Background: Autoimmune cytopenias are significant complications that can occur after pediatric solid organ transplants and often tacrolimus-induced. A variety of treatment methods have previously been investigated, however, little research has focused solely on autoimmune cytopenias in pediatric cardiac transplantation. The purpose of this study was to assess the outcomes of common treatment methods utilized in pediatric heart transplant patients presenting with autoimmune cytopenias.
Methods: This single center retrospective study included all pediatric patients from 0-18 years at the time of transplant, who were diagnosed with autoimmune cytopenias following heart transplant between January 1996 and July 2019.
Results: Thirteen patients (6.9%) out of 188 total heart transplant patients were diagnosed with autoimmune cytopenia (AIC). All patients received immune modulation (primarily tacrolimus) to prevent rejection and 7 of 13 patients (53%) had preceding viral infection. The median time from transplant to the diagnosis of the first episode of AIC was 3.6 years (IQR 0.7-4). The most common immune cytopenia was autoimmune hemolytic anemia (AIHA) (10/13, 76.9%). Multi-lineal cytopenias (Evans syndrome) were common and occurred in 8 cases (8/13, 61.5%; 5 cases with two lines and 3 cases with three lines). Five patients (5/13, 38.5%) were diagnosed with a single immune cytopenia. First-line therapy with steroids and/or IVIg was only successful in four cases (4/13, 30.8%): two patients with AIHA required only pulse steroids treatment; one patient with adenovirus infection and pancytopenia, and another patient with autoimmune thrombocytopenia (AITP) responded to high dose IVIg only. Overall, the majority of the cases, 8 patients (8/13, 62%) failed steroids and/or IVIg, and required second-line therapy with anti-CD20+ rituximab to achieve sustained response. This group included all 4 patients with isolated autoimmune neutropenia (AIN).
Conclusions: Tacrolimus-induced AIC often requires treatment in patients with heart transplant. A subset of patients develops AIC that can be multi-lineal and require second-line therapy. In our cohort, rituximab resulted in excellent response in first-line refractory cases.. Response rates varied between treatment types and the type of autoimmune cytopenia.
pediatric autoimmune hemolytic anemia, thrombocytopenia, neutropenia, evans syndrome, rituximab, heart transplant
Autoimmune cytopenias (AICs), such as autoimmune hemolytic anemia (AIHA), autoimmune thrombocytopenia (AITP), and autoimmune neutropenia (AIN), are known potential complications following pediatric solid organ transplantation (SOT) [1]. These have been shown to be linked to a variety of factors, including infections, ABO incompatibility, and immune dysregulation [1,2]. AICs can be difficult to treat, particularly in children. Prior studies have asserted that the prolonged use of immunosuppressive medications (ISMs) to prevent allograft rejection in SOT patients may be responsible for the development of immune cytopenias [1]. In addition, the higher levels of immunosuppression often required for cardiac transplant recipients [2], predispose them to a higher incidence of cytopenias at a relatively younger age than liver or kidney transplant patients [3]. In the subgroup of intended ABO-mismatched transplants, immune hemolysis is reported to be highest in heart-lung (70%) compared to liver (29%) and kidney (9%) transplants [4,5].
The most common approach to treatment of AICs involves alteration of immune suppression. The calcineurin inhibitor, tacrolimus, is the preferred drug for immune suppression as it allows for sparing of long-term steroid usage during the first-year post-transplant, and has proven to be highly effective in blocking cytotoxic T-cell survival and interleukin-2 productions [4]. However, the incidence of AIC has been shown to be highest with the use of tacrolimus compared to other immunosuppressive drugs [5]. In fact, switching from tacrolimus to an mTor inhibitor has been shown to result in improvement in AIC following SOT. However, due to a higher risk of organ rejection, this may not be the optimal approach in most patients. Other therapies for AIC that have been employed include steroids, intravenous immunoglobulin (IVIg) [first-line therapy], and more recently rituximab [second-line therapy]. No single agent has been identified as gold standard, and there is limited data regarding the use of RTX for autoimmune cytopenia in pediatric heart transplant patients. In this study, we describe a series of 13 pediatric heart transplant patients at our center who developed AICs post-transplant, and their clinical response to first (steroids, HD-IVIg), and/or second-line therapy with rituximab.
We included in our study all pediatric patients who developed immune cytopenias following heart transplantation between January 1996 and July 2019 at our institution. The study was approved by our Institutional Review Board, and informed consent was not required for this retrospective study. One patient who received a second transplant at our hospital, but whose first transplant was performed at another institution, was excluded from further analysis. The presence of one or more immune cytopenias was verified via laboratory results showing an absolute neutrophil count (ANC) of less than 500 cell/ul (AIN), hemoglobin below 8 g/dl (AIHA), or platelet below 100 x 109 count/L (ITP). We used these lower arbitrary cut-off counts to exclude patients with mild cytopenia, a common phenomenon in pediatric heart transplant patients. Laboratory evidence of immune phenomenon included a positive Coombs test, the presence of warm or cold autoantibodies, anti-neutrophil antibodies, or anti-platelet antibodies.
Response to therapy for AIN was defined as in previous studies [6]: for AIN, by an increase of greater than or equal to 500 cells/ul for ANC; for AIHA, by a 2 g/dl increase in hemoglobin or the attainment of transfusion independence in a patient who was previously transfusion dependent; and for AITP, by using the International Consensus Group criteria which outlines a response to ITP as any platelet count greater than or equal to 30 x 109 L, in the absence of bleeding, that is confirmed on two or more separate occasions at least 7 days apart [6]. Since most patients received additional therapies, such as blood and platelet transfusions, and granulocyte colony stimulation factor (GCSF, Neupogen), transient response was not considered a clinical response. Sustained response for at least one month without further need for these additional therapies was defined as response to therapy.
A total of thirteen patients met criteria for inclusion in the study. Table 1 shows characteristics and demographic data for all 13 patients. The median age at transplantation was 1.8 years (Range 0.2-16). The median time from transplant to the diagnosis of the first immune cytopenia was 3.6 years (IQR 0.7-4). The median follow-up time for all patients was 2.3 years (Range 1.3 – 9.3)
Table 1. Patient demographics and characteristics of type of immune cytopenia and treatment
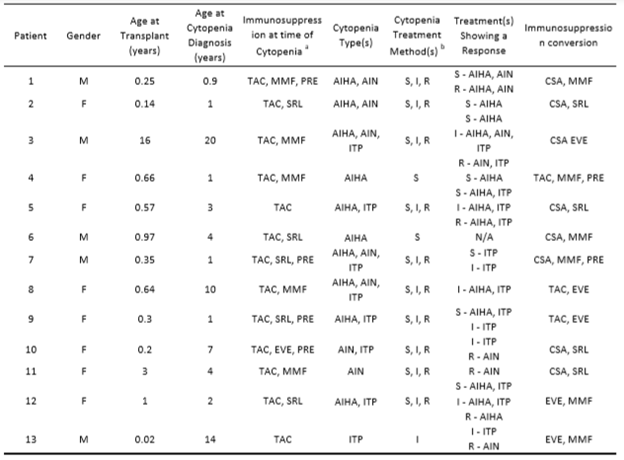
TAC = tacrolimus, MMF = mycophenolates, PRE = prednisone, SRL = sirolimus, EVE = everolimus, AIHA = autoimmune hemolytic anemia, AIN = autoimmune neutropenia, ITP = autoimmune thrombocytopenia, S = solumedrol, I = intravenous immunoglobulin, R = rituximab
Five patients (5/13, 38.5%) were diagnosed with a single immune cytopenia. Five patients (5/13, 38.5%) were diagnosed with two immune cytopenias, and three patients (3/13, 23.1%) were diagnosed with three immune cytopenias. These 8 patients (8/13, 61.5%) with multi-lineal cytopenias belong to the subgroup of Evans’ Syndrome. The majority of patients (10/13, 76.9%) were evaluated by a hematologist for the diagnosis of immune cytopenia. The most common immune cytopenia was AIHA (10/13, 76.9%) with warm IgG-mediated AIHA being the most common subtype. Of the patients with ITP, five (5/7, 71.4%) were noted to have anti-platelet antibodies. Four (4/6, 66.6%) of the AIN patients had anti-neutrophil antibodies. Each patient was tested for infections upon presentation of AIC, and 7 of 13 patients (54%) had viral infections within 2 weeks of presentation: Rhinovirus/Enterovirus alone (3), Coronavirus (2), Respiratory syncytial virus + Rhinovirus/enterovirus (1) and adenovirus (1).
The most common features of an immune cytopenia were a laboratory abnormality (8/13, 61.5%), followed by fever (4/13, 30.8%), fatigue (2/13, 15.4%), bleeding (2/13, 15.4%), and difficulty with breathing (1/13, 7.69%).
All 13 patients were receiving tacrolimus at the time of diagnosis of AIC (Table 1). If cytopenia was refractory to first-line treatment with steroids and/or IVIg, then tacrolimus was changed to cyclosporine or an mTor inhibitor as the primary immunosuppression. Nine (9/13) patients transitioned from tacrolimus to another primary immunosuppressive agent following the diagnosis of immune cytopenia. Of the patients who were taken off tacrolimus, 4 patients required re-initiation of tacrolimus by the end of the study. Patient #10 was transitioned from everolimus to tacrolimus to allow for healing of an incised abdominal pustule, cultured positive for pseudomonas. Upon healing of the pustule, the patient reverted to everolimus.
All thirteen patients required pharmacological treatment for immune cytopenia(s) secondary to chronic and/or severe course of disease. Of the 3 variants of AIC, 50% (5 of 10) of patients with AIHA showed a response to first line therapy with steroid and IVIg. The remaining 5 patients required treatment with rituximab, with 60.0% (3/5) response rate. The combination of steroids with IVIg was the initial treatment for AITP, but sustained response was only seen in three patients (3 of 8, 37.5%). Rituximab was used in the 5 non-responders with a 60.0% (3/5) response rate. Isolated AIN was refractory to first line treatment with steroids plus IVIg, but showed an extremely high response rate 100% (4/4) to rituximab.
There were significant events during treatment for immune cytopenia in our patients. One patient who was diagnosed with both thrombocytopenia and neutropenia, developed pseudomonas sepsis during therapy, that was successfully treated. A patient who was presented with Evans syndrome, with all three cytopenias, developed worsening renal insufficiency during therapy. One patient diagnosed with AIHA developed metapneumovirus pneumonia during therapy. Another patient developed glomerulonephritis during the therapy for AIHA. One patient experienced an acute kidney injury and acute hypoxemic respiratory failure during therapy for all three cytopenia types. There was a total of 45 hospitalizations between the thirteen patients associated with the diagnosis and treatment of cytopenia, an average of 3.5 admissions per patient. The most common reasons for hospitalization were fever plus neutropenia (7/45, 15.6%), fever alone (5/45, 11.1%), and allograft rejection (3/45, 6.7%). Only five out of thirteen patients received red cell transfusions (5/13, 38.5%). One patient who received an ABO incompatible organ continued tacrolimus primary immunosuppression while being treated for immune cytopenia. Three other patients remained on tacrolimus due to concern for rejections.
AIC may occur among patients with heart transplant and chronic immune modulation, especially with the use of tacrolimus [5]. The most common approach to treatment of tacrolimus-associated AICs is alteration of immune suppression. The calcineurin inhibitor, tacrolimus, is the most commonly used primary immunosuppressive medication as it allows for less dependence on long-term steroid usage during the first-year post-transplant, and has proven to be 100 times more effective at blocking cytotoxic T-cell generation and interleukin-2 production, compared to cyclosporine [4]. In fact, switching from tacrolimus to an mTor inhibitor has been shown to result in improvement in AIC following SOT. However, due to a higher risk for organ rejection, this may not be the optimal treatment in most patients. Other AIC therapies that have been employed include steroids, intravenous immunoglobulin (IVIg), and more recently rituximab. No single agent has been identified as gold-standard, and there is limited data regarding the use of rituximab for autoimmune cytopenia in pediatric heart transplant patients. In this study, we describe a series of 13 pediatric heart transplant patients at our center that developed AICs post-transplant in an effort to determine the treatment response.
Autoimmune hemolytic anemia was the most common cytopenia in our patients. A large subset of patients developed multi-lineal AIC and required second line therapy. Specifically, 61.5% of patients required treatment with rituximab to achieve therapeutic response, which suggests a failure rate of nearly 62% to standard first-line treatment with steroids and/or IVIg. In our cohort, rituximab resulted in excellent response in first-line refractory cases.
Of note that while some studies have debated the efficacy of IVIg, one study found a response rate to IVIg of 40%, with children more likely to respond than adults [7,8]. Our study supported this practice with a response rate of 38.5% to IVIg and steroids.
Schoettler M, et al. reported that pediatric patients who developed immune cytopenias following solid organ transplantation did not respond well to traditional first-line therapies. In their series, steroids and IVIG had a response rate of 50% or less in all immune cytopenias. They concluded that, although these agents are often initially used as treatment, other therapeutic options should be considered early when these patients present [3].
Although small numbers, the four patients in our study who presented with isolated AIN, all showed good response to rituximab (100.0%). This is especially interesting as prior studies have shown a possible connection between the use of Rituximab and the subsequent development of neutropenia [9,10]. Because rituximab was administered in multiple doses that were weekly apart per our institutional protocol, it was difficult to determine if a patient needed all four doses or if a patient could have received fewer doses. Nonetheless, the clinical response after administration of rituximab has been sustained for over 6-18 months, the time when B cell function recovery is expected.
Most SOT immunosuppression regimens utilize tacrolimus or cyclosporine with an antimetabolite, usually mycophenolate, in order to limit the side effects associated with calcineurin inhibitor use [3,6,7]. Our immunosuppressive regimen for heart transplantation utilizes tacrolimus as the primary agent. All thirteen patients were on tacrolimus at the time of their cytopenia diagnosis. Tacrolimus disrupts negative thymic selection and down-regulates regulatory T cells, leading to an increased number of auto-reactive T cells [3]. This is postulated to be the mechanism for development of autoimmunity [4]. In our series, most patients were switched from tacrolimus to sirolimus, everolimus or cyclosporine as the primary immunosuppression, and we believe this may have contributed to resolution of cytopenias in some of the patients.
In summary, most of our cytopenia patients did not respond well to standard first-line therapies with corticosteroids and IVIg, however they appeared to respond better to Rituximab.
In all, it appears that each autoimmune cytopenia has a variable response to standard first-line therapy. According to our data, transitioning immunosuppression from tacrolimus to cyclosporine or an mTor inhibitor, and/or early use of rituximab in refractory cases of AIC appeared to be the most optimal therapy for these patients.
In the future, immune studies may help to distinguish patients at risk for refractory AIC while on immune suppressive therapy with tacrolimus or other agents directed against T cell function. In addition, patients with cardiac transplant may have primary or secondary thymic dysfunction due to partial or total thymectomy that may contribute to the pathogenesis of AIC. Lastly, AIHA may also develop secondary to blood antigen incompatibility.
The limitations of our study relate to the retrospective nature of the study. There is the inherent selection bias since the events and outcomes had already occurred before the study. We can therefore only describe association, not causation or therapeutic efficacy. The small sample size also certainly affects generalizability of our results and findings. Although a head-to-head comparison between response rates to steroids, IVIg or rituximab would be interesting, we could not perform such a comparison in our study population because of the retrospective study design. Several patients experienced more than one immune cytopenia and were counted in multiple immune cytopenia subgroups, hence various treatment methods overlapped. It is also a common practice to administer steroids and IVIg concurrently for autoimmune cytopenias. As such, it is difficult to say which treatment method produced a specific response in those patients. If a response was seen during the standardized time to initial response following each treatment period, then the associated treatment type was given credit for said response. Consequently, some treatment types may have been credited with a response that may have been influenced by another treatment type. Since non-immune cytopenias are common in heart transplant patients, this study is skewed towards patients with the most severe cytopenias, and may under-estimate the actual incidence of immune cytopenias. A future larger multi-institutional study is warranted to confirm our findings.
None.
None.
This project was approved by the Johns Hopkins All Children’s Hospital Institutional Review Board.
Sydney Stahlman: Data acquisition and manuscript writing
Bethany Wisotzkey: Study design, Data acquisition, Supervision and Manuscript writing
Jennifer Carapellucci: Data acquisition, manuscript writing
Abigail Doyle: Data acquisition, supervision
Laura Reeger: Data acquisition, supervision
Anthony Gomez: Data acquisition, supervision
Jennifer Leiding: Study concept, manuscript writing
Jolan E Walter: Manuscript writing
Alfred Asante-Korang: Study design, supervision and manuscript writing
- Avdimiretz N, Seitz S, Kim T, Murdoch F, Ursche S, et al (2018) Allergies and autoimmune disorders in children after heart transplantation. Clin Transplantation 32: e13400. [Crossref]
- Tubman VN, Smoot L, Heeney MM (2007) Acquired immune cytopenias post-cardiac transplantation response to rituximab. Pediatr Blood Cancer 48: 339-344. [Crossref]
- Schoettler M, Elisofon SA, Kim HB, Blume ED, Rodig N, et al. (2015) Treatment and outcomes of immune cytopenias following solid organ transplant in children. Pediatr Blood Cancer 62: 214-218. [Crossref]
- Niviann BM, Healey M, Hsu E (2017) Immunosuppression in the pediatric transplant recipient. Semin Pediatr Surg 26: 193-198. [Crossref]
- Kanellopoulou T (2017) Autoimmune hemolytic anemia in solid organ transplantation – the role of immunosuppression. Clin Transplant 31: 313031. [Crossref]
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, et al. (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenia purpura of adults and children. Report from an international working group. Blood 113: 2386-2393. [Crossref]
- De Simone P, Carrai P, Coletti L, Ghinolfi D, Petruccelli S et al. (2018) Everolimus vs mycophenolate mofetil in combination with tacrolimus: a propensity score-matched analysis in liver transplantation. Transplantation Proc 50: 3615-3620. [Crossref]
- Zanella A, Barcellini W (2014) Treatment of autoimmune hemolytic anemias. Haematologica 99: 1547-1554. [Crossref]
- Lucchini E, Zaja F, Bussel J (2019) Rituximab in the treatment of immune thrombocytopenia: what is the role of this agent in 2019? Haematologica 104: 1124-1135 [Crossref]
- Weissmann-Brenner A, Brenner B, Belyaeva I, Lahav M, Rabizadeh E (2011) Rituximab associated neutropenia: description of three cases and an insight into the underlying pathogenesis. Med Sci Monit 17: CS133-137. [Crossref]

