Mammary myofibroblastoma (MFB) is a rare benign mesenchymal tumor. We present a rare case of this benign mesenchymal breast tumor in a menopausal woman. The woman was admitted to the hospital and the ultrasound of the left breast revealed a firm tumor mass, measuring 1,1 cm in diameter, suspicious for malignancy. Radical mastectomy was performed. After histological and immunohistochemical examination of the tumor, the diagnosis was benign mixed mesenchymal tumor of the mammary gland – mammary type myofibroblastoma with granular cell myoblastoma component. We discuss the differential diagnosis between myofibroblastoma and other benign and malignant mammary gland mesenchymal tumors. Pathologists should be aware of the wide morphological spectrum revealed by MFB to avoid a misdiagnosis of malignancy.
mammary myofibroblastoma, differential diagnosis, mesenchymal tumors, immunohistochemistry
Mammary myofibroblastoma (MFB) is a rare benign mesenchymal tumor [1,2]. The name myofibroblastoma is used for the first time from Wargots, in 1987 [3]. Extramammary MFBs are reported in the literature and they are usually located in the inguinal area in elderly man [1]. These extramammary tumors are characterized by morphological and immunohistochemical features similar to the MFBs in the mammary gland. The tumor can occur in the skin of the breast, in soft tissues or in mammary lymph nodes [2,4,5]. The tumor is described in postmenopausal women and in older men. Children and young people are rarely affected. A thoughtful differential diagnosis is necessary to exclude different malignant mesenchymal tumors that resemble MFB morphologically.
We present here a case of 52-year-old woman who had found a painless tumor in her left breast 2 months before admission to the hospital. The physical exam revealed a firm tumor mass, measuring 1,5 cm in diameter, in the upper median quadrant of the left breast. There were no enlarged lymph nodes in the left axilla. Fibrocystic plaque was found at the border between the upper median and lateral quadrant of the right breast. Normal local status of the right axilla was reported. The ultrasound showed poorly demarcated hypoechogenic mass in the upper median quadrant of the left breast, measuring 11 mm, infiltrating the mammary parenchyma and the adipose tissue. The left axillary lymph nodes were with hypoechogenic periphery and hyperehogenic center, some of them were enlarged, measuring 8/9/11 mm and suspicious for metastatic.
A frozen section was performed during surgery and the result was “invasive tumor, suspicious for malignancy”. Radical left mastectomy was performed with axillary lymph nodes dissection. The histological examination of the symptomatic lesion (11 mm) revealed a tumor, composed of nests of epitheloid tumor cells with monomorphous nuclei with occasional nucleoli, areas with spindle tumor cells in a fibrous background with hyaline change, areas with mature adipocytes. Peripheral nerves were surrounded by tumor nests (Figure 1). Scattered lymphocytic infiltrates were also found. PAS reaction revealed single positive granules in a few tumor cells (Figure 2). The described histological findings are observed in both mammary MFB and granular cell myoblastoma. Immunohistochemical analysis was performed. The tumor cells were positive for: S-100 protein (Figure 3), CD68 (Figure 4), Vimentin (Figure 5). The proliferative index detected with Ki67 is low (1-2%) (Figure 6). The tumor cells were negative for ER, PR and HER. The histological examination of the dissected lymph nodes revealed sinus histiocytosis, follicular hyperplasia and lipomatosis. Metastases were not found.
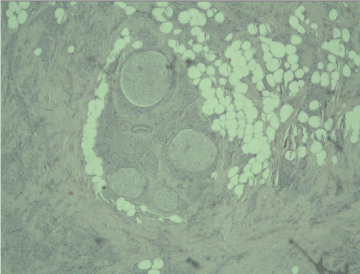
Figure 1. Peripheral nerves surrounded by nests of tumor cells, Н&E, x10
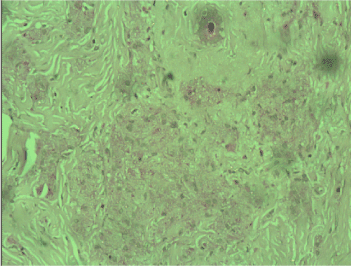
Figure 2. PAS reaction revealed single positive granules in a few tumor cells x40
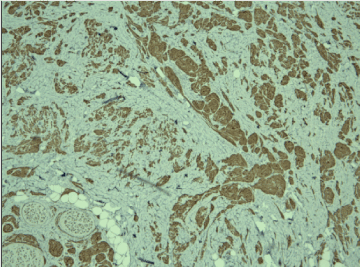
Figure 3. Positive cytoplasmic reaction for S-100 protein in tumor cells, x10
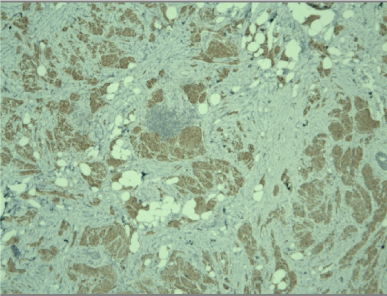
Figure 4. Positive cytoplasmic reaction for CD68 in tumor cells, x10
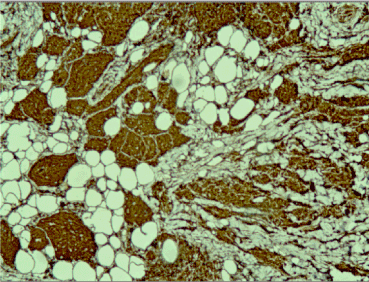
Figure 5. Positive cytoplasmic reaction for CD68 in tumor cells, x20
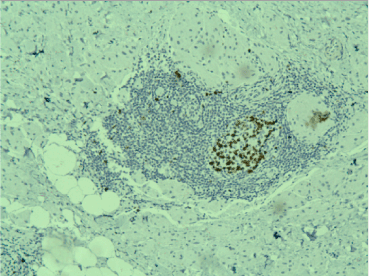
Figure 6. Positive nuclear reaction for Ki67 in a germinal center in a lymphoid follicle, low Ki67 in the tumor cells, x20
The final diagnosis was benign mixed mesenchymal tumor of the mammary gland – mammary type myofibroblastoma with granular cell myoblastoma component.
Mammary MFB is composed of monotonous spindle cells with myofibroblastic differentiation without nuclear atypia, rare or no mitotic figures. Usually, the tumor presents as a small single lesion, measuring between 1 to 4 cm in diameter, but larger tumors are also described [6,7]. Mammary MFB is a benign unencapsulated mesenchymal tumor, composed of fascicles of spindle cells with myogenic and myofibroblastic differentiation, hyalinized collagenous stroma and variable amount of adipose tissue [5]. There are several morphological variants of MFB: epitheloid, fibrous, deciduoid-like, cellular, collagenous, lipomatous, myxoid, infiltrative [8]. The immunohistochemical analysis of the spindle cells shows positive expression for CD34 and Desmin [1,4]. A genetic link between mammary MFB, cellular angiofibroma and spindle cell lipoma was shown in some cases, all showing a loss of genetic material from the 13q14 region, as indicated by monoallelic deletion of RB1 and FOXO1 [9]. The genetic relationship between cellular angiofibroma, mammary-type myofibroblastoma and spindle cell lipoma, and their similar morphological characteristics suggest that these tumors share common histogenesis [2,9,10].
Lipomatous MFB is composed of mature monotonous adipocytes that lack nuclear pleomorphism. The cellular areas in the tumor include spindle and oval-shaped cells with morphological and immunohistochemical features typical for classic MFB. Lipomatous MFB should be differentiated from other benign lesions, like fibromatosis, nodular fasciitis and spindle cell lipoma, as well as from malignant spindle cell tumors, like spindle cell liposarcoma, malignant spindle cell myoepithelioma, low grade fibrosarcoma/malignant fibrous histiocytoma [11].
Myofibroblasts are normally present in the stroma of the mammary gland. Different spindle cell lesions originate from these cells, including reactive proliferations, benign and malignant neoplasms [12]. Although many of these lesions are uncommon, they are a diagnostic challenge for the pathologists. Histologically, mammary MFB may resemble an aggressive tumor, like low grade sarcoma with myofibroblastic differentiation [13], malignant variant of solitary fibrous tumor [14], mammary myofibrosarcoma [15], low grade periductal stromal sarcoma [16] and even spindle cell carcinoma [17]. Primary mammary myofibrosarcoma is composed of cellular and less cellular areas of spindle cells and hyalinized collagenous stroma. The tumor cells are positive for Vimentin, SMA, Fibronectin, Bcl-2, and negative for Desmin, laminin, collagen type IV, S-100 protein, C-kit, CD34 [15,18]. The proliferative index is 30% using Ki67 [18]. Low grade periductal stromal sarcoma is composed of spindle cells arranged around ductal structures in abundant mesenchymal stroma. The spindle cells show severe atypia and pleomorphism, occasionally, vacuolated cytoplasm, and moderate mitotic activity. The surrounded ducts and lobules are with minimal deformities and without phylloid-like characteristics. The tumor cells are positive for SMA, CD34 and Vimentin and show focal positivity for CD10 [16,17].
Spindle cell carcinoma should be differentiated from various spindle cell lesions in the mammary gland. Moreover, different variants are described, including fibromatosis-like variant, which presents a diagnostic challenge. The tumor cells are positive for basal Cytokeratins, and negative for CD34, some tumors show positive reaction for nuclear β-catenin [17]. It is important to differentiate MFB from metaplastic sarcomatoid carcinoma. There are two variants of the latter described in the literature – monophasic and biphasic. In the monophasic variant the mesenchymal component dominates, but there are still focal areas with spindle cells positive for Cytokeratins. The biphasic variant should be differentiated from sarcomatous carcinoma, myoepithelial carcinoma and malignant phylloides tumor [19]. The metaplastic sarcomatoid carcinoma is a malignant tumor, characterized by infiltrative growth, the mitotic activity is low, focal areas with hyaline and myxoid change are present [20].
A simplified approach has been proposed by Tse et al. to evaluate the spindle cells and epithelial cells in the spindle cell lesions in the mammary gland. The spindle cells can show bland morphology or can be pleomorphic, and they may be mixed with a benign or malignant epithelial component. The authors divided the spindle cell lesions into four groups [21]:
- Biphasic lesions with predominant spindle cell component with benign epithelial (ductal) component e.g., fibroadenomas, Phyllodes tumors, pseudoangiomatous stromal hyperplasia, adenomyoepithelioma.
- Biphasic lesions with predominant spindle cell component with malignant epithelial (ductal) component e.g., biphasic metaplastic carcinoma with a ductal component.
- Monophasic lesions with pure pleomorphic spindle cells only e.g., monophasic metaplastic carcinoma, sarcomas like angiosarcoma, malignant fibrous histiocytoma.
- Monophasic lesions with pure bland spindle cell only e.g., fibromatosis like metaplastic carcinoma, fibromatosis and other unusual conditions like dermatofibrosarcoma protuberance.
A definitive diagnosis of MFB can be reached in core biopsies, but spindle cell lesions require careful histological and immunohistochemical analysis, an active search for epithelial elements and sufficient clinical data [21].
Spindle cell lesions in the mammary gland present a diagnostic dilemma and thoughtful differential diagnosis is needed. It is very helpful if the diagnosis can be reached in core biopsies, because different tumors require different therapeutic approach. We conclude that, if spindle cell lesions with myofibroblastic differentiation and admixed adipose tissue and mast cells are observed, the diagnosis of MFB should be considered, and immunohistochemistry is helpful for the accurate diagnosis.
- McMenamin ME, Fletcher CD (2001) Mammary-type myofibroblastoma of soft tissue: a tumor closely related to spindle cell lipoma. Am J Surg Pathol 25: 1022-1029. [Crossref]
- Hox V, Vander Poorten V, Delaere PR, Hermans R, Debiec-Rychter M, et al. (2009) Extramammary myofibroblastoma in the head and neck region. Head Neck 31: 1240-1244. [Crossref]
- Wargotz ES, Weiss SW (1987) Myofibroblastoma of the breast: sixteen cases of a distinctive benign mesenchymal tumour. Am J Surg Pathol 11: 493-502. [Crossref]
- Wei Q, Zhu Y (2011) Collision tumor composed of mammary-type myofibroblastoma and eccrine adenocarcinoma of the vulva. Pathol Int 61: 138-142. [Crossref]
- Yoo CC, Pui JC, Torosian MH (1998) Myofibroblastoma associated with bilateral gynecomastia: a case report and literature review. Oncol Rep 5: 731-733. [Crossref]
- Mele M, Jensen V, Wronecki A, Lelkaitis G (2011) Myofibroblastoma of the breast: case report and literature review. Int J Surg Case Rep 2: 93-96. [Crossref]
- Abeysekara AM, Siriwardana HP, Abbas KF, Tanner P, Ojo AA (2008) An unusually large myofibroblastoma in a male breast; a case report. J Med Case Rep 2: 157. [Crossref]
- Magro G (2008) Mammary Myofibroblastoma. Arch Pathol Lab Med 132: 1813-1820.
- Flucke U, van Krieken JH, Mentzel T (2011) Cellular angiofibroma: analysis of 25 cases emphasizing its relationship to spindle cell lipoma and mammary-type myofibroblastoma. Mod Pathol 24: 82-89. [Crossref]
- Magro G, Righi A, Casorzo L, Antonietta T, Salvatorelli L, et al. (2012) Mammary and vaginal myofibroblastomas are genetically related lesions: fluorescence in situ hybridization analysis shows deletion of 13q14 region. Hum Pathol 43: 1887-1893. [Crossref]
- Magro G, Michal M, Vasquez E, Bisceglia M (2000) Lipomatous myofibroblastoma: a potential diagnostic pitfall in the spectrum of the spindle cell lesions of the breast. Virchows Archiv 437: 540-544. [Crossref]
- Brogi E (2004) Benign and malignant spindle cell lesions of the breast. Semin Diagn Pathol 21: 57-64. [Crossref]
- Mentzel T, Dry S, Katenkamp D, Fletcher CD (1998) Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol 22: 1228-1238. [Crossref]
- Yang LH, Dai SD, Li QC, Xu HT, Jiang GY, еt al. (2014) Malignant solitary fibrous tumor of breast: a rare case report. Int J Clin Exp Pathol 7: 4461-4466. [Crossref]
- Taccagni G, Rovere E, Masullo M, Christensen L, Eyden B (1997) Myofibrosarcoma of the breast: review of the literature on myofibroblastic tumors and criteria for defining myofibroblastic differentiation. Am J Surg Pathol 21: 489-496. [Crossref]
- Tomas D, Janković D, Marusić Z, Franceschi A, Mijić A, et al. (2009) Low-grade periductal stromal sarcoma of the breast with myxoid features: Immunohistochemistry. Pathol Int 59: 588-591. [Crossref]
- Lee AH (2008) Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathology 52: 45-57. [Crossref]
- Stark M, Hoffmann A, Xiong Z (2011) Mammary myofibrosarcoma: case report and literature review. Breast J 17: 300-304. [Crossref]
- Al-Nafussi A (1999) Spindle cell tumours of the breast: practical approach to diagnosis. Histopathology 35: 1-13. [Crossref]
- Kinkor Z, Svitáková I, Ryska A, Kodet R, Hrabal P (2002) Metaplastic spindle-cell (fibromatosis-like) carcinoma of the breast-report of 4 cases. Cesk Patol 38: 164-168. [Crossref]
- Tse GM, Tan PH, Lui PC, Putti TC (2008) Spindle cell lesions of the breast--the pathologic differential diagnosis. Breast Cancer Res Treat 109: 199-207. [Crossref]






