Microvascular angina refers to chest pain associated with coronary microvascular dysfunction (CMD). A new paradigm in cardiology was entrenched when the WISE studies demonstrated that angina may be present in patients with non-obstructive coronary artery disease (NOCAD). Statistically, there is a higher number of cases in women. The prevalence of NOCAD has been perceived in recent years reaching up to 20-30% of all coronary artery disease CAD. 60% of the patients with angina symptoms may not present any sign of obstructive coronary artery disease (OCAD). In 2015, the COVADIS group established diagnostic criteria for microvascular angina. Recent studies used these criteria to design randomized clinical trials to elucidate the prevalence, diagnosis, treatment and prognosis of this entity. Conventional invasive angiography suggests that up to two-thirds of patients with NOCAD may have CMD, however this test does not allow direct visualization of the microcirculation. Non-invasive imaging modalities such as Positron Emission Tomography (PET) enable a direct and accurate assessment of coronary microvascular function. Thus, PET is becoming an indispensable tool in the evaluation of suspected CMD.
In 1973, Harvey Kemp described what he called ‘cardiac syndrome X’ in patients with chest pain, ischemic electrocardiographic changes and absence of epicardial obstruction [1]. Invasive coronary angiography is considered the gold standard for the diagnosis of OCAD. Despite the well-established link between OCAD and myocardial ischaemia, a significant proportion of patients with signs and symptoms suggestive of myocardial ischaemia do not have significant flow limiting lesions on coronary angiography. Thus, abnormalities in the coronary microcirculation are thought to be the cause of their angina symptoms [2-4].
In the last two decades, noninvasive techniques for assessing CMD have evolved. PET can identify CMD noninvasively by assessing reductions in hyperemic myocardial blood flow (MBF) in absolute terms (milliliters per minute per gram) and / or myocardial flow reserve (MFR) for the noninvasive identification and characterization of CMD as an important functional substrate for angina symptoms [5]. Notably, the identification and characterization of CMD by PET flow studies provides relevant prognostic information that can affect the treatment decision process [5,6]. CMD is characterized by a low Coronary Flow Reserve (CFR) – the ratio of coronary flow at maximum vasodilation divided by the basal coronary flow [7].
Given the importance of microvascular disease, we reviewed the current literature on CMD and microvascular angina, focusing on its pathophysiology, clinical presentation, risk factors, and prognosis.
NOCAD prevalence has increased in recent years, accounting for 20 to 30% of all CAD, approximately two thirds of these cases present in women. Sara, et al. followed 1,439 patients with chest pain, finding that 66% of the patients presented with CMD [8]. Ford, et al. studied a total of 391 patients with angina during the CorMicA clinical trial, of which 151 (39%) did not show any sign of OCAD [3]. Based on the results mentioned above, it is clear that NOCAD is a common problem that can explain a substantial proportion of cases of chest pain in the absence of epicardial lesions.
The coronary arterial system is formed by multiple vessels, each type presenting different characteristics and functions. Epicardial arteries are mostly conductive vessels and are regulated by both myocardial wall stress and endothelial function. On the other hand, pre-arterioles and arterioles are the main vascular resistance determinants. Pre-arterioles are regulated by flux and pressure, while arterioles by cardiac metabolism byproducts. Finally, the primary function of capillary vessels is to control the fluid exchange within the tissue. [9,10]. The maximal myocardium oxygen extraction is obtained during rest, thus, oxygen delivery depends mostly on dynamic vascular resistance changes to maintain a constant coronary flow; these homeostatic mechanisms prevent myocardial ischemia [9,11].
Furthermore, CMD is mediated by various subendocardial hypoperfusion pathogenic mechanisms, which occur mostly during stress, and can potentially cause myocardial ischemia. Rahman, et al. identified two groups of vascular impairments in these patients: structural and functional abnormalities; each one with a different pathophysiological pattern, and presenting itself either as a single isolated abnormality, or together [12] (Figure 1).
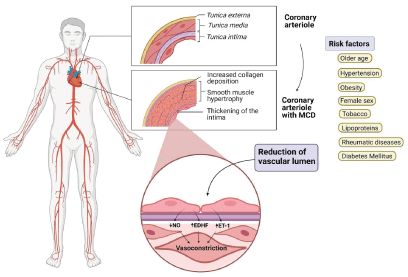
Figure 1. Pathophysiology of CMD
MCD: microvascular coronary dysfunction; NO: nitric oxide; EDHF: endothelial-derived hyperpolarizing factor; ET-1: endothelin-1.
Structural abnormalities
Structural abnormalities – also known as microvascular remodeling – are characterized by morphological changes, causing intraluminal obstruction, increasing minimal coronary resistance, and reducing CFR. These changes turn hyperemic dilation ineffective [10-14]. Furthermore, the ability to adapt to physical stress and exercise is affected by dysfunction in the synthesis of nitric oxide (NO) by the endothelium; causing elevated coronary pressure during strenuous physical activities. This leads to an increase in the afterload and the myocardium’s oxygen demand, resulting in a sustained elevated coronary vascular tone. [11].
Functional abnormalities
Functional abnormalities comprise elevated basal blood flow, which is secondary to a decreased resting microvascular resistance. Consequently, this increases myocardial perfusion during rest, and thus decreases the vasodilatory capacity during stress [15]. This dysfunction can be endothelium-dependent or independent [9,11,16]. Endothelium-dependent abnormalities are caused by an alteration in intrinsic pathways involved in endothelial relaxation, such as the production of NO [16] or vasoconstrictive agents such as endothelin-1 [9,16]. On the other hand, an alteration in adenylate cyclase pathway causes a decreased vasodilation capacity, independent from the endothelial function [16].
Some authors believe that increased basal blood flow is caused by an increase in myocardial oxygen demand during rest, and/or due to an excessive vasoconstrictive response that needs to be counteracted [11]. Broadly speaking, all the functional pathophysiological mechanisms in CMD cause a reduction in CFR, meaning they impede coronary blood flow from adapting correctly to the myocardial oxygen demand [9].
Camici and Crea classified CMD in 4 groups, depending on the patients’ clinical features [15] (Table 1 and Figure 2):
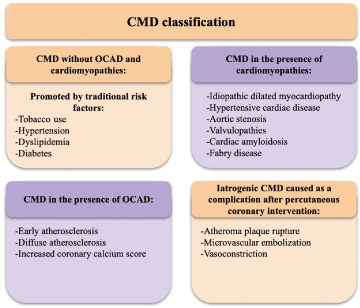
Figure 2. Classification of CMD according to comorbidities
CMD: coronary microvascular dysfunction; OCAD: obstructive coronary artery disease.
Table 1. Classification of CMD according to comorbidities
CMD without OCAD and cardiomyopathies |
This population tends to be asymptomatic, although patients may present with angina and low CFR measurements [15]. CMD in this group leads eventually to CAD, and atherosclerotic lesions, culminating in atherothrombosis and myocardial infarction [13] |
CMD in the presence of cardiomyopathies |
Both patients with primary (genetic) and secondary (acquired) cardiomyopathies can suffer from reduced CFR, indicating CMD [15]. Left ventricle hypertrophy (LVH) and microvascular remodeling are essential factors in the development of microvascular dysfunction in these patients. [12,13]. |
CMD in the presence of OCAD |
The extension and severity of CMD in patients with acute or stable chronic coronary syndromes, are related to the variation in the clinical manifestations of this population. Microcirculatory damage – both structural and functional – in these patients can cause anginal symptoms, or the so-called "no-reflow" phenomenon (impeded blood flow to an ischemic tissue after reperfusion therapy) [13]. |
Iatrogenic CMD |
Vasoconstriction or arterial embolization can occasionally occur secondary to successful reperfusion therapy. This vasoconstriction is mediated by alpha-adrenergic receptors activation. The embolisms are secondary to ruptured atheroma plaques that expel material down into the blood torrent and cause microcirculatory damage [13]. |
Clinical Manifestations
In clinical practice, it is frequent to encounter patients who refer to chest pain without evidence of OCAD. Up to 60% of patients with angina symptoms may not show any signs of OCAD [17]. Jaskanawal, et al. found in 2015 that over two thirds of patients with angina and no evidence of OCAD, had some degree of microvascular dysfunction [15]. Based on these findings, it has been theorized that a large proportion of patients with angina symptoms present coronary microvascular dysfunction, even if no apparent epicardial arteries are compromised.
Moreover, CMD patients commonly stay asymptomatic during long periods of time. Nonetheless, in symptomatic patients, the classical clinical features are stable angina, angina equivalents (e.g., dyspnea), persistence of symptoms during the recovery phase after exercise, and even heart failure [4,18] The physical examination of these patients is usually normal. [9]
In an effort to develop a standardized diagnostic system, the COVADIS group created a series of diagnostic criteria for microvascular angina [18] that are shown on table 2.
Table 2. Diagnostic criteria for microvascular angina.
Definitive microvascular angina is diagnosed only if criterias 1, 2, 3 and 4 are present.
1. Symptoms of myocardial ischemia |
- Effort and/or rest angina
- Angina equivalents (e.g., dyspnea, fatigue, diaphoresis, dizziness, indigestion, pain in locations other than the chest, among others)
|
2. Absence of OCAD (obstruction <50% or fractional flow reserve >0.80) confirmed by: |
- Coronary computed tomography angiography (CTA)
- Invasive coronary angiography
|
3. Objective evidence of myocardial ischemia |
- Ischemic ECG changes during an episode of chest pain
- Stress-induced chest pain and/or ischemic ECG changes, with or without the presence of transient/reversible abnormal myocardial perfusion and/or wall motion abnormality
|
4. Evidence of impaired coronary microvascular function |
- Impaired coronary flow reserve
- Coronary microvascular spasm
- Abnormal coronary microvascular resistance indexes (e.g., IMR N25)
- Coronary slow flow phenomenon
|
Risk factors associated with CMD are similar to those classically related to OCAD. On this matter, numerous studies have reported age as an important risk factor for the development of microvascular dysfunction. It has been theorized that vascular remodeling and progressive decrease in coronary flow allow the development of coronary microvascular dysfunction [19]. For instance, in a cohort study, Sara, et al. observed that older patients had abnormal responses to the administration of acetylcholine and adenosine, as well as higher prevalence of abnormalities in coronary microcirculatory function [17].
The association between tobacco use and cardiovascular disease is undeniable. Various studies have documented that the values of both CFR and MBF are diminished in smokers; Kaufmann, et al. for example, demonstrated a 21% reduction in CFR values in smokers compared with controls [19]. It is also important to note that in smokers, MBF values are normalized after a month of quitting.
The presence of systemic hypertension is associated with microvascular remodeling (tunica media hypertrophy, periarteriolar fibrosis, etc.) and alterations in vessel function, such as decreased CFR and MFR levels [19,20]. Decreased CFR has been associated with elevated levels of lipoproteins such as low density lipoprotein (LDL), and increase in cholesterol levels [19]. Lee, et al. demonstrated that LDL-C levels are an independent factor associated with a CFR value of <2 [21].
Endothelial dysfunction is one of the main physiopathological characteristics of diabetes mellitus. Hyperglycemia, insulin resistance and chronic inflammation have been associated with the development of CMD by altering the production of endothelial protective factors, such as NO, and therefore decreasing CFR values. [21,17]. Patients with type 1 and 2 diabetes mellitus have a higher prevalence of microvascular dysfunction, which gives them an increased risk of adverse cardiovascular events; occurring even before the onset of macrovascular complications [22]. Lee, et al. showed that higher Hb1Ac levels are an independent factor associated with a value of CFR < 2 [21,23].
Different studies have reported other risk factors associated with the appearance of microvascular dysfunction, including female sex, obesity, and rheumatological diseases. [8,19,22,24]
Non-invasive techniques allow us to evaluate ischemia by detecting regional differences in perfusion or ventricular wall motion in epicardial perfusion zones (coronary territories). In patients with angina, the first evaluations should be performed with non-invasive methods (Figure 3) [25]. These methods include imaging techniques that use radiotracers, cardiac MRI, and contrasted echocardiography [26]. PET scan is the non-invasive gold standard technique for MBF quantification, maximum hyperemia and CFR [12,26-28]. PET scan's capacity to measure blood flow (ml/g/min) and its linear relation between blood flow and radiotracer signal intensity allow us to evaluate ischemia in early and asymptomatic stages [5,27]. The most used radiotracers are 13N-Ammonium and 82-Rubidium; while 15O-Water [6,26,27], and 18F-flurpiridaz are still in trial phases [29].
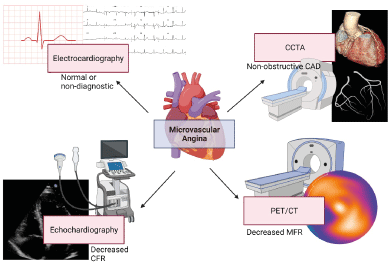
Figure 3. Non-invasive imaging techniques useful on the diagnosis of microvascular angina
CAD: coronary artery disease; CFR: coronary flow reserve; PET/CT: positron emission tomography-computed tomography; MFR: myocardial flow reserve
PET scans do not measure coronary epicardial blood flow directly; it actually measures myocardial blood flow. Thus, the term “myocardial blood flow reserve (MFR)” is the appropriate one for PET scans, and CFR should be reserved for invasive methods [29]. 13N-Ammonium and 82-Rubidium are currently the only FDA-approved radiotracers. 13N-ammonium generates high-quality images thanks to its high myocardial extraction (80%). 82-Rubidium's advantage is that it does not need a cyclotron to be produced [5]. Maximum hyperemia is achieved with endothelium-independent vasodilators, such as adenosine and regadenoson [25].
PET scan’s importance relies on its ability to calculate MFR accurately. Low values of MFR (<1.5), are strongly related to patient comorbidity [6,29-31]. The opposite is also true, a high MFR (>2.0) has a great negative predictive value for high-risk coronary artery disease (such as multivessel disease) [6,29,31].
Correlating results between non-invasive and invasive methods is important since hyperemic myocardial blood flow is decreased both on significant epicardial disease and microvascular dysfunction. [12,32] Abnormal MFR in patients without coronary artery disease have been associated with diastolic dysfunction and increased risk of hospitalization secondary to heart failure with preserved ejection fraction (HFpEF) [30].
PET scan has greater sensibility than single-photon emission computed tomography (SPECT) due to its higher counting rate, intrinsic attenuation correction, and quantitative evaluation of myocardial blood flow in absolute units (ml/g/min), which in turn allows for the calculation of MFR with BMF and hyperemic myocardial flow (Table 3). This quantification is useful for diagnosis and prognosis [33].
Table 3. PET-Scan strengths (left) and weaknesses (right)
\
PET-Scan strengths |
PET-Scan Weaknesses |
Most validated for myocardial blood flow and NOCAD evaluation [30] |
Limited by cost and availability [12,30] |
Shows many prognosis factors [30] |
Most radiotracers require a cyclotron for production (except for 82-Rubidium) [34] |
Relatively low radiation exposure thanks to low radiotracer half-life [30] |
Differentiation between microvascular disease and epicardial disease can be challenging [32] |
Kidney disease does not affect the study [30] |
Many comorbidities (such as diabetes, hypertension, age, tobacco exposure) can alter the recollected data [21] |
Good reproducibility and precision [30] |
Validity reduced by obstructive lesions >70% or abnormal coronary FFR [21] |
PET/CT combination allows for anatomic evaluation of coronary arteries [30] |
Hypoxic induced collaterals can reduce the manifestation of regional stress induced ischemia, reducing the validity [5] |
Table 4. Analysis of multiple studies for the prognosis of patients with microvascular dysfunction
Authors |
Study type |
Population studied (n) |
Measured variable |
Result |
P |
Murthy, M, et al. [24] |
Prospective cohort |
n=1218 |
MACE |
OR=0.80 [95% CI 0.75-0.86] for each 10% increase in CFR values. |
P < 0.0001 |
Taqueti, VR, et al. [35] |
Prospective cohort |
n=329 |
MACE/
heart failure hospitalization |
OR= 2.17 [95% CI, 1.34–3.52] for each CFR unit decreased. |
P=0.002 |
Taqueti, VR, et al. [36] |
Prospective cohort |
n=510 |
MACE |
Univariate analysis: OR 2.06 [95% CI 1.37-3.11] when CFR < 1.65.
Multivariate analysis: OR 1.78 [95% IC 1.16–2.74] when CFR >65 |
Univariate analysis: P=0.0006
Multivariate analysis:
P= 0.01 |
Taqueti, VR, et al. [37] |
Prospective cohort |
n=761 |
MACE |
Univariate analysis: OR 2.48 [95% CI, 1.45–4.24] for decreased CFR.
Multivariate analysis: OR 2.25 [95% CI, 1.31– 3.86] for decreased CFR. |
Univariate analysis:
P=0.001
Multivariate analysis:
P=0.003 |
Gupta A, et al. [38] |
Prospective cohort |
n=4029 |
CFR |
Univariate analysis: Adjusted OR 3.37 [95% CI 2.76-4.11], for each CFR unit decreased and 2.25 [95% CI 1.94-2.62], for each MBF unit decreased.
Análisis multivariado: Adjusted OR 1.83 [95% CI 1.47-2.27] for each CFR unit decreased and 1.35 [95% CI 1.13- 1.61] for each MBF unit decreased |
Univariate analysis:
P<0.001
P<0.001
Multivariate análisis:
P<0.001
P=0.001 |
Ziadi, MC, et al. [30] |
Prospective cohort |
n=677 |
Significant events and MACE |
Significant events: OR 3.3 [95% CI 1.1-9.5].
MACE: OR 2.4 [95% CI 1.4-4.4]. |
Significant events:
P<0.029
MACE:
P<0.003 |
Herzog, BA, et al. [31] |
Prospective cohort |
n=245 |
MACE and cardiovascular death in 1 year. |
MACE: 6.25% with altered CFR vs. 1.4% without.
Cardiovascular death: 3.1% with altered CFR vs 0.5% without. |
MACE:
P< 0.05
Cardiovascular death:
P< 0.05 |
Many authors have studied CMD´s role as an independent predictive factor for the development of major adverse cardiovascular events (MACE) and death related to cardiovascular events [35-38]. Since CMD is an infra diagnosed and probably frequent disease, it is crucial to analyze the prognosis of these patients [39].
Brainin P, et al. included six studies in their meta-analysis that evaluated epicardial endothelium dysfunction in diabetic patients with a positive stress test or angina and followed them for an average of 2.8-9.7 years. They found a total of 243 cardiovascular events, concluding that coronary endothelial dysfunction confers an increased risk for cardiovascular events (OR 2.38, 95% CI 1.74-3.25). This same meta-analysis included ten studies of patients with stable angina, hypertrophic cardiomyopathy, heart failure, atrial enlargement, and decreased CFR values. This analysis also reported an increased risk for cardiovascular events in patients with lower CFR values (OR 2.44, 95% CI 1.8-3.3) [40].
Gender variations
PET can differentially contribute to the evaluation of patients (especially women) with symptoms of myocardial ischemia [41]. A decreased CFR based on PET is associated with an increase in MACE frequency for approximately one year [30]. Furthermore, recent data has consistently shown that CFR PET measurements can distinguish patients at low or high risk of serious adverse events, including cardiac death beyond comprehensive clinical assessment, LVEF, or traditional semi-quantitative measures of stress-induced ischemia [29]. Between 50% to 60% of women with chest pain and NOCAD on coronary angiography may present an abnormal response on pharmacological stress tests with adenosine or acetylcholine with a reduction in coronary flow reserve during a direct assessment of coronary artery flow, or with SPECT or PET, is indicative of CMD [42].
However, Taqueti, et al. [42] reached out new information on the interaction of sex, CAD severity, and coronary vasomotor dysfunction in adverse events. While coronary angiography found that most men with severely impaired CFR had a CAD of ≥1 vessel, most women with a similarly impaired CFR demonstrated a CAD of ≤1 vessel. However, despite a lower likelihood of pre-test CAD and a lower burden of diagnosed angiographic CAD, women experienced a higher risk of cardiovascular events than men. In the adjusted analysis, up to 40% of this observed "sex gap" was mediated by CFR [42]. This implies that a very low CFR (<1.6) may be a critical link in understanding the hidden biohazard of ischemic cardiopathy among women.
In a review of nearly 400,000 coronary angiograms of women and men with chest pain complaints, only 37.6% have OCAD [17]. NOCAD is associated with adverse cardiovascular outcomes [43]. It is estimated that approximately half of NOCAD patients have CMD, and over the past two decades, it has become increasingly clear that a large proportion of NOCAD patients are women with CMD [44]. The WISE study concluded that cardiovascular death or myocardial infarction occurred in 12.8% of women with NOCAD and that the combined risk of death from myocardial infarction, heart failure, and stroke was more than 2% annually [45]. Furthermore, persistence or worsening of symptoms was common among the participants. Repeat coronary angiography was performed at a 1.8 times higher rate in women with NOCAD than in patients with OCAD, and one in five women were rehospitalized for cardiac symptoms [46].
Similarly, women are more likely to have myocardial infarction without OCAD [47], representing up to 14% of all acute myocardial infarctions [48]. Data from more than 750 hospitals in the USA (from 2007 to 2014) indicate that myocardial infarction without OCAD occurs in 10.5% of women with myocardial infarction (MI) compared to 3.4% of men [49].
Patients with MI without OCAD may also have a different cardiovascular risk profile than patients with OCAD because they are less likely to be diagnosed with hyperlipidemia [48] and diabetes [50]; however, a higher prevalence of hypertension has been reported [51]. Likewise, patients with myocardial infarction without OCAD report less angina before MI [51], and non-ST-elevation MI (NSTEMI) represent two-thirds of cases [48].
Although the prognosis for myocardial infarction without OCAD appears to be more favorable than MI with OCAD, it is not benign. In a systematic review, the analysis of 8 studies found that, despite a lower mortality rate than those with OCAD, myocardial infarction without OCAD was associated with a mortality rate of 4.7% in the following 12-months [48]. Furthermore, up to 25% of myocardial infarction patients without OCAD reported persistent angina after MI and experienced similar rates of hospitalization for angina as their counterparts with OCAD [51]. According to Planer, et al. in a cohort of 197 patients with myocardial infarction without OCAD, recurrent MI, as well as urgent revascularization, was significantly lower than in those with OCAD [52].
Recently, a clinical study that examined the impact of sex on the outcome after coronary angiography in patients with angina and NOCAD revealed that women were three times more likely to experience MACE within the first year after cardiac catheterization compared to men [46].
A history of previous preeclampsia (Pp) is a coronary risk factor [53], it also has been associated with a high prevalence of CMD. They are known to increase the risk for hypertension and diabetes; evidence suggests that both conditions also cause endothelial dysfunction during pregnancy, which may increase the risk of ischemic cardiopathy in later life regardless of hypertension or diabetes. Kul S et. found that these patients have a reduced CFR (which is a marker of CMD) and is related to an increased risk for cardiovascular events [54].
Certainly, all medical personnel should be fully aware that ischemic heart disease in women can be caused not only by atherosclerotic obstructive epicardial CAD but also by CMD.
Heart failure with preserved ejection fraction
Recently, CMD abnormalities associated with endothelial dysfunction and microvascular inflammation have been implicated as a possible pathogenic basis for HFpEF; a poorly understood clinical syndrome. [55-57]. Thus, CMD could explain other deleterious effects, such as exercise-induced myocardial ischemia and left ventricle subendocardial systolic dysfunction [58].
In the PROMIS study — the largest prospective multicenter study of CFR measurement in patients with HFpEF—, it was found a high prevalence of CMD in this group of patients, approximately 75% [57]. Furthermore, HFpEF is associated with increased NTproBNP (a marker of severity in HF), systemic endothelial dysfunction, and cardiac dysfunction. The comorbidities most closely associated with CMD were a history of smoking and atrial fibrillation. Microvascular dysfunction may be a promising composite risk marker and a therapeutic target in HFpEF [57].
CMD is associated with poor outcomes in patients with HFpEF [59]. Taqueti, et al. demonstrated that in symptomatic patients without OCAD with preserved LVEF, an altered CFR was associated with future diastolic dysfunction and MACE, especially HFpEF events, including hospitalization for HFpEF. Furthermore, patients with diastolic dysfunction and impaired CFR demonstrated a greater risk of hospitalization for HFpEF [56]. Undoubtedly, the evidence from the PROMIS-HFpEF trial [57] and the study by Taqueti, et al. [56] illustrates the associations between a decreased (abnormal) CFR with parameters of myocardial injury and diastolic dysfunction, suggesting the presence of endothelium-independent CMD in patients with HFpEF. More recently, Yang et al. [60] demonstrated that, in patients with HFpEF without OCAD, both endothelium dependent and independent CMD is common. Diastolic dysfunction correlates with endothelium-independent microvascular dysfunction. However, it cannot be identified only by clinical markers or comorbidities. The presence of CMD in HFpEF is associated with an increased risk of death, [60] so more studies are needed to better define the role, pathophysiology, evaluation, and treatment of coronary microvascular disease in patients with HFpEF.
Diabetes Mellitus
Patients with metabolic syndrome and those with diabetes mellitus (DM) have a higher risk of developing OCAD and heart failure. [61]. In recent years, CMD associated with diabetes has been widely explored [62]. Picchi A, et al. demonstrated that CMD is common in patients with type 2 DM [62]. Similarly, Sara JD, et al. showed that up to 72.1% of patients with diabetes have some type of CMD [63]; demonstrating that CMD is prevalent among an unselected population of patients with type 2 DM who present with chest pain and NOCAD on coronary angiography [63].
The relationship between glycemic control and CMD is not well established. As CMD is common in diabetes and is related to adverse cardiovascular events, particularly among women [64], the potential link between glycemic control and cardiovascular morbidity and mortality could be explained and mediated, in part, by CMD. However, this requires further investigation with prospective studies. Therefore, risk prevention strategies in patients with type 2 diabetes could include therapies specifically directed at CMD [63].
A significant proportion of patients who suffer chest pain and undergo diagnostic coronary angiography do not manifest significant obstructive coronary lesions. This group of patients have a combination of functional and structural anomalies in their microvascular circulation which are related to endothelial dysfunction. It is of great importance to suspect and diagnose CMD accurately using the criteria established by the COVADIS group, as this disease has been historically under-diagnosed and is associated with higher cardiovascular morbidity and mortality. Patients with clinical manifestations associated with cardiac ischemia in the absence of obstructive coronary lesions must undergo further testing. To date, there is no consensus on how to stratify risk in these patients, nonetheless, invasive and non-invasive tests can be used to assess CFR. The development of these diagnostic techniques has permitted in recent years to achieve a better diagnostic evaluation in patients who suffer from CMD and MVA.
Based on the review conducted on the current literature, we can conclude that there is a lack of properly conducted clinical trials to evaluate these groups of patients. Therefore, it is fundamental to encourage future researchers to develop new protocols aiming to establish new diagnostic and therapeutic standards of care in CMD and MVA.
- Kemp HG (1973) Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol 32: 375-376 [Crossref]
- L B, CNB M, J W, C S, E H, C P, et al. Even “WISE-R?”-an Update on the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation. Curr Atheroscler Rep 22: 35. [Crossref]
- Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, et al. (2018) Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol 72: 2841-2855. [Crossref]
- Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, et al. (2015) Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv 8: 1445-1453. [Crossref]
- Schindler TH, Schelbert HR, Quercioli A, Dilsizian V (2010) Cardiac PET Imaging for the Detection and Monitoring of Coronary Artery Disease and Microvascular Health. JACC Cardiovasc Interv 3: 623-640. [Crossref]
- Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, et al. (2018) Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med 59: 273-293. [Crossref]
- MA G, VL M, M D, AG M-G, R S, DL B. Association of Isolated Coronary Microvascular Dysfunction With Mortality and Major Adverse Cardiac Events: A Systematic Review and Meta-Analysis of Aggregate Data. J Am Heart Assoc 9: e014954. [Crossref]
- Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, et al. (2015) Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv 8: 1445-1453. [Crossref]
- Taqueti VR, di Carli MF (2018) Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol 72: 2625-2641. [Crossref]
- Pries AR, Reglin B (2017) Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J 38: 478-488. [Crossref]
- Rahman H, Ryan M, Lumley M, Modi B, McConkey H, et al. (2021) Coronary Microvascular Dysfunction Is Associated With Myocardial Ischemia and Abnormal Coronary Perfusion During Exercise. Circulation 140: 1805-1816. [Crossref]
- Vancheri F, Longo G, Vancheri S, Henein M (2020) Coronary Microvascular Dysfunction. J Clin Med 9: 2880. [Crossref]
- Rahman H, Demir OM, Khan F, Ryan M, Ellis H, et al. (2020) Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol 75: 2538–2549. [Crossref]
- Schindler TH, Dilsizian V (2020) Coronary Microvascular Dysfunction: Clinical Considerations and Noninvasive Diagnosis. JACC Cardiovasc Imaging 13: 140-155. [Crossref]
- PG C, FC (2007) Coronary microvascular dysfunction. N Engl J Med 356: 830-840. [Crossref]
- Shome JS, Perera D, Plein S, Chiribiri A (2021) Current perspectives in coronary microvascular dysfunction. Microcirculation 24: e12340. [Crossref]
- Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, et al. (2010) Low Diagnostic Yield of Elective Coronary Angiography. N Engl J Med 362: 886-895. [Crossref]
- Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, et al. (2018) International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 250: 16-20. [Crossref]
- Chen C, Wei J, AlBadri A, Zarrini P, Merz CNB (2016) Coronary Microvascular Dysfunction - Epidemiology, Pathogenesis, Prognosis, Diagnosis, Risk Factors and Therapy. Circ J 81: 3-11. [Crossref]
- O R, SD R, PG C (2014) The blunting of coronary flow reserve in hypertension with left ventricular hypertrophy is transmural and correlates with systolic blood pressure. J Hypertens 32: 2465-2471. [Crossref]
- DH L, HJ Y, YS C, CS P, JH P, et al. (2010) Coronary flow reserve is a comprehensive indicator of cardiovascular risk factors in subjects with chest pain and normal coronary angiogram. Circ J 74: 1405-1414. [Crossref]
- RE K, TJ G, JC K, AHEM M, SE E-S (2020) The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc Res 116: 817-828. [Crossref]
- Sara JD, Taher R, Kolluri N, Vella A, Lerman LO, et al. (2019) Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc Diabetol 18: 1-12. [Crossref]
- Shaw J, Anderson T (2015) Coronary endothelial dysfunction in non-obstructive coronary artery disease: Risk, pathogenesis, diagnosis and therapy. Vasc Med 21: 146-155. [Crossref]
- V K, A C, PG C, C B, J E, et al. (2021) An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 16: 1049-1069. [Crossref]
- Bravo PE, Carli MF di, Dorbala S (2017) Role of PET to Evaluate Coronary Microvascular Dysfunction in Non-ischemic Cardiomyopathies. Heart Fail Rev 22: 455. [Crossref]
- Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, et al. (2015) Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC: Cardiovasc Imaging 8: 210-20. [Crossref]
- Pelletier-Galarneau M, Dilsizian V. Microvascular Angina Diagnosed by Absolute PET Myocardial Blood Flow Quantification. Curr Cardiol Rep 22: 1-9. [Crossref]
- VL M, M N, CR F, J H, M G, et al. (2011) Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 124: 2215-24. [Crossref]
- Mathew RC, Bourque JM, Salerno M, Kramer CM (2020) Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC: Cardiovasc Imaging 13: 1577-1590. [Crossref]
- VR T, SD (2016) The role of positron emission tomography in the evaluation of myocardial ischemia in women. J Nucl Cardiol 23: 1008-1015. [Crossref]
- M P-G, P M, G EF (2021) Quantification of PET Myocardial Blood Flow. Curr Cardiol Rep 21: 11. [Crossref]
- Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 23: 1187-1226. [Crossref]
- Löffler AI, Bourque JM (2015) Coronary Microvascular Dysfunction, Microvascular Angina, and Management. Curr Cardiol Rep 18: 1-7. [Crossref]
- VR T, R H, VL M, M N, CR F, et al. (2015) Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 131: 19-27. [Crossref]
- Majmudar MD, Murthy VL, Shah Rv, Kolli S, Mousavi N, et al. (2015) Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging 16: 900. [Crossref]
- VR T, BM E, VL M, M G, CR F, J H, et al. (2015) Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 131: 528-535. [Crossref]
- A G, VR T, TP van de H, NS B, PE B, et al. (2017) Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation 136: 2325-2336. [Crossref]
- Rojas-Sernaque JK, Barajas-Paulin A, Hernandez-Sandoval S, Espinola-Zavaleta N, Rosas EA. MICROVASCULAR CORONARY DYSFUNCTION A DIAGNOSTIC CHALLENGE AND MANAGEMENT. Journal of the American College of Cardiology 77: 2677.
- Brainin P, Frestad D, Prescott E (2018) The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: A systematic review and meta-analysis. Int J Cardiol 254: 1-9. [Crossref]
- Taqueti VR, Dorbala S, Wolinsky D, Abbott B, Heller Gv, et al. (2017) Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease—state-of-the-evidence and clinical recommendations. J Nucl Cardiol 24: 1402-1426. [Crossref]
- Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, et al. (2017) Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation 135: 566-577. [Crossref]
- Merz CNB, Pepine CJ, Walsh MN, Fleg JL, Camici PG, et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA). Circulation 135: 1075-1092. [Crossref]
- Waheed N, Elias-Smale S, Malas W, Maas AH, Sedlak TL, et al. (2020) Sex differences in non-obstructive coronary artery disease. Cardiovasc Res 116: 829-40. [Crossref]
- Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, et al. (2013) Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: Findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J 166: 134-141. [Crossref]
- Sedlak TL, Lee M, Izadnegahdar M, Merz CNB, Gao M, et al. (2013) Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J 166: 38-44. [Crossref]
- Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, et al. (2019) Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 139: E891-908.
- Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF (2015) Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 131: 861-870. [Crossref]
- Mahajan AM, Gandhi H, Smilowitz NR, Roe MT, Hellkamp AS, et al. (2019) Seasonal and circadian patterns of myocardial infarction by coronary artery disease status and sex in the ACTION Registry-GWTG. Int J Cardiol 274: 16-20. [Crossref]
- de Ferrari GM, Fox KAA, White JA, Giugliano RP, Tricoci P, et al. (2014) Outcomes among non-ST-segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Heart J Acute Cardiovasc Care 3: 37-45. [Crossref]
- Grodzinsky A, Arnold Sv, Gosch K, Spertus JA, Foody JM, et al. (2015) Angina frequency after acute myocardial infarction in patients without obstructive coronary artery disease. Eur Heart J Qual Care Clin Outcomes 1: 92-99. [Crossref]
- Planer D, Mehran R, Ohman EM, White HD, Newman JD, et al. (2014) Prognosis of Patients With Non-ST-Segment-Elevation Myocardial Infarction and Nonobstructive Coronary Artery Disease. Circ Cardiovasc Interv 7: 285-93. [Crossref]
- Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V (2014) Pre-Eclampsia and Future Cardiovascular Risk Among Women: A Review. J Am Coll Cardiol 63: 1815-1822. [Crossref]
- Caliskan M, Turan Y, Caliskan Z, Gullu H, Ciftci FC, et al. (2015) Previous gestational diabetes history is associated with impaired coronary flow reserve. Ann Med 47: 615-623. [Crossref]
- Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, et al. (2017) The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 38: 473-477. [Crossref]
- Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, et al. (2018) Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 39: 840-849. [Crossref]
- Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, et al. (2018) Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 39: 3439-3450. [Crossref]
- Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, et al. (2016) Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction. Circ Cardiovasc Imaging 9: 10.1161/CIRCIMAGING.115.003754 e003754. [Crossref]
- Allan T, Dryer K, Fearon WF, Shah SJ, Blair JEA (2019) Coronary Microvascular Dysfunction and Clinical Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. J Card Fail 25: 843-845. [Crossref]
- Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, et al. (2020) Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 22: 432-441. [Crossref]
- Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, et al. (2016) Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 134: e535-e578. [Crossref]
- Picchi A, Capobianco S, Qiu T, Focardi M, Zou X, et al. (2010) Coronary microvascular dysfunction in diabetes mellitus: A review. World J Cardiol 2: 377. [Crossref]
- Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, et al. (2010) Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 56: 845-854. [Crossref]
- Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, et al. (2010) NOC Study. Journal of the American College of Cardiology 55: 2825-2832.
Editorial Information
Editor-in-Chief
Akira Sugawara
Tohoku University Graduate School of Medicine
Article Type
Review Article
Publication history
Received date: January 23, 2022
Accepted date: February 03, 2022
Published date: February 06, 2022
Copyright
©2022 Cuellar-Vargas J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Cuellar-Vargas J, Proano-Bernal L, Villa-Ramirez CA, Garcia-Arroyo A, Gurrola-Luna H, et al. (2022) Microvascular angina and microvascular dysfunction - Current review of the literature. Clin Res Trials 8: doi: 10.15761/CRT.1000362



