Migraine is a common neurological disorder characterized by nausea, vomiting, photophobia, aching, fever, pain and chills. Triptans are selective serotonin agonists which are used to manage migraine. Almotriptan malate and naratriptan hydrochloride are triptans currently used in the form of tablets. Orally administered dosage forms such as tablets may be problematic for patients experiencing nausea and vomiting which are often associated with migraine. The microneedle-assisted transdermal drug delivery of these triptans may improve patient compliance. A vertical six-celled, static Franz diffusion cell system was used to conduct in vitro permeation experiments on porcine ear skin to determine the influence of microneedle on the transdermal delivery of almotriptan malate and naratriptan hydrochloride. LC-MS analysis was performed using an Agilent 1200 series high performance liquid chromatography system in combination with an Agilent time of flight mass spectrometer (TOF-MS) system model 6230 (Agilent, Santa Clara, CA, USA). A reversed phase liquid chromatography column (Agilent Zorbax Eclipse Plus C18, 100 mm X 2.1 mm, 3.5 µm), was utilized for chromatographic separation. Confocal laser scanning microscopy was used to characterize the depth of the microchannels created after stainless-steel microneedle roller application. Transdermal flux of both triptans was calculated from the linear portion of the average cumulative amount of drug versus time curve. The mean passive flux of almotriptan malate was 13.044 ± 0.32 µg.cm2.h, while the mean flux following microneedle roller application was 11.281 ± 0.22 µg.cm2.h. The mean flux values for naratriptan hydrochloride following passive administration and microneedle roller application were 0.88 ± 0.29 µg.cm2.h and 4.18 ± 1.39 µg.cm2.h, respectively. Statistical analysis was performed using the Student’s t-test (GradPad Prism 7). A statistically significant difference (p<0.05) between microneedle-treated porcine skin samples compared to untreated skin samples was found for the transdermal flux values of naratriptan hydrochloride. Solid stainless-steel microneedle rollers enhanced the transdermal delivery of naratriptan hydrochloride. In contrast, transdermal flux values obtained for almotriptan malate indicate that differences between microneedle-treated and untreated skin samples were not statistically significant (p>0.05).
migraine, anti-migraine drugs, naratriptan hydrochloride, almotriptan malate
Migraine is a common neurological disorder that affects numerous individuals globally [1]. Females are three times more likely to suffer from migraine in comparison with males and the disorder is more prevalent in individuals between the ages of 25 and 55 years [2,3]. Symptoms of migraine include: nausea, vomiting, photophobia, aching, fever and chills. Migraines are usually due to cranial vasodilation and/or the release of pro-inflammatory sensory neuropeptides from the trigeminal system [4]. Although not much is known about the etiology of migraine current studies indicate that the pathophysiology of the disorder maybe linked with both neural and vascular mechanisms. Activation of the trigeminovascular system triggers the release of vasodilators which contribute to the headache associated with migraine [4]. Vasoactive neuropeptides induce vasodilation and are located within the trigeminal neurons. The neuropeptides mediating vasodilation include: calcitonin gene-related peptide (CGRP), substance P, and neurokinin A [5]. CGRP is the most critical neuropeptide involved in the generation of migraine [4]. The release of vasoactive neuropeptides from the trigeminal nerve terminals induces inflammatory reactions in the meningeal blood vessels [4]. Furthermore, the relationship between 5-hydroxytrptamine (5-HT) and migraine pathophysiology is supported by the increased level of 5-hydroxyindoleacetic acid, a major metabolite of 5-HT during migraine [5].
Triptans act by binding to selective serotonin receptors such as the 5-HT 1B and 1D receptors [5]. The 5-HT 1B receptors are located in the intracranial blood vessels within smooth muscle cells while the 5-HT 1D receptor is expressed in the trigeminal nerve fibers [4]. The activation of these receptors is thought to produce cranial vasoconstriction and provide pain relief [5]. Triptans have three modes of action which contribute to their anti-migraine effects (6): vasoconstriction of intracranial extracerebral vessels, inhibition of vasoactive neuropeptide release by the trigeminal terminals, and nociceptive neurotransmission inhibition [6].
Triptans are commonly available as tablets for oral administration [7]. However, many migraine patients fail to comply with oral triptans because oral treatments may be ineffectual for patients who are experiencing nausea, vomiting, and gastric-stasis which are often associated with migraine [6]. Due to the limitations of oral triptans, transdermal drug delivery may be a more effective alternative [7]. The long-term goal of this research project is to develop a microneedle-assisted transdermal system for the delivery of both triptans: almotriptan malate and naratriptan hydrochloride.
There are several advantages of transdermal drug delivery which include: avoidance of gastrointestinal irritation, prevention of the first-pass effect, and the provision of stable drug plasma concentration over a prolonged period [5]. Furthermore, transdermal delivery provides a pain-free mode of drug administration. However, not all drugs are suitable for transdermal delivery [8]. For a drug to cross the skin barrier and be delivered into the bloodstream, it must have certain physicochemical properties such as low molecular weight and optimal partition coefficient [9]. The major challenge to transdermal delivery is the low drug permeability through the stratum corneum [10].
The stratum corneum (SC) is the outermost layer of the epidermis and consists of 15-20 layers of corneocytes which are embedded in a lipid matrix [10,11]. The aqueous pores in the hydrophilic regions of the SC help to facilitate the transcutaneous diffusion of penetrants [12]. There are several ways of overcoming the SC barrier including sonophoresis, iontophoresis, prodrugs, microemulsions and microneedles (MN) [10,13].
MNs are micron-sized needles that can increase the skin penetration of medications by creating micropores which enhance transdermal diffusion of drugs [14]. MNs are appealing to patients with needle-phobias because MNs do not stimulate the nerves associated with pain [11]. A common disadvantage of MNs is that only a small amount of the drug may be given each time administration compared to when using hypodermic needles. MN have been fabricated in a range of different sizes, shapes, and materials [8]. Solid, coated, dissolving, hollow, and hydrogel-forming MNs currently being investigated [10,11,15]. When using solid MNs, the drug is applied to the skin after the creation of micro-pores [11,13]. MNs can be coated with the drug, where the drug is dissolved off and then the MN are removed [11]. MNs can also dissolve release the drug payload upon insertion into skin (11). In contrast, hollow MNs can be used to inject liquid drugs into the skin through a needle bore (11, 13). Hydrogel-forming microneedles (HFMNs) are usually not loaded with drugs but typically take in the skin’s interstitial fluid upon insertion to form channels between dermal microcirculation and an attached patch-type drug reservoir(13). In this research project, we will use solid microneedles for the transdermal delivery of almotriptan malate and naratriptan hydrochloride across porcine ear skin. Microneedle-assisted transdermal delivery can potentially enhance the skin permeability for drug compounds and may lead to a statistically significant increase in transdermal flux values.
Almotriptan malate is a sulfonamide triptan which is used for the management of migraines with or without aura (2). has the molecular weight of 333.465 g/mol (16). The drug selectively binds to and activates serotonin 5-HT 1B and 1D receptors, which are localized in the central nervous system (6). This triggers intracranial blood vessel constriction which relieves the pain associated with a migraine (2). Almotriptan malate can relieve symptoms such as including nausea, vomiting, or photophobia [3]. A transdermal delivery system for almotriptan malate may be therapeutically useful.
Naratriptan hydrochloride is another triptan drug utilized for the treatment of migraine. It has the molecular weight of 335.464 g/mol [17]. This triptan acts as a selective agonist for 5-HT 1B and 5-HT 1D receptors involved in mediating vascular constriction which provides pain relief from migraine [2]. The formulation of naratriptan hydrochloride into a transdermal patch will improve patient compliance. In this project, porcine ear skin was used as a substitute for human skin due to comparable permeation properties. The SC thickness for porcine skin is 21-25 µm and this is of the same order of magnitude with the SC thickness for the human skin (10-25 µm) (18-20). Density of hair follicles between porcine skin and human skin has been found to be 20/cm2 and 14-32/cm2, respectively [19,21]. Porcine skin has been frequently used in several in-vitro skin permeation studies [21,22].
In this research project, the influence of stainless-steel microneedle rollers on the transdermal delivery of almotriptan malate and naratriptan hydrochloride across pig ear skin in-vitro was studied.
Materials
Almotriptan malate, naratriptan hydrochloride, phosphate buffer saline (PBS, pH 7.4) were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). PBS was used as a reconstitution agent for almotriptan malate and naratriptan hydrochloride. The NanoPure Infinity Ultrapure water system (Barnstead, Dubuque, IA, USA) was used to obtain deionized water for all experiments. Stainless-steel microneedle rollers with a density of 192 microneedles and microneedle length of 500 µm were obtained from Pearl Enterprises LLC (Lakewood, NJ USA). Porcine ears were purchased from Pel-Freez Arkansas LLC (Rogers, AR, USA).
Methods
Skin Preparation: The Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC) of Touro University, Mare Island-Vallejo, CA, USA approved the experiments. Porcine ears were thawed and carefully shaved with an electric hair clipper. An electric dermatome (Nouvag®, Goldach, Switzerland) was used to prepare split-thickness skin and the thickness of the skin samples was measured with a Digimatic Micrometer (Mitutoyo, Tokyo, Japan). The dermatomed skin samples were stored at -20 °C until further use.
In-vitro Diffusion Studies: A vertical Franz diffusion system (PermeGear, Hellertown, PA, USA) was utilized to conduct transdermal permeation studies. The six cells consisted of the top donor and lower receptor compartments. To help maintain uniform drug distribution the receptor compartments were magnetically stirred. Each of the cells also had a sampling port and the system possessed a circulating water jacket at 37 °C to simulate human body temperature (ThermoFisher Scientific, Waltham, MA, USA). The cells had a diffusion area of 1.77cm2 and a receptor volume of 12mL.
Microneedle-treated and untreated porcine ear skin samples were used for this experiment. A stainless-steel microneedle roller was applied to porcine skin to create microchannels. The microneedle roller was applied 15 times to each skin sample in the vertical, diagonal, horizontal directions. The roller was applied and measured at a force of 20lbs using a weighing scale. Three of six compartments of the Franz diffusion cell system contained microneedle treated skin samples while the remaining three consisted of untreated samples, serving as controls. Porcine ear skins were mounted between the upper donor and lower receptor compartments. Vacuum grease was utilized (Dow Corning, Midland, MA, USA) along with a metal clamp to keep the porcine samples in place and prevent loss of the drug solution. Skin samples were placed with the stratum corneum surface facing towards the donor cell. Diffusion experiments for both drugs were replicated six times each (n=6) and each experiment was conducted using 1 mL of either almotriptan malate or naratriptan hydrochloride. We placed 1ml of the drug solution in the donor compartment. To help reduce evaporation. the donor compartments and sampling ports were covered with parafilm and aluminum foil. At intervals of 2 hours for a 12-hour period, 1mL aliquots of the receptor solution were taken. The solutions were placed in vials for liquid chromatography-mass spectrometry (LC–MS) analysis. After sampling, the receptor compartment was refilled with an equal volume of fresh PBS (1mL). Using the LC-MS data, the average cumulative amount of drug permeated was plotted as a function of time for both triptans. The slope of the steady state portion of the cumulative amount of drug permeated over time was used to calculate the transdermal flux values.
Liquid chromatography-Mass Spectrometry(LC-MS): Quantitative analyses of the medications in the samples were carried out using LC-MS .-MS was performed using an Agilent 1200 series liquid chromatography system in combination with an Agilent time of flight mass spectrometer (TOF-MS) model 6230 (Agilent, Santa Clara, CA, USA). Chromatographic separation was achieved using a reversed phase liquid chromatography column (Agilent Zorbax Eclipse Plus C18, 100 mm X 2.1 mm, 3.5 µm) with the temperature maintained at 30 °C (Agilent, Santa Clara, CA, USA). The mobile phase consisted of 0.1% formic acid in acetonitrile. A sample volume of 5 µL of mobile phase B was injected via the linear gradient program, starting from 3% to 20% in three minutes. The mobile phase B was then increased from 20% to 100% in two minutes. The mobile phase flow rate was 0.3 mL min-1 with an injection volume of 5 µL. The reconstructed ion current mode with a m/z of 336.16 was used to perform the quantification of both triptans.
Microchannel Depth Characterization by Confocal Laser Scanning Microscopy (CLSM): CLSM was performed at the Van Winkle Lab housed in the Center for Health and the Environment of the University of California, Davis to characterize the depth of the microchannels created by the stainless-steel microneedle roller. Confocal images were taken of both microneedle-treated and untreated skin samples. After the microneedle roller application, porcine skin samples were treated with 200 uL of fluorescent dye, Alexaflour 594 (Thermo Fisher Scientific, Waltham, MA, USA). The sample was then blotted with Kimwipes (Kimberly-Clark Professional, Roswell, GA, USA) to remove excess dye. Strips of microneedle-treated porcine skin were cut into sections, embedded with OCT medium (Sakura Finetek Inc., Torrance, CA, USA) and placed onto Tissue-Tek Cryomolds (Sakura Finetek Inc., Torrance, CA, USA). All samples were cryosectioned to 10 µm thick vertical sections using a Leica CM1950 Cryostat (Leica Biosystems, Buffalo Grove, IL, USA) and transferred onto glass slides in preparation for CLSM. Confocal images of microneedle-treated and untreated skin samples taken using a Leica TCS LSI laser scanning confocal microscope. The frame was set at 1024 X 1024 pixels with a magnification of 5X. Excitation was carried out at 590 nm and emission at 617 nm. Untreated porcine samples were also treated with Alexaflour 594, cryosectioned and examined using the Leica TCS LSI confocal laser scanning microscope.
Data Analysis: The transdermal flux of both triptans was determined from the slope of the average cumulative amount versus time curve. For each drug experiments were replicated six times and sample dilution was accounted for by using the Hayton-Chen equation [37].
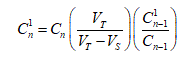
For the Hayton–Chen equation, 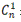 is the corrected concentration and Cn is the measured
is the corrected concentration and Cn is the measured
concentration in the nth sample. VTstands for the total volume of the receptor fluid (12 mL) and Vsis the volume of sample withdrawn from the receptor fluid (1 mL). 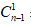 represents the corrected concentration in (n-1)th sample while
represents the corrected concentration in (n-1)th sample while 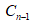 is the measured concentration in (n-1)th sample.
is the measured concentration in (n-1)th sample.
Statistical Analysis: GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) was used to perform statistical analysis. Results with a p-value of less than 0.05 was considered to be statistically significant. To plot the graphs, the mean amount (n=6) with corresponding standard deviations was used for both almotriptan malate and naratriptan hydrochloride.
Microchannel Formation
Confocal laser scanning microscopy was used to compare both microneedle-treated and untreated skins. Untreated porcine skin samples were used as controls to compare against microneedle-treated samples and determine whether microchannels were formed after microneedle roller application. In Figure 1A, it is seen that the microneedle roller successfully penetrated the stratum corneum to create micropores which are visible. The pore constructed by the microneedle roller is displayed in Figure 1A with a microchannel depth of 215 µm. Unlike Figure 1A, the untreated porcine skin displayed in Figure 1B displays no microchannels.
Figure 1. Chemical structures of (A) almotriptan malate and (B) naratriptan hydrochloride.
Figure 2. Stainless-steel microneedle roller with microneedles 500 µm in length and density of 192 needles.
In-vitro Diffusion Study
Transdermal flux values of both naratriptan hydrochloride and almotriptan malate were calculated from the linear portions of the average cumulative amount versus time curves. The flux for naratriptan hydrochloride across microneedle-treated pig skin was 4.18 1.39 µg.cm2.h while that for passive diffusion was 0.88 ± 0.29 µg.cm2. h. There were statistically significant differences (p<0.05) in the transdermal flux values of naratriptan hydrochloride across microneedle-treated porcine samples compared to passive diffusion. Passive penetration rates for naratriptan hydrochloride were low compared to microneedles therefore solid stainless-steel microneedles enhanced the transdermal delivery of this drug. In contrast, there were no statistically significant differences between the transdermal flux values of almotriptan malate across microneedle-treated pig skin. The passive and microneedle-treated flux obtained for almotriptan malate was 13.044 ± 0.32 and 11.281 ± 0.22, respectively.
The effect of microneedles on the percutaneous transport of almotriptan malate and naratriptan hydrochloride was investigated. Microchannel visualization with confocal microscopy confirmed that the microneedles pierced the pig ear skin. Banga, et al. reported that the application of a microneedle roller created microchannels in the skin allowing for enhanced transdermal drug delivery [23]. Methylene blue staining and histological sectioning were used to confirm microchannels while confocal laser scanning microscopy was used to characterize microchannel depth after microneedle roller application [23]. In our project, confocal microscopy images confirmed that the stainless-steel microneedle roller created microchannels across porcine skin r (Figure 3). Despite the presence of microchannels, there was no statistically significant increase in the percutaneous absorption of almotriptan malate (p<0.05).
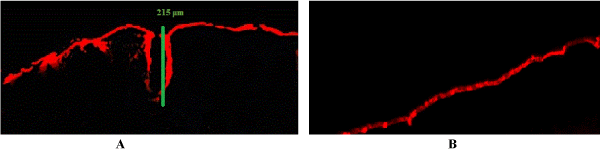
Figure 3. Images of porcine skin samples by confocal laser scanning microscopy (CLSM). (A) Microneedle treated porcine skin showing a single microchannel depth of 215 μm (B) Untreated porcine skin.
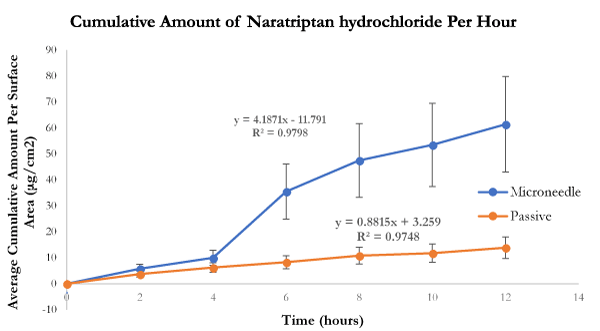
Figure 4. In-vitro average cumulative amount versus time curve of naratriptan hydrochloride across microneedle-treated and untreated porcine ear skin over 12 hours.
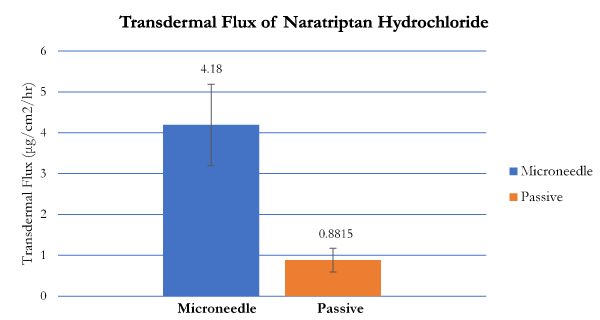
Figure 5. Transdermal flux of naratriptan hydrochloride across microneedle-treated and untreated porcine ear skin over 12 hours.
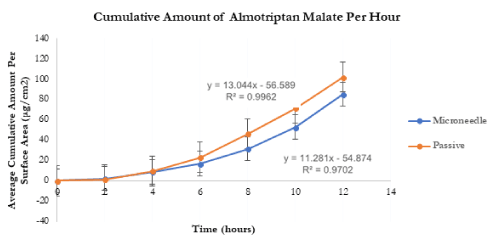
Figure 6. In-vitro average cumulative amount versus time curve of almotriptan malate across microneedle-treated and untreated porcine ear skin over 12 hours.
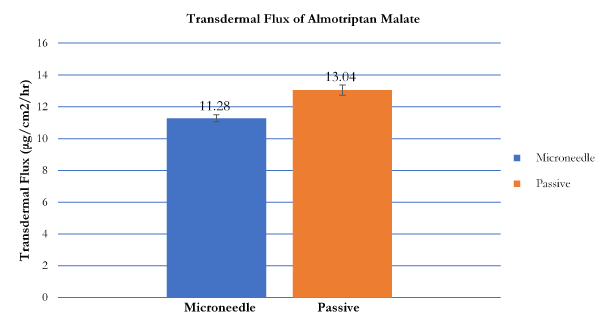
Figure 7. Transdermal flux of almotriptan malate across microneedle-treated and untreated porcine ear skin over 12 hours.
Triptans are commonly taken orally in the form of tablets. Patients with chronic migraine take medications more than once a day [7]. Almotriptan malate is available in 6.25mg tablets with a maximum daily dosage of 25mg while naratriptan hydrochloride tablets are available in 1mg or 2.5mg tablets with a maximum daily dosage of 5mg [7]. However, due to the symptoms associated with migraine such as vomiting, and nausea, patients may find it challenging to ingest tablets. Other disadvantages include the presystemic metabolism, degradation by enzymes and acid in the stomach, and erratic absorption [8]. Therefore, a microneedle transdermal delivery system would be useful in improving patient compliance. Arya, et al. tested dissolving microneedle patches on human subjects and found that the patches were well tolerated with no pain and swelling, and self-administration rates of microneedle patches were high [25]. In a review y Arya, et al, it was observed that influenza vaccine delivery using a microneedle patch was comparable to hypodermic injections [26]. Needle-phobia is a challenge with many patients and having a microneedle patch would be would be beneficial. Arya et al. also stated that the microneedle patch was preferred by patients over a hypodermic needle [25].
Table 1. Transdermal flux values (µg/cm2/h ± SD) of naratriptan hydrochloride and almotriptan malate following microneedle roller application. Passive flux values served as controls (n = 6).
Solution Name |
Passive (µg/cm2/h) |
Microneedle (µg/cm2/h) |
P- Value |
Naratriptan hydrochloride |
0.881 ± 0.293 |
4.187 ± 1.396 |
0.0054 |
Almotriptan malate |
13.044 ± 0.32 |
11.281 ± 0.22 |
0.6053 |
For almotriptan malate, the difference in flux values between microneedle-treated and passive was not statistically significant (p<0.05). Several factors may have caused this phenomenon. The effectiveness of a microneedle-assisted transdermal delivery system is dependent on the properties of the skin, the microneedles, and the drug. Physiochemical properties of the compound such as molecular weight, melting point, and partition coefficient all influence the rate of transdermal drug delivery. Generally, low molecular weight medications permeate the skin at a faster rate in comparison with high molecular weight drugs. Furthermore, the intercellular multilamellar bilayers that comprise that skin lipid barrier comprise alternating lipid and aqueous phases [38]. Thus, it is important that drugs for transdermal delivery possess both aqueous and lipid solubilities [38]. An ideal transdermal drug delivery candidate would have an aqueous solubility of greater than 1 mg/mL, partition coefficient (log P) between 1-3, melting point of less than 200 °C [27]. It has been observed that compounds with log P values below 1 are too hydrophilic to effectively disrupt the stratum corneum while those greater than 3 are too hydrophobic resulting in low therapeutic plasma concentrations [27]. The melting point of a compound is indirectly proportional to its lipophilicity and solubility in skin lipids [13]. A low melting point will allow for faster penetration and optimal transdermal delivery. Almotriptan malate has a molecular weight of 335.466 Daltons, log P value of 1.6, and a melting point of 170 °C [7]. However, the interactions between the drug, microneedles and the skin are complex. Several investigators have studied the relationship between the physicochemical properties of compounds and skin permeability [28,29]. Only a few variables were studied in the Potts-Guy equation but currently those variables now include properties such as dipole moment and polar surface area [28,29]. Clearly, these are complex interactions worthy of further investigation.
Microneedle length, density, insertion force and time can all influence the permeation rates of compounds across the skin. The low almotriptan malate flux values for microneedle-treated skin samples for almotriptan malate may be due to the microneedle length. Microneedles that were 500 μm in length were used for this study. Increasing the length may increase the penetration rate of almotriptan malate. In the study by Nalluri, et al. 500 μm and 1000 μm microneedles were compared for the in vitro permeation of levodopa across porcine ear skin [30]. It was observed that longer microneedle arrays resulted in enhanced permeation rate [30]. The use of 500 μm microneedle arrays led to a lower permeation rate of levodopa probably due to the inability of the shorter microneedles to create deep enough pores [30]. Another study by Li, et al. reported that the microneedle-assisted transdermal delivery effectively increased antibody delivery when microneedle length, microneedle array, and drug concentration were increased [31]. Banga, et al. confirmed that an increase in microneedle length from 500 μm to 1100 μm and 1400 μm enhanced microchannel depth [14]. The results showed that an increase in needle length from 500μm to 1100μm and 1400μm significantly increased microchannel depth in the skin [24]. It is important to consider these factors for future studies as this may assist in enhancing the delivery of almotriptan malate. The study conducted by Calatayud-Pascul, et al. confirmed that therapeutic drug levels for almotriptan were obtained using an iontophoresis transdermal delivery system [32]. Iontophoresis uses an electrical current to increase drug penetration through the skin Using iontophoresis in combination with microneedles may sometimes lead to enhanced drug delivery and permeation rates. In this scenario, micropores are created and the electrical current pushes medications into those microchannels [33]. Sachdeva, et al. found that iontophoresis treatment delivered three times more drug concentration compared to microneedle treatment [33]. The combination of enhancement technologies such as microneedles with iontophoresis may prove to be effective for the transdermal delivery of almotriptan malate.
The low flux levels of almotriptan hydrochloride across microneedle-treated skin samples may also have been the result of the “bed of nails” effect. The phenomenon is associated with high microneedle density and close needle-to-needle spacing which reduces the penetration and efficiency of microneedles [34,35]. Yan, et al. studied microneedles densities ranging from 400 to 11,900 needles/cm2 [36]. Exerting the same force on the microneedle arrays, the study found that arrays with less than 2000 needles/cm2 increased drug flux greater than higher needle density arrays of 5625 needles/cm2 [36]. It has been observed that when microneedle density is high, the pressure exerted by each microneedle is significantly reduced preventing proper penetration [34]. Applying the same force to a microneedle array of lower density may lead to a more enhanced drug flux.
Unlike almotriptan malate, skin pretreatment with microneedle rollers helped to enhance transdermal permeation for naratriptan malate. The molecular weight of naratriptan hydrochloride is 371.924 Dalton, it has a log P value is 1.6, and melting point is 246 °C [7]. These properties may facilitate transdermal delivery of the drug following the application of microneedles. Further in-vitro studies are needed to determine the influence of variables such as drug concentration, microneedle length, density and design, on the transdermal flux of these triptans. Solid stainless-steel microneedle rollers were used for this experiment. Therefore, it would be interesting to compare flux enhancement with different types of microneedles for almotriptan malate transdermal delivery instead of solely using solid microneedle rollers. The combination of microneedles with other techniques such as iontophoresis and sonophoresis may also increase permeation rates. Future in-vivo studies can also be performed to test the efficiency of a microneedle-assisted transdermal system for both triptans: almotriptan malate and naratriptan hydrochloride.
This research was carried out to study the influence of microneedles on the transdermal delivery of almotriptan malate and naratriptan hydrochloride. For naratriptan differences in flux values of microneedle-treated and untreated porcine skin samples using solid microneedles were statistically significant (p<0.05). Conversely, for almotriptan malate there was not a statistically significant increase in transdermal flux across porcine skin following the application of a microneedle roller (p>0.05).
We thank Patti Edwards of the Cellular and Molecular Imaging Lab at UC Davis for her assistance with confocal laser scanning microscopy for microchannel visualization images. This work was supported by Touro University California, Vallejo, CA, USA.
This project was conceptualized by Kevin B. Ita; Diffusion experiments were performed by Iqra Ahmad under the supervision of Kevin B. Ita; LC-MS was conducted by Inna E. Popova; The data was analyzed by Kevin B. Ita and Iqra Ahmad; Matthew J. Morra provided the LC-MS equipment and laboratory; Iqra Ahmad and Kevin B. Ita wrote the paper.
The authors have no conflicts of interest to declare.
- Ingledue VF, Mounsey A (2014) PURLs: treating migraine: the case for aspirin. J Fam Pract 63: 94-96. [Crossref]
- Sandrini G, Perrotta A, Arce Leal NL, Buscone S, Nappi G (2007) Almotriptan in the treatment of migraine. Neuropsychiatr Dis Treat 3: 799-809. [Crossref]
- Wang SJ, Chen PK, Fuh JL (2010) Comorbidities of migraine. Front Neurol 1: 16. [Crossref]
- Aggarwal M, Puri V, Puri S (2012) Serotonin and CGRP in migraine. Ann Neurosci 19: 88-94. [Crossref]
- Pierce MW (2010) Transdermal delivery of sumatriptan for the treatment of acute migraine. Neurotherapeutics 7: 159-163. [Crossref]
- Pini LA, Brovia D (2004) Different characteristics of triptans. J Headache Pain 5: 109-111.
- Adelman JU, Belsey J (2003) Meta-analysis of oral triptan therapy for migraine: number needed to treat and relative cost to achieve relief within 2 hours. J Manag Care Pharm 9: 45-52. [Crossref]
- Ita K (2015) Transdermal Delivery of Drugs with Microneedles-Potential and Challenges. Pharma 7: 90-105.
- Singh I, Morris AP (2011) Performance of transdermal therapeutic systems: Effects of biological factors. Int J Pharm Investig 1: 4-9.
- Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26: 1261-1268. [Crossref]
- Kim YC, Park JH, Prausnitz MR (2012) Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 64: 1547-1568. [Crossref]
- Larrañeta E, McCrudden MTC, Courtenay AJ, Donnelly RF (2016) Microneedles: A New Frontier in Nanomedicine Delivery. Pharma Res 33: 1055-1073.
- Zaid Alkilani A, McCrudden MTC, Donnelly RF (2015) Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharma 7: 438-470.
- Nguyen HX, Banga AK (2015) Enhanced skin delivery of vismodegib by microneedle treatment. Drug Deliv Transl Res 5: 407-423. [Crossref]
- Prausnitz MR (2017) Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu Rev Chem Biomol Eng 8: 177-200.
- Mathew NT, Finlayson G, Smith TR, Cady RK, Adelman J, et al. (2007) Early Intervention With Almotriptan: Results of the AEGIS Trial (AXERT® Early Migraine Intervention Study). Headache 47: 189-198.
- Maddineni J, Walenga JM, Jeske WP, Hoppensteadt DA, Fareed J, et al. (2006) Product Individuality of Commercially Available Low-Molecular-Weight Heparins and Their Generic Versions: Therapeutic Implications. Clin Appl Thromb Hemost 12: 267-276.
- Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, et al. (2016) Skin models for the testing of transdermal drugs. Clin Pharmacol 8:163-176.
- Flaten GE, Palac Z, Engesland A, Filipovic-Grcic J, Vanic Ž, et al. (2015) In vitro skin models as a tool in optimization of drug formulation. Eur J Pharm Sci 75: 10-24.
- Lademann J, Richter H, Meinke M, Sterry W, Patzelt A (2010) Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles? Skin Pharmacol Physiol 23: 47-52. [Crossref]
- Jacobi U, Kaiser M, Toll R, Mangelsdorf S, Audring H, et al. (2017) Porcine ear skin: an in vitro model for human skin. Skin Res Technol 13:19-24.
- Gray GM, Yardley HJ (1975) Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res 16: 434-440. [Crossref]
- Kalluri H, Kolli CS, Banga AK (2011) Characterization of Microchannels Created by Metal Microneedles: Formation and Closure. AAPS J 13: 473-481.
- Nguyen HX, Banga AK (2015) Enhanced skin delivery of vismodegib by microneedle treatment. Drug Deliv Transl Res 5: 407-423. [Crossref]
- Arya J, Prausnitz MR (2016) Microneedle patches for vaccination in developing countries. J Control Release 240: 135-141.
- Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, et al. (2017) The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390: 649-658.
- Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF (2016) Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Materials Science and Engineering 104: 1-32.
- Moss GP, Gullick DR, Cox PA, Alexander C, Ingram MJ (2006) Design, synthesis and characterization of captopril prodrugs for enhanced percutaneous absorption. J Pharm Pharmacol 58: 167-177. [Crossref]
- Tsakovska I, Pajeva I, Al Sharif M, Alov P, Fioravanzo E (2017) Quantitative structure-skin permeability relationships. Toxicology 387: 27-42. [Crossref]
- Nalluri BNSK, Valluru SSA, Uppuluri CT, Shaik AS (2015) Microneedle Assisted Transdermal Delivery of Levodopa. Indian Journal of Pharmaceutical Education and Research 50: 287-294.
- Li G, Badkar A, Nema S, Kolli CS, Banga AK (2009) In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharma 368: 109-115.
- Calatayud-Pascual MA, Balaguer-Fernández C, Serna-Jiménez CE, Del Rio-Sancho S, Femenía-Font A, et al. (2011) Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int J Pharma 416:189-194.
- Sachdeva V, Zhou Y, Banga AK (2013) In vivo transdermal delivery of leuprolide using microneedles and iontophoresis. Cur pharma biotech 14:180-193.
- Moga KA, Bickford LR, Geil RD, Dunn SS, Pandya AA (2013) Rapidly–Dissolvable Microneedle Patches Via a Highly Scalable and Reproducible Soft Lithography Approach. Adv Mater 25: 5060-5066.
- Gomaa YA, El-Khordagui LK, Garland MJ, Donnelly RF, McInnes F (2012) Effect of microneedle treatment on the skin permeation of a nanoencapsulated dye. J Pharm Pharmacol 64: 592-1602. [Crossref]
- Yan G, Warner KS, Zhang J, Sharma S, Gale BK (2010) Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int J Pharma 391: 7-12.

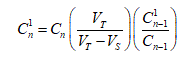
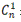 is the corrected concentration and Cn is the measured
is the corrected concentration and Cn is the measured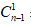 represents the corrected concentration in (n-1)th sample while
represents the corrected concentration in (n-1)th sample while 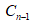 is the measured concentration in (n-1)th sample.
is the measured concentration in (n-1)th sample.





