021 Garriga-Baraut T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Garriga-Baraut T, Jorda Alba P, Hernandez Suarez HR, Gonzalez Perez R, Melgar Perez J, et al. (2021) Observational study on the tolerability of cluster immunotherapy schedules in patients sensitized to house dust mites. Trends Med 21: DOI: 10.15761/TiM.1000285
