Aims: To value the antioxidant effect of vitamin C on atherogenesis induced by oxidative stress, using nitric oxide, superoxide dismutase and nitrotyrosine as biomarkers.
Main methods: Wistar rats were used: (A) Control; (B) Proinflammatory Induction for 30 days, (C) Proinflammatory Induction for 30 days+Vitamin C, (D) Proinflammatory Induction for 60 days and (E) Proinflammatory Induction for 60 days+Vitamin C. Proinflammatory Induction with adrenaline (0.1 mg/day/rat). Vitamin C (2,14 mg/day/rat) administered 20 days in (C) and 50 days in (E). Nitric oxide (NO) (µM) and superoxide dismutase(U/ml) were estimated employing spectrophotometry, nitrotyrosine (nM) employing Elisa and histopathological sections were analyzed by optical microscopy. ANOVA was used for quantitative variables and square Chi was used for categorical variables, significance p<0.05 was stablished in all cases.
Key findings: In groups (B) (12.23±1.14) and (D) (17.84±1.7) NO decreased compared with (A) (22.46±1.24) (p<0.001, p<0.01). NO normalization was found in (E) (21.78±1.9). Superoxide dismutase showed significantly increased in (B) (159.33±5.56) and (D) (241±5.6) compared to (A) (128.7±5) (p<0.01, p<0.001). Similar augment showed groups (C) (185.12±6.3) and (E) (298.75±3.17) compared with (A) (p<0.01, p<0.001). Nitrotyrosine was increased in (B) (5.03±0.1) and (D) (5.31±0.12) according to proinflammatory induction and decreased in (C) (2.28±0.32) when compared with (B) (p<0.001). Similar response showed (E) (0.73±0.4) compared with (D) (p<0.001). In groups (B) and (D) showed endothelial denudation, intimal thickening and vascular layers disorganization. Group (E) showed a reversal of the lesions described.
Significance: nitrotyrosine is a useful marker of peroxynitrite production and oxidative damage to endothelial cells. Vitamin C reversed the oxidative stress but did not reverse the atherogenic lesions in endothelial.
nitrotyrosine, superoxide dismutase, oxidative stress, atherogenesis, vitamin C
Cardiovascular diseases are the leading cause of morbidity and mortality in the occidental worldwide [1]. Ross proposed his inflammatory theory [2] in which postulated that the endothelial alteration is response at harmful stimuli. The established and emerging risk factors for cardiovascular disease [3] contribute to the atherogenic process initiated in the vascular wall due to imbalance in the homeostatic endotelial balance [4], characterized by the movement of cells from light into the vascular wall. The persistence of proinflammatory state stimulate the production of acute phase proteins such as fibrinogen, which high concentrations reflects inflammatory activity and endothelial dysfunction, both parts of the vascular disease [5]. Hyperfibrinogenemia induce pro-oxidative processes mediated by cytokines such as tumor necrosis factor, and these attract lipoproteins into subendothelial space and initiate a progressive oxidation [6]. This oxidative process, induce the expression of adhesion molecules that facilitate the access of monocytes and further processing to monocyte-macrophage residents, generating more free radicals and thus further oxidized lipoproteins, allowing to progress of the atherogenic lesion [7-9]. In this condition of vascular dysfunction and oxidative stress, nitric oxide (NO) synthesized in endothelial cells exerts its toxic effect by binding to the superoxide radical and/or hydrogen peroxide forming peroxinitritos [10,11]. Moreover, reactive nitrogen species such as peroxynitrite and nitrogen dioxide mediates nitration of tyrosine residues in proteins associated with biomembranes forming 3-nitrotyrosine. This represents an oxidative post-translational modification that disrupts the physiological NO signaling and metabolism drift towards pro-oxidative processes [12,13]. 3-Nitrotyrosine is thought to be a relatively specific marker of oxidative damage mediated by peroxynitrite. The formation of nitrotyrosine represents a specific peroxynitrite-mediated protein modification; thus, detection of nitrotyrosine in proteins is considered as a biomarker for endogenous peroxynitrite activity. The peroxynitrite-driven oxidation and nitration of biomolecules may lead to atherogenic process [13]. Nitrotyrosine formation would assess the toxic effect of NO involved in the disruption of cell signaling process inducing reversible damage difficulting obtain an additional risk stratification in the population [14]. The enzyme superoxide dismutase (SOD), is an endogenous protection against free radicals and behaves as major antioxidant enzyme; its function is to remove superoxide dismutation ion due prevent water and oxygen reactions with biological molecules. In a oxidative stress situation, SOD is overwhelmed by excessive production of oxygen and nitrogen reactives species,so it is unable to exert its enzymatic function to preserve the bioavailability of NO and keep the tone vascular [15].
Exogenous antioxidants like vitamin C (or ascorbic acid) could collaborate to standardize the enzymatic activity of SOD. Unlike endogenous antioxidants such as SOD, which neutralize the action of free radicals already formed, Vitamin C is a nonenzymatic group that interrupt the free radicals propagation due to inhibit the activation because react catalytically with them preventing chain reaction lipid peroxidation [16,17]. Humans do not synthesize this vitamin endogenously due to a genetic mutation that results in lack of a liver enzyme, L-gulonolactone oxidase, able of catalyzing the conversion of glucose to L-ascorbic acid [18]. Vitamin C or L-ascorbate is a glucose derivative, its hydroxyl groups associated with the double bond act as agents with high reducing power, allowing direct participate in oxygen reduction and functioning as donor substrate lipid peroxidation reactions. Besides being a water-soluble substance is an important antioxidant in the extracellular fluids and their molecular mechanism of action allows to consider it as an effective antioxidant.
In this context, this paper evaluates the antioxidant vitamin C effect in vascular oxidative stress, using NO, SOD and nitrotyrosine as biomarkers in rats with induced atherogenesis.
Male adults Wistar rats were housed at room temperature (20 ºC±2 ºC) with food and water ad libitum. After weaning, the animals were randomly assigned to single sex groups of 20 rats. The investigation was carried out according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institute of Health (No. 8023, revised 1996). Besides, the Medical School Ethics Committee (National University of Córdoba, Argentina) approved the experimental animal procedures. Total of 120 rats were used grouped into the following experimental conditions:
A) control (without intervention)
A-I) control + Vitamin C (without intervention and administration of vitamin C)
B) Induction proinflammatory (IP) for 30 days (with subcutaneous injection of adrenaline)
C) IP for 30 days + vitamin C (with subcutaneous injection of adrenaline and administration of vitamin C)
D) IP for 60 days (with subcutaneous injection of adrenaline)
E) IP for 60 days + vitamin C (with subcutaneous injection of adrenaline and administration of vitamin C)
No deaths and no animals were excluded in any of the groups studied. Considering previous work from our laboratory, proinflammatory induction (IP) was performed with subcutaneous injection of adrenaline (0.1 mg/day/rat) for 30 and 60 days [19,20]. Drug treatment was performed by administering vitamin C (2.14 mg/day/rat), equivalent to 500 mg given in dose human [18] from day 10 of the first IP and for a period of 20 to 50 consecutive days corresponding to 30 and 60 days of IP. It was administered orally, with the aid of a syringe adapted with 1 ml probe at its end, allowing to deposit the indicated dose of vitamin in the esophagus preventing it be regurgitated by the animal.
Blood was obtained at 72 hours of the last IP, coinciding with the 30 and 60 days, samples were centrifuged at 3000 rpm for 15 minutes to obtain the plasma and red cell lysing.
Fibrinogen (mg/dL) was determined by Ratnoff and Menzie’s method [21]. Nitrites; such reference nitric oxide (NO) (uM) by Griess’ reaction [22] and SOD activity (U/ml) was assayed in red cell lysates using a Randox Kit [23]. Both parameters were quantified in a MetroLab 1600 spectrophotometer. Nitrotyrosine (nM) was determined with Elisa using reagents Oxis technique Research [24].
Furthermore, in all groups were selected 300 cuts of thoracic aortic from origin to the last portion, because in rats has shown that injuries are preferably in the aortic portion, unlike humans where commonly they found in abdominal aorta. The aorta was severed from its origin to the last rib portion; 30 sections 4 μm making each cut, selected by simple blind. The processed for histopathology material preserved in formalin bufferizado 10%, it was stained with hematoxylin-eosin (HE) and examined by light microscopy with a 40X and 60X magnification. To stratify the degree of injury endothelial cells and intimal thickness control in thoracic aortas analyzed and classified as follows: endothelial denudation: Mild: <5%, moderate: between 5% -10% and severe:> 10%; intimal thickening measured in microns was classified as: mild: <5 µm; moderate: between 5-10 μm and severe:> 10 µm.
Statistical analysis
Quantitative variables were analyzed using ANOVA and post hoc test Hotelling. For the results of pathology Chi Square test was used for categorical variables, p<0.05 was considered as significant.
The proinflammatory status was confirmed by significantly increased in fibrinogen levels in the groups with IP for 30 days (B) (289±12.8 mg/dL) and IP for 60 days (D) (337±9.4 mg/dL) compared to the control (A) (199±11.2 mg/dL) (p<0.001). In addition, it was found that antioxidant administration for 50 days (E) (225±13 mg/dL) was reduced fibrinogen levels compared to (D) (p<0.001) (Figure 1a).
At the same time, a control group was performed with administration of vitamin C (A-I) in order to test whether oxidative stress biomarkers were modified by the antioxidant drug. The administration of vitamin C did not alter the levels of NO (23.89±1.7 µM) and SOD (122.3±4.36 U/ml), in addition nitrotyrosine was not quantified and electron microscopy did not show changes, otherwise when referring to the control group is done we will stand referring to the group (a) without intervention, to avoid redundancy of results.
When the bioavailability of NO was analyzed, a significant decrease in the groups with IP for 30 days (B) (12.23±1.14 µM) and IP for 60 days (D) (17.84±07.01 µM) compared to control (A) was evident (22.46±1.24 µM) (p<0.001, p<0.01 respectively). In addition, it was confirmed in the treaty for 20 days (C) group antioxidant administration failed to increase levels of NO (19.89±2.3 µM) to the values of the control group (A) (p<0.01). Different behavior expressed the treatment group for 50 days (E) (21.78±1.9 µM), which did not show difference compared to the control group (A) (Figure 1b).
The enzymatic activity of SOD in groups with IP for 30 days (B) (159.33±5.56 U/ml) and IP for 60 days (D) (241±5.6 U/ml) significantly increased compared to the control (A) (128.7±5 U/ml) (p< 0.01, p<0.001, respectively). In addition, it was found that the persistence of pro-inflammatory state (D), significantly increases the SOD enzyme response compared to group IP for 30 days (B) (p<0.001). Similar behavior showed the groups that received treatment with vitamin C for 20 days (C) (185.12±03.06 U/ml) and 50 days (E) (298.75±3.17 U/ml), in which increased activity of SOD significantly contrasted with (A) (p<0.01, p<0.001, respectively). Also objectify the prolonged antioxidant treatment group (E) expressed significantly increased SOD activity regarding the treaty for 20 days (C) (p<0.01) group (Figure 1c).
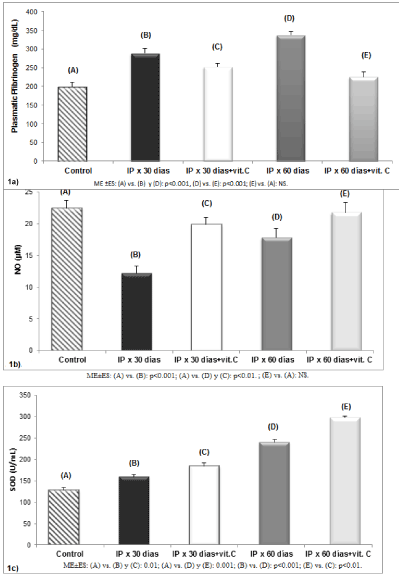
Figure 1. (a) Measurements of plasma fibrinogen in rats with proinflammatory induction and antioxidant treatment. (b) Nitric oxide levels in rats with proinflammatory induction and administration of vitamin C. (c) Effect of vitamin C on the enzymatic activity of superoxide dismutase in rats with proinflammatory induction (n=20 in each group)
Quantification of oxidative stress nitrotyrosine found in proinflammatory groups for 30 days (B) (5.03±0.1 nM) and 60 days (D) (5.31±0.12 nM) expressing an increase in concentration according to the persistence of the IP. On the other hand, a significant decrease in nitrotyrosine was saw in animals with antioxidant treatment for 20 days (C) (2.28±0.32 nM) compared to the untreated group (B) (p<0.001). Similar response showed the lot with administration of vitamin C for 50 days (E) (0.73±0.4 nM) contrasted with the IP group for 60 days (D) (p<0.001). At the same time, we noted that prolonged drug treatment group (E) decreased nitrotyrosine levels compared to animals treated with vitamin C for 20 days (C) (p<0.001) (Table 1).
Table 1. Quantification of nitrotyrosine in rats with proinflammatory induction and antioxidant treatment
Groups |
Nitrotyrosine (nM) |
Control (A) |
- |
IP x 30 days (B) |
5.03±0.1 |
IP x 30 days+vit.C (C) |
2.28±0.32 |
IP x 60 days (D) |
5.31±0.12 |
IP x 60 days+vit.C (E) |
0.73±0.4 |
ME ±ES: (B) vs. (C): p<0.001, (B) vs. (D): NS; (C) vs. (E): p<0.001; (D) vs. (E): p<0.001.
Proinflammatory induction (IP).
Mean and standard error (ME±ES).
Moreover, when 300 thoracic aorta tissue sections by light microscopy in group (B) (Figure 2a), 79.33% of same showed multiple sectors of endothelial denudation were analyzed and 66% of the intimal thickening. Similar lesions presented histopathology analyzed animals with IP for 60 days (D) (Figure 2b) where 283 cuts (94.33%) showed endothelial denudation and disorganization of the vascular layers with increased extracellular matrix was observed in 245 of 300 cuts studied (81.67%), expressing significant changes compared to the group (B) (p<0.01).
Animals treated with vitamin C for 20 days (C) (Figure 2c) showed persistence of endothelial denudation in 201 of 300 (67%) and intimal thickening in 186 slices (62%), showing a partial regression of vascular lesions compared with the group (B) (p<0.02). When histological sections in the group with prolonged antioxidant treatment (E) were analyzed, we observed a recovery in endothelial denudation in 196 (65.33%) and intimal thickening in 204 (68%) of the 300 courts studied, regarding the group (D) (p<0.01) as shown in Figure 2d. Optical microscopy of the control group (A) is displayed in Figure 2e (Table 2).
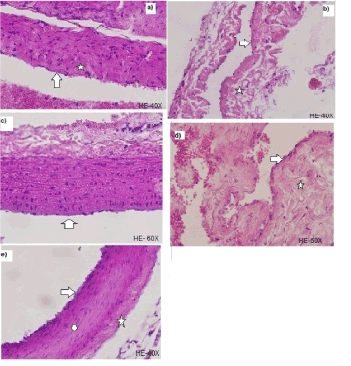
Figure 2. (a) Histological section of the thoracic aorta for the IP group for 30 days (B) where multiple sectors endothelial denudation (arrow) and intimal thickening (star) are displayed. (b) Histological section of the thoracic aorta for the IP group for 60 days (D) where endothelial denudation (arrow) and disorganization of the vascular layers with increased extracellular matrix (Star) was evident. (c) Histological section of the thoracic aorta for the IP group for 30 days+Vitamin C (C) which showed persistence of endothelial denudation (arrow) and intimal thickening (star). (d) Histological section of the thoracic aorta for the IP group for 60 days+Vitamin C (E) where a recovery in endothelial denudation (arrow) and intimal thickening (star) was observed. (e) Histological section of the thoracic aorta for the control group (A) where endothelium is observed (arrow) and adventitious unscathed (circle) and wall with several (star) elastic boundary layers
Table 2. Classification of lesions in rats with proinflammatory induction and vitamin C administration
Groups |
Endothelial Denudation |
Injury in other vascular layers |
IP x 30 days (B) *300 |
Mild: 16% Moderate 51% Severe: 33% n=243** |
Mild: 20% Moderate: 32% Severe: 48% n=198** |
IP x 30 days +vit. C (C) *300 |
Mild: 56 % Moderate: 21 % Severe: 23% n=201** |
Mild: 38 % Moderate: 22% Severe: 40 % n=186** |
IP x 60 days (D) *300 |
Mild: 17% Moderate: 27% Severe: 56% n=283** |
Mild: 13% Moderate: 25% Severe: 62% n=245** |
IP x 60 days +vit. C (E) *300 |
Mild: 75 % Moderate: 21 % Severe: 04% n=196** |
Mild: 66 % Moderate: 22 % Severe: 12 % n=204** |
*Histological sections studied by group.
**Numbers of cuts with anatomopathological modifications.
Endothelial Denudation: (B) vs. (D): p<0.01; (B) vs. (C): p<0.02; (E) vs. (D): p<0.01.
Intimal Thickening: (C) vs. (D): p<0.01; (B) vs. (C): p<0.02; (D) vs. (E): p<0.01.
Proinflammatory induction (IP).
Our results showed that the fibrinogen concentration increases in response to endothelial injury and in accordance with the persistence of pro-inflammatory stimulus. On this way it would demonstrate its active participation in early stages of atherosclerosis [20]. Fibrinogen´s measurements in plasma could be used as biological marker of atherogenesis regardless its role as cardiovascular risk factor [3,25].
A decrease in the bioavailability of NO in groups with IP originates a proinflammatory state and a loss of the homeostatic mechanisms. Thus, the persistence of inflammatory stimulus would be the beginning of endothelial dysfunction, inducing the process of oxidative stress and altering the delicate balance between vasodilators and vasoconstrictor factors [26,27]. NO has antithrombotic and antiatherogenic properties so, the NO decrease result in an impaired endothelium-dependent vasodilation, and also in the acceleration and accentuation of atherogenic process.
Multiple processes would be associated with pro-inflammatory and oxidative stress; therefore, the endothelial nitric oxide synthase can generate both superoxides instead of NO, or NO and superoxide and at the same time [28]. The NO molecule has a mismatched electron that allows it to react rapidly with oxygen and other reactive oxygen species. The reaction of NO with the superoxide ion produces peroxynitrite. This reaction occurs at a rate three times higher than the SOD is able to dismantle the superoxide ion. This results in a decrease in NO bioavailability with inhibition of its physiological functions [12,14]. The oxidative inactivation of NO results in excessive peroxynitrite formation, this is potent biological oxidant, whose pathophysiological effects accentuate the vascular lesions as we observed in Figures 2a and 2b.
Moreover, in this research we observed an increment in SOD activity versus time of proinflammatory induction, demonstrating an adaptive response to oxidative stress present in the vascular wall [29]. Inflammatory signals activate the endothelium using superoxide radical chain reaction generating free radicals which initiate lipid peroxidation causing loss in its structure and function cellular [30] as observed in pathologic sections of IP groups (Figures 2a and 2b).
In addition, the quantification of nitrotyrosine in groups with IP indirectly demonstrate the production of peroxynitrite and oxidative damage in endothelial cells, it would be the "mark" that would exempt when vascular tissue is damaged during atherogenic process [13,31]. The presence of nitrotyrosine in our research, only observed in animals with proinflammatory induction therefore normally not synthesized.
Consistent with the plasma results, showed histopathological studies in groups with IP endothelial denudation, leaving the exposed collagen platelet aggregation facilitating further reflecting focal inflammatory activity level of the vessel wall. Also, it noted that the persistence of proinflammatory state generated thickening vascular intima and increase in the extracellular matrix, probably as a result of a number of physiological stimuli that are an attempt to maintain tissue vascular homeostasis. These lesions showed that IP is associated with endothelial histopathological changes of Type I, II and III of the Stary [32] classification.
Those results demonstrate that the antioxidant mechanism exerted by the SOD was insufficient. Vitamin C prolonged treatment showed anti-inflammatory effect with reduced reactive oxygen and nitrogen species, increasing the bioavailability of NO and inhibing the formation of peroxynitrite. The L-ascorbic acid donates two electrons from a double bond between carbons at positions 2 and 3, this action would preserve the physiological functions of NO by intracellular superóxide ion chelation [33,34].
The SOD activity increased in treated animals and this suggests that vitamin C to scavenge free radicals formed during the atherogenic process, restoring the enzymatic response of SOD [12,18]. In addition, the antioxidant capacity of this vitamin in the treated groups, showed a decrease in levels of nitrotyrosine, indirectly demonstrating that peroxynitrite formation would be controlled at the endothelial level. Thus, there would cooperation of antioxidant systems to reverse the pathophysiological state leading to the atherogenic process.
Similarly, the pathological study showed partial restitution of the injuries described in the group treated for 50 days. Ascorbic acid strengthens the permeability barrier by improving endothelial dysfunction, preventing lipoprotein oxidation by neutralizing free radicals and inhibiting the formation of peroxynitrite [35-37]. The partial regression of the lesions observed in different groups with treated IP, is related to the ability of vitamin C to inhibit the differentiation and proliferation of vascular smooth muscle cells in the areas of vascular damage. It is likely required extended treatment periods to exert more definitive antioxidants effects.
The authors declare that there is no conflict of interests regarding the publication of this paper.
The authors gratefully the financial support of:
- Secretary of Science and Technology (SECYT) of National University of Cordoba
- Science and Technology (CYCYT) of National University of La Rioja.
- MINCYT, Ministry of Industry of the Province of Córdoba.
- Guterbaum T, Gaedeb P (2011) Intervención sobre múltiples factores de riesgo para prevenir la enfermedad cardiovascular. Un enfoque basado en la evidencia. Rev Esp Cardiol 64: 173–174.
- Ross R (1999) Atherosclerosis is an inflammatory disease. Am Heart J 138: S419-420. [Crossref]
- Wang S, Liu J, Wu DI, Pang X, Zhao J, et al. (2015) Pro-inflammatory effect of fibrinogen on vascular smooth muscle cells by regulating the expression of PPARα, PPARγ and MMP-9. Biomed Rep 3: 513-518. [Crossref]
- Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801-809. [Crossref]
- Carbayo Herencia JA, Simarro Rueda M, Artigao Ródenas LM, Divisón Garrote J, Caldevilla Bernardo D, et al. (2013) Relación entre el proceso inflamatorio y la mortalidad de origen cardiovascular y por todas las causas en un estudio de cohortes prospectivo de base poblacional. Clin Invest Arterioscl 25: 56-62.
- Moya M, Campana V, Gavotto AC, Spitale L, Simes J, et al. (2005) Simvastatin: pharmacological response in experimental hyperfibrinogenaemias. Acta Cardiol 60: 159-164.
- Bryk D, Olejarz W, Zapolska-Downar D (2017) The role of oxidative stress and NADPH oxidase in the pathogenesis of atherosclerosis. Postepy Hig Med Dosw (Online) 71: 57-68. [Crossref]
- Bobryshev YV, Nikiforov NG, Elizova NV, Orekhov AN (2017) Macrophages and Their Contribution to the Development of Atherosclerosis. Results Probl Cell Differ 62: 273-298. [Crossref]
- Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E (2013) Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol 62: 1541-1551. [Crossref]
- Upmacis RK (2008) Atherosclerosis: A Link Between Lipid Intake and Protein Tyrosine Nitration. Lipid Insights 2008: 75. [Crossref]
- Ozkanlar S, Akcay F (2012) Antioxidant Vitamins in Atherosclerosis– Animal Experiments and Clinical Studies. Adv Clin Exp Med 21: 115–123.
- Förstermann U (2010) Nitric oxide and oxidative stress in vascular disease. Pflugers Arch 459: 923-939. [Crossref]
- Radi R (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 46: 550-559. [Crossref]
- Diers AR, Broniowska KA, Hogg N (2013) Nitrosative stress and redox-cycling agents synergize to cause mitochondrial dysfunction and cell death in endothelial cells. Redox Biol 1: 1–7. [Crossref]
- Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R (2009) Novel aspects of oxidative stress in cardiovascular diseases. Circ J 73: 201-207. [Crossref]
- Ellulu MS (2017) Obesity, cardiovascular disease, and role of vitamin C on inflammation: a review of facts and underlying mechanisms. Inflammopharmacology 25: 313-328. [Crossref]
- Honarbakhsh S, Schachter M (2009) Vitamins and cardiovascular disease. Br J Nutr 101: 1113-1131. [Crossref]
- Aguirre R, May JM (2008) Inflammation in the vascular bed: importance of vitamin C. Pharmacol Ther 119: 96-103. [Crossref]
- Palma JA, Enders J, de Oliva PP (1981) Effects of epinephrine on plasma fibrinogen levels in rats submitted to tissue injury. Experientia 7: 780-782.
- Baez MC, Táran MD, Campana V, Simes JC, Pons P, et al. (2009) Oxidative stress markers in atherogenesis induced by hyperfibrinogenemia. Arch Cardiol Mex 79: 85-90. [Crossref]
- Ratnoff OD, Menzie C (1951) A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med 37: 316-320. [Crossref]
- Muñoz-Fuentes RM, Vargas F, Bobadilla NA (2003) Assay validation for determining nitrites and nitrates in biological fluids. Rev Invest Clin 55: 670-676. [Crossref]
- Woolliams JA, Wiener G, Anderson PH, McMurray CH (1983) Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci 34: 253-256. [Crossref]
- ter Steege JC, Koster-Kamphuis L, van Straaten EA, Forget PP, Buurman WA (1998) Nitrotyrosine in plasma of celiac disease patients as detected by a new sandwich ELISA. Free Radic Biol Med 25: 953-963. [Crossref]
- Baez MC, Tarán M, Culasso JM, Balceda A, Scribano MP, et al. (2013) Nuevo rol para una vieja proteína: fibrinógeno. Hematologia 17: 21-25.
- Delgado Roche L, Martínez Sánchez G, Díaz Batista A (2009) Determinación de marcadores de estrés oxidativo en pacientes con enfermedades cardiovasculares. Acta Bioquím Clín Latinoam 43: 307-313.
- Tousoulis D, Briasoulis A, Papageorgiou N, Tsioufis C, Tsiamis E, et al. (2011) Oxidative stress and endothelial function: therapeutic interventions. Recent Pat Cardiovasc Drug Discov 6: 103-114. [Crossref]
- Grassi D, Desideri G, Ferri C (2011) Cardiovascular Risk and Endothelial Dysfunction: The Preferential Route for Atherosclerosis. Curr Pharm Biotechnol 2: 1343-1353. [Crossref]
- Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R (2008) Atherosclerosis and oxidative stress. Histol Histopathol 23: 381-390. [Crossref]
- McCord JM (2008) Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Response 6: 223-238. [Crossref]
- Huang H, Koelle P, Fendler M, Schröttle A, Czihal M, et al. (2014) Induction of inducible nitric oxide synthase (iNOS) expression by oxLDL inhibits macrophage derived foam cell migration. Atherosclerosis 235: 213-222. [Crossref]
- Fuster V, Gotto AM, Libby P, Loscalzo J, McGill HC (1996) Matching the intensity of risk factor management with the hazard for coronary disease events. Pathogenesis of coronary disease: the biologic role of risk factors. J Am Coll Cardiol Medline 27: 964-976. [Crossref]
- García GA, Cobos C, Rey CA, Mejía OR, Casariego CA, et al. (2006) Biología, patobiología y bioclínica de la actividad de oxidorreducción de la vitamina C en la especie humana. Rev CES Med 20: 53-72.
- Traber MG, Stevens JF (2011) Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med 51: 1000-1013. [Crossref]
- Frikke-Schmidt H, Lykkesfeldt J (2009) Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin Pharmacol Toxicol 104: 419-433. [Crossref]
- May JM, Qu ZC (2010) Ascorbic acid prevents increased endothelial permeability caused by oxidized low density lipoprotein. Free Radic Res 44: 1359-1368. [Crossref]
- Zhang PY, Xu X, Li XC (2014) Cardiovascular diseases: oxidative damage and antioxidant protection. Eur Rev Med Pharmacol Sci 18: 3091-3096. [Crossref]


