Abstract
Maintenance of normal epithelial ion and water transport in the lungs includes providing a thin layer of surface liquid that coats the conducting airways. This airway surface liquid (ASL) is critical for normal lung function in several ways but, perhaps most importantly, is required for normal mucociliary clearance and bacterial removal. Preservation of the appropriate level of hydration, pH, and viscosity for the ASL requires the proper regulation and function of a battery of different types of ion channels and transporters. They are situated in the membranes of the cells, and they are protein molecules containing aqueous pores that can open and shut to permit ion flow through cell membrane. This paper covers two aspects of the subject. First, the role of ion channels in cell physiology and second, how alterations in ion channel/transporter function often led or contributes to bronchopulmonary pathologies.
Keywords
Ion channels, respiratory channelopathies, recombinant DNA technology, patch clamp technique, viroporins
General and History
Ion channels are crucial components in the activity of living cells. Since they may be either open or closed, they can exert control over this ion movement by switching it on or off (change from close to open took no more than 3 µm) (Figure 1). The change really is effectively instantaneous. The humans’ cells are in contact with moderately salty solutions as body fluids of the blood or the intercellular spaces. These extracellular solutions usually contain a relatively high concentration of sodium and chloride ions, and much lower concentrations of potassium, calcium, and other ions. The plasma membranes act as a barrier separating the cell contents from the outside, so that the ionic concentration inside the cell can be maintained at levels appreciably different from those in the extracellular fluids. There is also an electrical potential difference between the cytoplasm and the external medium. This combines with the ionic concentrations gradient to make an electrochemical gradient across the plasma membranes for each ion species, the ion will flow down the gradients into or out of the cell [1]. The plasma membranes are composed of two layers of tightly packed lipid molecules with the polar heads on the outside and their hydrophobic tails inside (Figure2). The bilayer is about 30 Å thick (1 Ångström=0.1 nm. 1 nm=10-9m). This structure is not readily permeable to polar molecules each as sugar or amino acids or to charged particles such as sodium or chloride ions. Such particles can pass through the membrane only via special protein molecules embedded in it. Ion channels form one group of these proteins; they permit rapid flow of ions across the membrane. Other membrane transport proteins may act as carriers for ions or other substances, transporting them at much lower rates than do channels, and sometimes up in electrochemical or concentrations gradient [2].
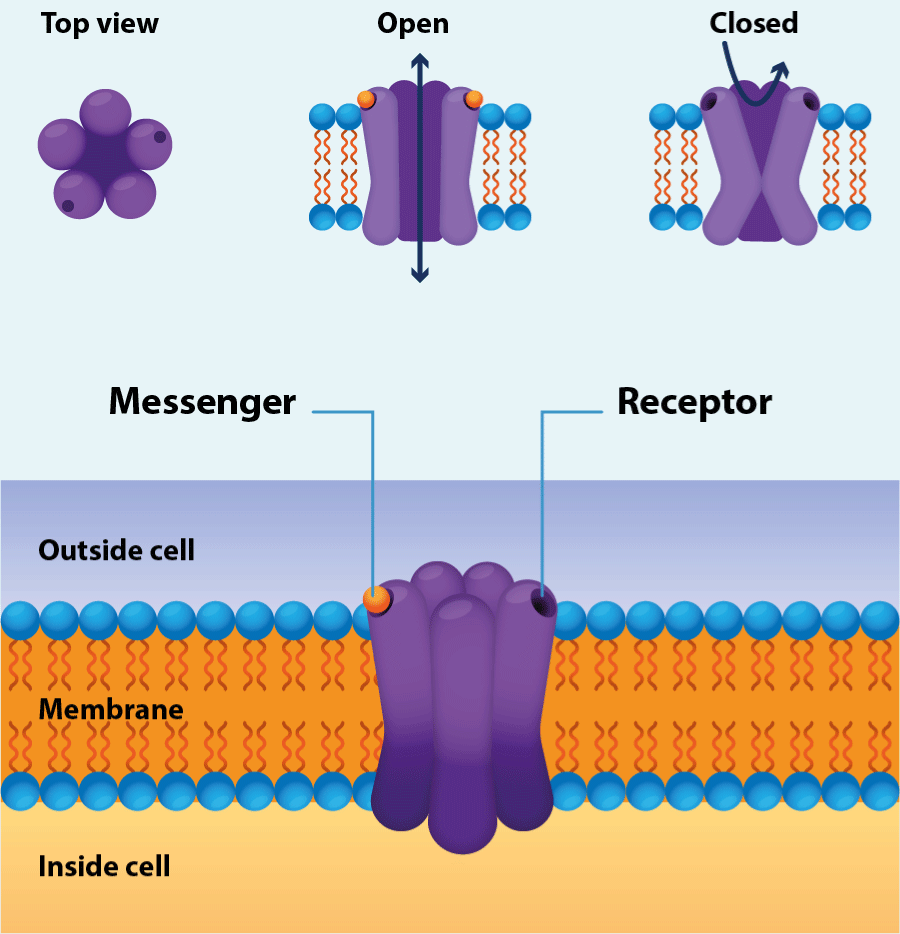
Figure 1.Ion channel
Structure of the channel (Designua/Shutterstock).
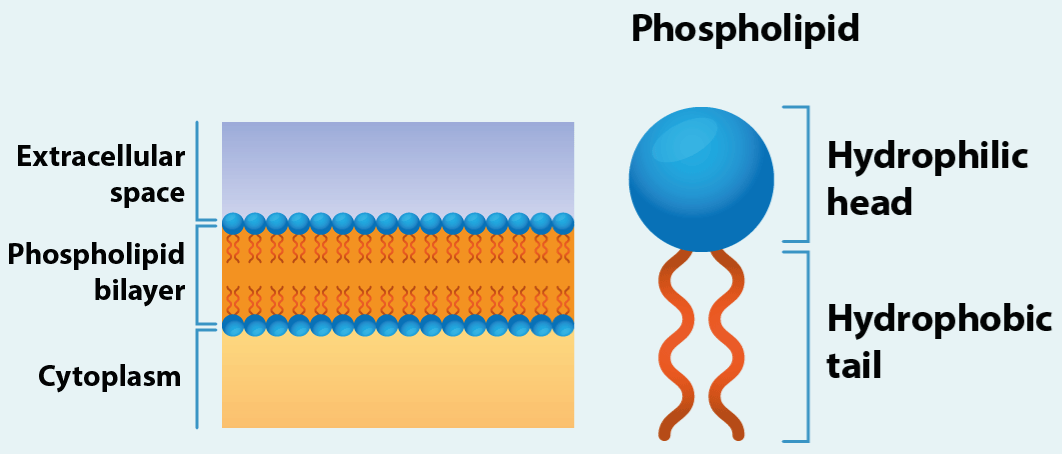
Figure 2.The plasma membrane
The plasma membranes are composed of two layers of tightly packed lipid molecules with the polar heads on the outside and their hydrophobic tails inside.
An ion channel is usually composed of merely a few protein molecules, sometimes of only one (Figure3). It contains a central aqueous pore that can be opened by conformational change to allow ions to flow through the channel at rates up to 100 million ions per second when it is open. This ion movement forms an electrical current that is sufficient to be measured by suitable techniques; hence we can observe the activity of an individual channel, and so of an individual molecule or molecular complex, just as it happens. Ion channels vary considerably in their gating (gating is an allosteric process, involving a conformational change in the channel protein) by which the factors that make them open or close. Some channels are opened by combination with chemical outside or inside the cell, such as neurotransmitter (external ligands) or cytoplasmic messenger molecules (internal ligands). Other are opened by changes in the voltage across the membrane, and yet others by sensory stimuli of various kinds. Channels show selectivity in the ions to which they are permeable. Some of them will permit only particular ions to pass through, such as sodium, potassium, calcium, or chloride ions; others are selective for broader group of ions, such monovalent cations, or cations in general [3].
The Figure4 illustrates what channels do with the examples of the pancreatic β cell. The β cells are concerned with the production and secretion of insulin, the hormone that controls blood sugar levels. Although the insulin secretion that we know today can be much more elaborate than what is shown in the figure3, it serves to exemplify the concerted action of two different receptors in a physiological process and two different gates, and as already in the decade of in the 90s, the preponderant role of receptors in human physiology was clear and how their manipulation allowed therapeutic options for diabetes mellitus. Two types of ion channels in the plasma membrane are involved in this. The ATP sensitive potassium channel close when the internal concentration of ATP (adenosine triphosphate) rises to an appropriate level. The voltage gated calcium channels open when the membrane potential of the cell becomes less negative by enough. When the blood glucose levels are low, the ATP sensitive potassium channels in the β cell are open to that potassium ions can flow out of the cell, and this ensures that the membrane potential is kept at negative value. When blood glucose rises after meal, uptake of glucose into the cell leads to a rise in its ATP levels, and this makes the potassium channel close. This leads to a change in the membrane potential to less negative value (depolarization), and this in turn promotes the opening of voltage-gated calcium channels. The resulting inflow of calcium ion concentration, which then acts as a trigger for the release of insulin from the cell. For some patients one approach is to use drugs that will block the ATP sensitive potassium channels and to promote opening of the calcium channels [4].
Figure 3.Ion channel pore
Note the oligomerization of the protein that makes up the channel, which allows visualization of the pore.
In their classic study of the nature of the nerve impulse in 1952, Alan Hodgkin and Andrew Huxley provided a mathematical description of the flow of sodium and potassium ions through the nerve axon membrane. It seemed like that the change in ionic permeability were associated with the movement of some electrically charges particles within the membrane. They deduced there must be many ions moving across the membrane for each movable membrane charge. They therefore proposed that the ionic currents were localized at sites (“active patch” as they called them) in the membrane. It is these sites that later becomes known as voltage-gated sodium and potassium channels [5,6]. Hodgkin and Richard Keynes investigated the potassium permeability of nerve axons in 1955. They found that the movements of radioactive potassium ions across the membrane could be explained if the ions passed through narrow pores in single file, ant they used the word “channel” to describe these ideas [7]. The breakthrough came in 1976 with the development of the patch clamp technique by Erwin Neher and Bert Sakmann. They used a glass microelectrode with polished tip that could be applied of the surface of a cell to isolate a small patch of membrane. The voltage across this patch was held steady (“clamped”) by a feedback amplifier so that they could measure the currents flowing through the individual ion channel in it. This revolutionary technique proved to be increasingly productive, especially after further technical improvements. In 1992 Neher and Sakmann were awarded the Nobel Prize for Physiology and Medicine for their work [8-10]. The openings and closings of channels are stochastic events. This means that, as with many other molecular processes, we can predict when they will occur only in terms of statistical probabilities.
The other great technical advance in the study of ion channels has been the use of the methods of recombinant DNA technology (also known as gene cloning or genetic engineering) to investigate the structure. Its essential purpose is to take a single DNA molecule or fragment and from this produce large numbers of molecules with identical sequences. Such DNA fragment is joined with vector DNA sequences (plasmids or bacteriophages), which can replicate when introduced into a host cell (Escherichia coli). The single recombinant DNA molecule, vector plus DNA fragment, is then replicate in the host cell so that large numbers of identical DNA molecules are produced. The amino acid sequence of the subunits of the nicotinic acetylcholine receptor channel were determined in 1982 by teams led by Shosaku Numa, Jean-Pierre Changeux and others, and the of the voltage-gated sodium channel followed two years later. Sequence gives us strong clues about structures, and knowledge of the structure of channels give us an increasing understanding of the way they work [11,12]. With the recent technical breakthroughs in structural biology, especially in cryo-electron microscopy (cryo-EM), many new high-resolution structures of ion channel targets have been solved, shedding light on the molecular mechanism of action of the insecticides and resistance mutations [13]. The role of ion channels in immune-related diseases is the subject of vigorous investigation [14]. The era of cloning initiated our understanding of ion channels at the molecular level, an understanding that has been further expanded by the crystal structures, spectroscopy, computational chemistry, nuclear magnetic resonance, and single molecule fluorescence that then followed [15]. A pattern emerged for Na+, K+, and Ca2+channels in that there was a basic structure of six putative transmembrane segments, repeated four times in the same polypeptide for Na+ and Ca2+ channels. Biochemical and biophysical experiments confirmed that the basic six transmembrane segments plus a re-entrant loop were common in all these voltage-gated channels [16]. The real understanding of an ion channel at the molecular level will only happen when the kinetics and steady state of the channel function can be predicted from the dynamics of the structure. The possible treatments to channel-associated disease will be accelerated if we understand in detail how channels are implicated in the physiology of the cell, and if we could design modifications that restore normal function. The latter will be aided by a description of the molecular basis of channel function.
Back To Basic
The existence of electrical charges is one of the fundamental features of the universe. Single charges are associated with single subatomic particles: they are unitary and either positive or negative. Charges of the same sign repel each other and those of opposite sign attract each other. The attractive force between positive and negative charges means that it requires an appreciable amount of energy to separate them, and that thus energy is released when they are allowed to come together again. Consequently, we find that the normal state of matter is to be electrically neutral, with equal numbers of positive and negative charges. Living cells can also utilize the energy available from the attractive force between electric charges of opposite sign, and this may be evident ultimately in the flow of ions through ion channels. Current electricity is concerned with the flow of charge from one place to another. Current will only flow from one point to another if there is a potential difference (V or E) between the two points and a conducting path between them. Potential differences are measured in volts (V) [1]. One of the triumphs of twentieth chemistry has been to explain the properties of chemical elements in terms of their atomic structures. Protons have positive charges and electrons have negative charges. So, the nucleus of an atom, composed of protons and neutrons, possesses a positive charge (equal to its atomic number) that is balanced by the negative charges on the cloud of electrons surrounding. Chemical bonding between separate atoms involves only the electrons in their outermost shell; these are therefore known as the valence electrons. A covalent bond is formed when a pair of valence electrons is shared between two atoms (water). A different type is the ionic bond. In sodium chloride, for example, each sodium atom has lost the single electron in its outer shell and each chloride atom has gained one. Both atoms are now ions: the sodium ion has a positive charge (cation), since it has lost an electron, and chloride ion has a negative charge (anion) since it has gained one. There is now also strong electrostatic attraction between the two ions and serves to make their state of ionization energetically favorable. In the ionic bond we have a complete transference of charges from one atom to another. In hydrogen chloride, the centre of gravity of the negative charges in the molecule is separated in space from that of the positive charges. The molecule thus has an electric dipole in it and described as being polar. Many organic substances are soluble in water because they form bonds with it. Glucose and other sugars are examples of this. Other compounds are not very soluble and are to clusters together in an aqueous environment. The effective attractive force between such molecules is sometimes called the hydrophobic bond [17]. The atoms forms ions (Michael Faraday) (cations and anions) and in the sodium chloride example they can form crystals. The size of an ion may well be crucial important in determining whether it can pass through a particular ion channel [18].
Molecules in liquid are in constant motion due to thermal agitation. Ions or other particles in solution or in suspension in water will be buffeted from all directions by the impact of water molecules (random movement called Brownian motion). In 1909 Einstein showed that diffusion results from the sum of single particles moving about at random under thermal agitation [19]. But the net movement will go from the region of higher concentration (H) to that of lower concentration (L). This is what is meant by diffusion. The rate of the net movement from H to L is proportional to the area of interface through which they are moving, and the concentration gradient (Fick´s law diffusion) [20]. The total gradient is called electrochemical gradient, and the total movement is called electro-diffusion. The solute ions will still cross from one side to the other under the influence of the electrochemical gradient, but the rate at which they do so will be greatly affected by the characteristics of the pores. We can apply these ideas to living cells. The plasma membrane is the barrier to ion movement, and ion channels are the aqueous pores through which the ions can move. The direction in which they move is determined by the electrochemical gradient, however the rate at which ions move across the membrane is determined by a few factors: the magnitude of the gradient, the nature of the ion, permeability and selectivity of the channels, the number of ions channels present per unit area of membrane, and the proportion of them that are opens. The larger the membrane capacitance, the grater the number of ions that must move to produce a particular potential.
The electrochemical gradient
To exemplify, we use the potassium channels and a potassium salt (K+ A-) in two different concentrations on both sides of a membrane. If the channels are closed then there can be no movement of ions across the membrane, so there is not excess of positive charges over negative charges in either of the compartments, and hence there is no potential difference between them. A new situation arises if the channels suddenly open. Potassium ions flow down their concentration gradient through the channels from compartment with high concentration (1st) to the compartment of low concentration (2nd). Now there will be some depletion of positive charges in the compartment with the highest original concentration of the salt (1st) and some excess of positive charges in the one with the lowest concentration (2nd). This cannot be compensated for by a corresponding movement of negative charge since the ion channels do not allow the A- anion to be through. Consequently, a potential difference arises between the two compartments. The 2nd compartment being positive than the 1st compartment. But this potential difference itself now affects the movement of the potassium ions K+: the excess of positive charges in compartment 2ndtends to drive back from 2nd to 1st. The system soon reaches an equilibrium position where the concentration gradient tending to push the potassium ions from 1st to 2nd is balanced by the electrical gradient tending to push them in the opposite direction. The potential difference between the compartments at this point is called the equilibrium potential for potassium ions (EK+) [21].
Each living cell is bounded by a plasma membrane, which separates the contents of the cell from the external medium in which it lives. The concentrations of ions inside the cell are almost different from those in the external medium [22,23]. The cell must be electrically neutral, so that the total numbers of positive and negative charges must be effectively equal. Consequently, the concentrations of anions and cations must be balance in the respect. It is usual for the intracellular negative charges to be associated largely with a variety of organic anions to which the plasma membrane is not permeable. Therefore, there is an ionic gradient. How is it handled? In the case of sodium ions, the answer is clear: there is an active transport system that continually extrudes sodium ions from the cell: the sodium pump. It is carried out by membrane-bound sodium-potassium ATPase [24]. Three sodium ions are extruded, and two potassium ions are drawn into the cell for each ATP molecule that is broken down. The potassium ion concentration gradient arises in part from the uptake associated with the activity of the sodium pump, and partly by a passive movement of potassium ions through open potassium-selective channels. In many cells chloride ions are largely passively distributed. The negative membrane potential ensures that the internal chloride concentration is much less than outside the cell. Calcium ions are actively extruded from most cells, and they are also sequestered in intracellular compartments such as the endoplasmic reticulum and the mitochondria. This leads to very low concentration of free calcium ions, typically in the region of 0.1 µM or less. Most cells possess a membrane potential, a voltage across the plasma membrane such that the inside is usually some tens of millivolts negative to the outside (-60-90 mv depending on the cell, tissue and species studied). It is possible explain this potential in terms of ionic gradients. At rest the microelectrode records a resting potential of about -90 mV (millivolts) from the muscle cell. When the motor axon serving it is stimulated, a depolarization (a reduction in the membrane potential to a less negative value) of several mV occurs. It is produced by the flow of ions through the acetylcholine receptor channels. It is possible to determine the relations between membrane potential and ionic conductance. In an area of the membrane containing many channels, each of which may be open or closed at any instant, the total number of channels open will fluctuate in a random fashion from one moment to the next. Consequently, the currents flowing through the membrane will also fluctuate from one moment to the next [25].
Structure and function
Function creates the organ and disuse atrophy. One of the fascinating features of biology is the way the structures interact with the functions. Ions channels are composed one of or more protein molecules. A common pattern is to have an oligomeric arrangement (Figure 3) whereby the channel consist of a rosette of four, five or six similar subunits with an aqueous pore at its centre (Figure1). Each subunit, like all protein molecules, is a chain of amino acids linked together in linear sequence by peptide bonds. There are 20 different amino acids and there may be some hundreds of residues arranged in a unique sequence in the molecules of any protein. The amino acid sequence determines the nature of the protein [26]. The amino acid sequence of a protein is sometimes known as its primary structure. The term secondary structure applies to the arrangement of short lengths of the chain, as α-helix and β-helix and sheet structure. Tertiary structure applies to the arrangement of the whole chain in single subunit, and quaternary structure applies to the overall shape of the whole molecule complex, including all its subunits. To exemplify we use the voltage gated channels. The channel pore in this superfamily is formed from the union of four similar subunits, or domain of a single protein chain in the case of sodium and calcium channels. The four principal subunits or domain have a characteristic structure (Figure5). In each of them there are six member-crossing segments, S1 to S6, which appear to be α-helices. There is also a section between S5 and S6, known as the SS1-SS2 loop or the H5 or P region, which probable form part of the lining of the pore. The S4 segment has a remarkable structure every third amino acid residue is positively charged. It seems likely that the movement of these positive charges is in some way responsible for the sensitivity of the channel to changes in membrane potential.
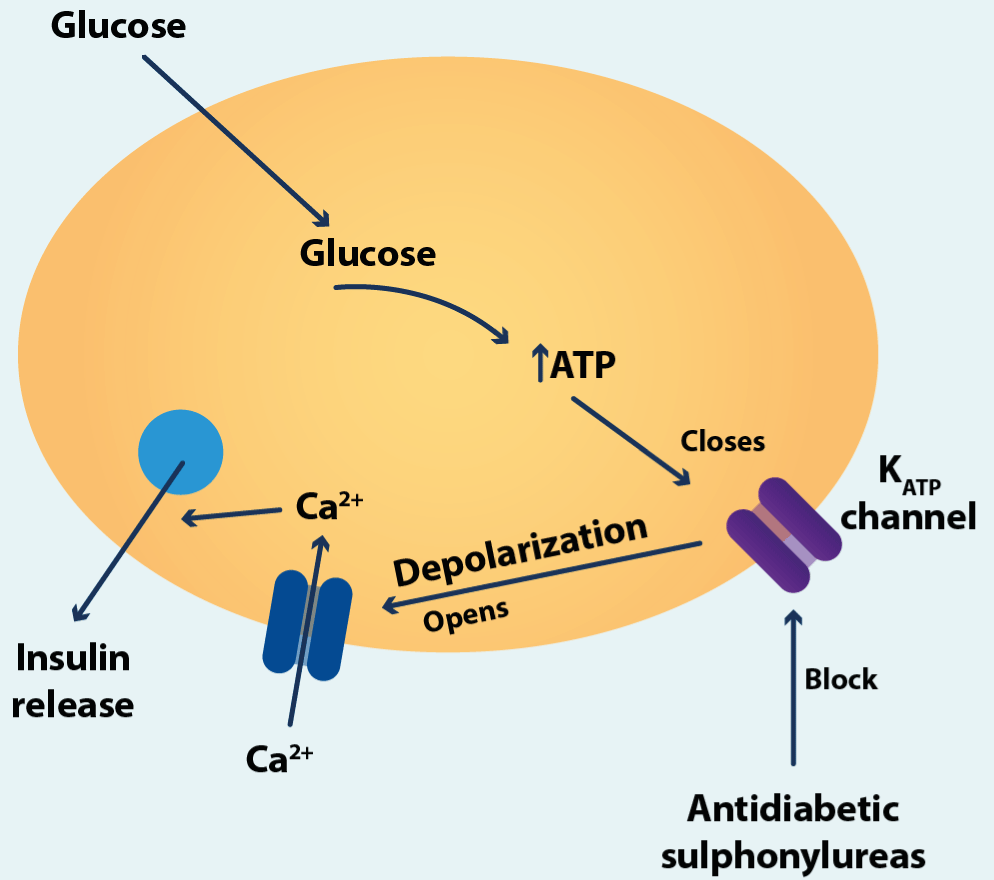
Figure 4.Pancreatic β cell
How ATP-sensitive potassium channels and voltage-gated calcium channels are involved in the secretion of insulin from pancreatic β cells (see text).
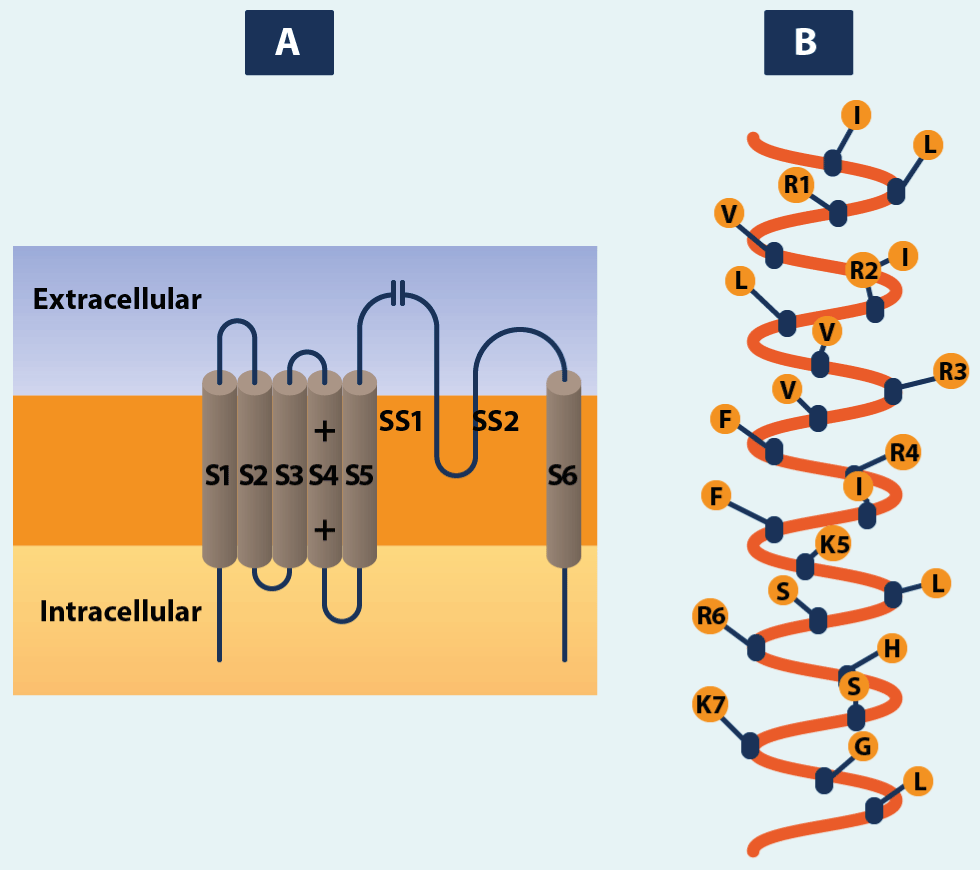
Figure 5.Characteristic structures in voltage-gated channels and their relatives
Each subunit or domain contains six transmembrane α-helices, shown in A as cylinders, with the rest of the nearby peptide chain drawn as a line connecting them. The membrane-associated segment SS1-SS2 occurs, and probably forms part of the lining of the pore. The S4 segment contains the positively charged amino acid residues arginine (R) or lysine (K) at every third position. This is shown for the potassium channel in B.
Since calcium ions act as intracellular messenger for a variety of cellular processes (cellular signaling systems), this means that calcium channels are intimately involved in intracellular control system in addition to any effect they may have on the membrane potential. For this reason, there are calcium channels in the intracellular membranes involved with the movement of calcium within cells. For example, the IP3 receptor (Inositol 1,4,5-triphosphate receptor, abbreviated as IP3R). IP3 is an important second messenger in many cellular operations [27-29]. Not all potassium channels are opened solely by changes in membrane potential. An important group found in many cells contains those that are opened by an increase in the intracellular calcium ion concentration, such as might be brought about by calcium inflow through voltage-gated calcium channels or calcium release from intracellular stores [30,31].
Another important aspect of how structure shape’s function is the fact that la conductance show saturation, i.e., it rises at first quite rapidly with increasing concentration, then more slowly, and finally it reaches a maximum. Clearly there is an upper limit on the rate at which ions can pass through the channel. The most likely explanation is that each ion combines with one or more binding sites in the channel pore during its passage through it, and these binding sites can each bind only one ion at time. The pores do not have the same diameter along their length. There is a narrower region where the ions are in more intimate contact with the channel proteins. The permeant organic ions all contain NH2-or OH-groups, whose hydrogen atoms can form hydrogen bonds with adjacent oxygen atoms in the channel wall [32]. Water molecules are about 3 Å in diameter. They can probably form hydrogen bonds with the polar groups of some of amino acids lining the pore, hence it is generally assumed that they can pass through open channels [33].
Modulation is a term usually used to describe some agent o process that modifies the gating of a channel. The probability of a channel opening may be altered by factors other than the primary gating trigger. The attachment of phosphate group to proteins or their detachment from them is if major important in cell biochemistry. This process is called phosphorylation. It is brought about by the action of protein kinase, an enzyme that transfers a phosphate group from ATP to the protein:
 Protein-kinase
Protein-kinase
protein + ATP -------------------------» protein-P + ADP
Dephosphorylation is the removal of the phosphate group by hydrolysis to leave the protein and inorganic phosphate.
 phosphatase
phosphatase
protein-P + H2O ---------------------» protein + Pi
There are several different types of protein kinase, distinguished by the different ways in which their activity is controlled. For example, cAMP-dependent protein kinase A (PKA). In the B2-adrenoreceptor-mediated smooth muscle relaxation the PKA phosphorylates different proteins with the consequent smooth muscle relaxation. PKA phosphorylates the potassium channels favoring the outflow of the same out of the cell, depolarization, and relaxation of smooth muscle (Figure6). In cholinergic mechanisms of smooth muscle contraction phosphorylation of myosin by MLCK (myosin regulatory light chain kinase) facilitates interaction between actin and myosin and contraction and PKC (protein kinase C) also because it phosphorylates calponin [34]. These mechanisms are blocked by muscarinic antagonists (Figure7).
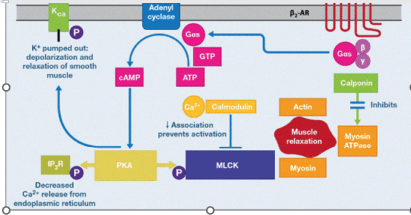
Figure 6.β2-adrenoreceptor-mediated smooth muscle relaxation: cell signaling
Smooth muscle relaxation is caused by binding of agonists (catecholamine, SABA, LABA or ULTRA-LABA) to the β2-adrenoreceptor. This is a Gαs-coupled receptor that stimulates adenylyl cyclase, resulting in the conversion of ATP to cAMP. Increased cAMP levels activate protein kinase A (PKA) which phosphorylates myosin regulatory light chain kinase (MLCK). This decreases its association with calcium/calmodulin. In addition, inositol triphosphate receptors (IP3R) on the endoplasmic reticulum (ER) are phosphorylated by PKA; this decreases Ca2+ release from the ER into the cytosol. The two pathways result in depolarization of the smooth muscle. (Adapted from Figure 4A of Johnson EN).
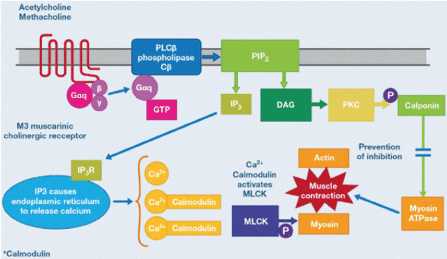
Figure 7. Cholinergic mechanisms of smoot muscle contraction
ACh binding to the M3 receptor results in guanosine triphosphate (GTP) binding to the Gαq subunit. GTP-bound Gαq stimulates phospholipase Cβ (PLCBβ), which cleaves phosphoinositolbisphosphate (PIP2) into inositol triphosphate (IP3) and diacyl glycerol (DAG). IP3 interacts with IP3 receptors (IP3R) on the ER and evokes Ca2+ release from the ER into the cytosol. Liberated Ca2+ binds calmodulin, forming a complex that activates MLCK. Phosphorylation (P) of myosin by MLCK facilitates interaction between actin and myosin and contraction. In addition, DAG activates protein kinase C (PKC), in an alternate mechanism of smooth muscle contraction. These mechanisms are blocked by muscarinic antagonists. (Adapted from Figure 4B of Johnson EN).
For 25 years, researchers have considered how the amino acid chains of channel proteins are arranged, and that precise knowledge of channel structure, should it become available, would allow molecular dynamics simulations of ion permeation through natural channels to be attempted. In recent days, bioinformaticians and biophysicists having the necessary expertise and interests in computer science techniques including versatile algorithms have started covering a multitude of physiological aspects including especially evolution, mutations, and genomics of functional channels and channel subunits. In these focused research areas, the use of artificial intelligence (AI), machine learning (ML), and deep learning (DL) algorithms and associated models have been found very popular [35]. Application of AI techniques in biological systems, e.g., ion channels, requires the development of specific algorithms and models capable of connecting with the complex, dynamic, and fluctuating natures of biomolecules involved in channel structures [36,37]. Although spanning a broad spectrum of families, ion channels can be roughly categorized into the voltage-gated, the ligand-gated, and others, based on activation mechanisms and structural similarities [38]. Ion channels have long been concerned as medically important drug targets. Increasing the ligand screening throughput and ligand binding selectivity are the main challenges in the search for highly potent ion-channel modulators. As the potential key to meeting these challenges, the practice of rational drug design has long been expected. With help from these computational methods, we expect the implementation of a more effective drug discovery paradigm in the coming future [39-41].
Ion Channels of the Lung
In this review, we focus on recent developments in understanding how ion channels maintain normal lung homeostasis and explorer the functional significance of these channels. The impairment of epithelial ion transport processes that alter the fluid composition/content are associated with several human diseases/pathologies, including cystic fibrosis (CF) [2], chronic bronchitis [3], chronic obstructive pulmonary disease (COPD) [42], bronchial asthma [43], pulmonary hypertension, pulmonary edema [44], primary ciliary dyskinesia [42] and Covid-19 [45].
A thin fluid layer that forms a continuous barrier between the organism and external environment covers the lining of the lung. Many upper airway epithelial cells are cuboidal cells with cilia [46]. These ciliated epithelial cells coordinate the beating of their cilia and govern ion transfer, and the efficiencies and fidelity of these two processes are necessary for proper mucociliary clearance (MCC). Efficient MCC requires that the ASL, which is composed of the mucous layer above and the periciliary liquid (PCL) below, is appropriately hydrated and of the right viscosity (mucus layer) to allow for the cilia to beat properly and move the mucus layer at an appropriate rate (Figure 8) [47]. There are approximately 200 cilia per cell, which beat 15-20 times per second and propel the gel at a speed of 1 mm/minute under normal conditions. Airway mucus is approximately isotonic with serum, consisting of 90-98% water, ions (at level like plasma), gel-forming mucins, and a wide array of other proteins, peptides, and small molecules. MCC is a part of the innate immune system that is responsible for trapping and clearing the airways from inhaled pathogens and other noxious particles. Although the trapped pathogens and particles are transported within the mucus layer, the composition and thickness, pH, and viscosity of the PCL determines the optimal ciliary beat and thus the effectiveness of mucociliary clearance [48]. The major function of the airway epithelium is to form an innate defense barrier, filter air, and kill microbes. The PCL characteristics are dependent on the active coordination between the cystic fibrosis transmembrane conductance regulator (CFTR), Ca2+-activated chloride channels (CaCC), chloride channel (CLC2), and the epithelial sodium channel (ENaC) (Figure9) [49,50]. The epithelium produces active secretion of chlorine and bicarbonate into the airway lumen. Both anions generate a lumen-negative voltage that draw sodium into the lumen, forming sodium chloride, and consequently the water flow towards the lumen. PLC is highly permeable to water and the volume of fluid is determined by the amount of sodium chloride in the lumen of the airway. The apical cyclic nucleotide-gated channels (CNG) may support ENaC function, whereas CLC2 chloride channel and CaCC, including the transmembrane domain 16a (TMEM16) channels, contribute to apical chloride secretion [51]. Chloride secretion is sustained by the basolateral Na+-K+-2Cl− cotransporter and HCO−3/Cl− anion exchanger, whereas basolateral Na+/H+exchanger (NHE) maintains intracellular pH [52]. Additionally, the basolateral membrane contains chloride channel ([BORC], [BIRC], [BCFTR) that along with [Kv7.1], [K7.5], [SK4], and [BKCa], [KCa3.1]) may modulate apical chloride secretion (Figure10) [53-56]. We must remember the nomenclature of the channels. First the ion in capital letter, then the activation mechanism in lower case letter. The first number (on the right) refers to the family. A point and then the second number to the subfamily. So Kv1.3refers to a voltage-gated potassium channel of one family and the third member (subfamily) of that one family [53].
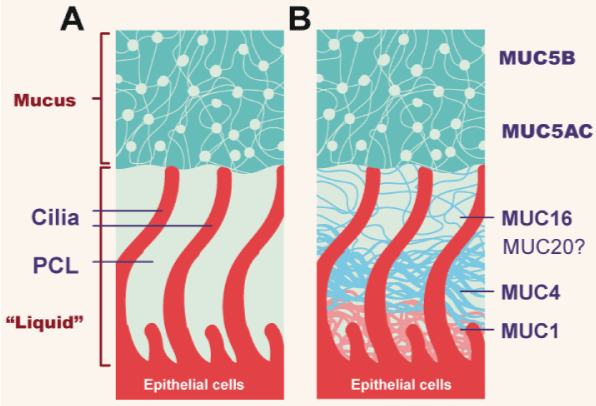
Figure 8. Topography of mucus clearance system
(A).Classic mucus layer “floating” on periciliary liquid layer. (B). Two-gel formulation, with secreted mucins (MUC5B and MUC5C) interpenetrating and residing over a brush-like periciliary gel layer (PCL) composed of MUC1,4,16, and possibly 20, tethered to cilial epithelial surface (Adapted from Button B).
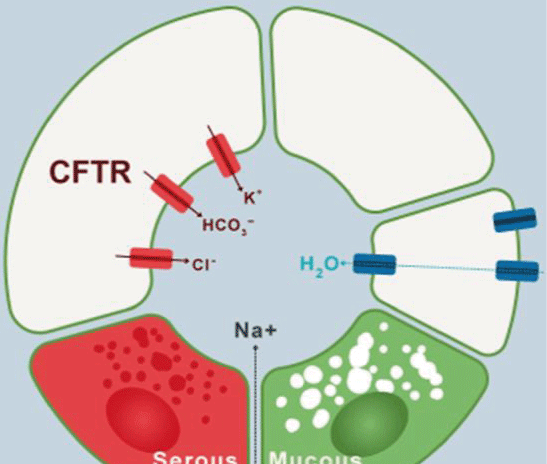
Figure 9.Ions transport mechanism in airway cells
The imagen shows clockwise the transport of electrolyte and water to the lumen of the airway in normal conditions (Modified from Lee and Foskett).
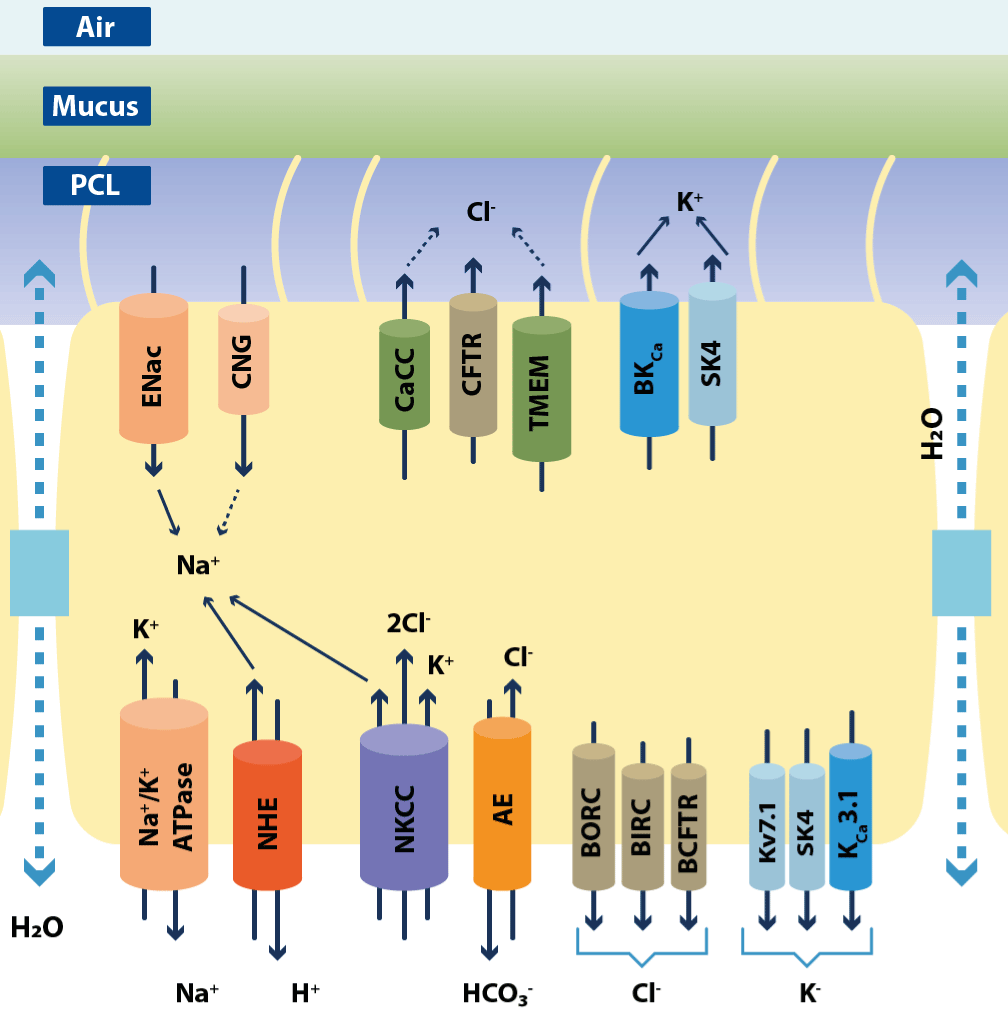
Figure 10.Ion channels and transporters that participate in regulation of lung periciliary liquid (PCL) homeostasis and mucociliary clearance by ciliated airway epithelia
Apical membrane sodium reabsorption, accompanied by chloride secretion along with passive H2O transport, provides the main mechanism responsible for PCL composition. The sodium reabsorption is mediated by coordinated function of epithelial sodium channel (ENaC, apical) and Na+-K+-ATPase (basal). However, apical cyclic nucleotide-gated channels (CNG) may contribute to sodium uptake as well. Furthermore, basal membrane located. CaCC (Ca2+-activated chloride channels), CFTR (cystic fibrosis transmembrane conductance regulator), TMEM (transmembrane domain), BKCa (large-conductance Ca2+-activated, voltage-dependent potassium channel), SK4 (small-conductance Ca2+-activated potassium channels), NHE (Na+/H+ exchanger), NKCC (Na+-K+-Cl- cotransporter) AE (anion exchanger), BORC (basolateral outward rectifying channel), BIRC (basolateral inward rectifying channel), BCFTR (basolateral CFTR-like channel), Kv7.1 (α-subunit of a voltage-dependent potassium channel), KCa3.1 (Ca2+-activated potassium channel). (Adapted from figure 1 of Bartoszweski R).
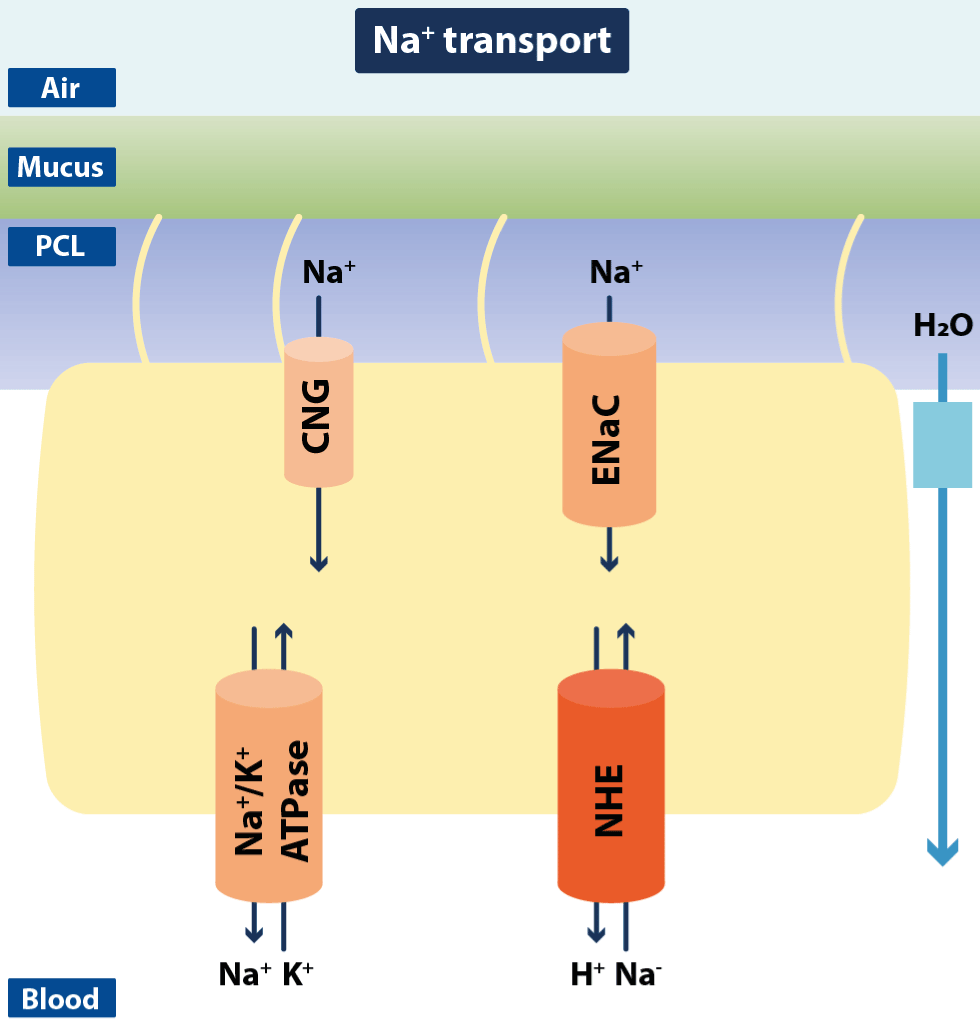
Figure 11.Schematic drawing of ciliated airway epithelial cells with Na+ channels and transporters
The figure shows transepithelial Na+ reabsorption mediated by concerted activity of apical epithelial Na+ channels (ENaC) and the basolateral Na+/K+ ATPase. Apical cyclic nucleotide-gated cation channels (CNG) might also contribute to Na+ reabsorption. Additionally, a Na+/H+ exchanger (NHE) has been identified in airway epithelial cells for regulation of intracellular pH (Modified from Hollenhorst MI).
The epithelial cells that form the alveolar epithelium (AE) are crucial for efficient gas exchange and are separated from the gas phase by a thin layer of alveolar lining fluid (ALF). The amount, volume, and composition of ALF are crucial for gas diffusion, and therefore the electrolyte composition of ALF is strictly controlled by AE-mediated ion transport [57]. The AE consist of two types of epithelial cells, alveolar type I (ATI) and alveolar type II (ATII) cells, that mediate vectorial ion processes despite their differences in function and morphology. Both ATI and ATII express ENaC, amiloride-insensitive CNG cation channels, potassium channels, CFTR, γ-aminobutyric acid type A (GABAA) channels, and voltage-gated chloride channels (CLC2 and CLC5) [58]. Furthermore, ATI cells express aquaporin 5 (AQP5) and exhibit the highest water permeability of any mammalian cell type. As in ciliated epithelial cells, fluid removal out of alveoli in the interstitium uses a transepithelial osmotic gradient created in AE cells by sodium uptake through the coordinated action of apical ENaC, CNG cation channels, and the basal ouabain-sensitive Na+/K+/ATPase. The fundamental objective at the level of the alveolar epithelium of the ionic gradient and the membrane potential is to keep the alveoli "dry" and prevent the formation of edema. However, the mechanism for chloride and potassium transport by AE cells is less clear [59].
The membrane potential of the apical membrane (Va) is governed by its major conductance, which are the apical sodium and chloride conductance, whereas the basolateral membrane potential (Vb) is largely governed by its potassium conductance. The primary ion transport function of lung epithelia is sodium absorption across apical sodium channels driven by the basolaterally located Na+/K+/ATPase. Recycling of potassium across the basolateral membrane results in a hyperpolarization of the cell, and chloride distributes passively across the apical membrane according to its electrochemical driving forces. Although there are a few other ion channels expressed in lung epithelium, including the apical hydrogen ion channel, their contributions to the membrane potentials can be safely neglected by the high sodium and chloride conductance [60].
Ion Channels and Some Respiratory Channelopathies
Due to comprehension and academic reasons, the Na+, Cl−, and K+ channels and transporters have been depicted in different cells although most of them are usually found in the same cell (Figure10)
Sodium channels
The driving force for apical sodium reabsorption in airway epithelial cells is provided by the Na+/K+/ATPase located along the basolateral membrane (Figure 11). The Na+/K+/ATPase is composed of a heterodimer of two subunits (α and β) [61]. The conversion of one ATP to ADP results in transport of three sodium ions out of the cell in exchange for two potassium ions pumped into the cell (along the basolateral membrane). The Na+-K+-ATPase activity is stimulated by sodium and potassium ions from the cytoplasmic and extracellular side, respectively, and an acute increase in intracellular sodium stimulates Na+/K+/ATPase activity and leads to a rapid export of intracellular sodium [61]. Thus, under physiological conditions, intracellular sodium is rate limiting for Na+/K+/ATPase activity, whereas the intracellular ATP concentration is not. ATP may become rate limiting, however, when its synthesis is decreased under pathological conditions such as during periods of hypoxia or oxidative stress [62]. Hypoxia is accompanied by a decrease of Na+/K+/ATPase function, reduces alveolar fluid clearance and an increased production of mitochondrial reactive oxygen species (ROS) leads to Na+/K+/ATPase endocytosis and ubiquitin-dependent degradation [63]. Therefore, under normal conditions the Na+/K+/ATPase maintains "dry" the alveoli. It´s underregulating promotes pulmonary edema. The function of the enzyme is vital to resolve pulmonary edema in pathological conditions. This ATPase located in the basolateral region when removing sodium, conditions the entry of sodium but through the apical part. This is the function of the sodium/potassium pump. The main mediator of transcellular sodium reabsorption is ENaC that is found at the apical membrane of human airway epithelia. Because ENaC is often considered the rate-limiting step of lung sodium homeostasis, it is not surprising that changes in sodium channel function that result from epigenetic or pathological factors often contribute to lung disease [64]. Hypercapnia can also decrease ENaC function by phosphorylating the Thr 615 (threonine) residue of the channel (mediated by ERK1 [extracellular signal regulatory kinase 1]). This would favor fluid retention and pulmonary edema. The use of a specific ENaC blocker could lead to ASL rehydration and increased ciliary function and mucociliary clearance. This data implies that ENaC inhibition may also be an effective strategy to treat other lung diseases such as COPD [65,66]. Recent studies have also shown that exposure to high levels of ROS results in increased amounts of oxidized glutathione in the ALF, and this reduces the ENaC activity in primary AE cells and impairs the lung fluid balance [67]. This last observation suggests that antioxidant-based therapies should be considered. Overexpression of the channel in the short term maintains stable blood pressure, intravascular volume, and electrolytes, but in the long term, it promotes mechanical myogenesis, sclerosis, remodeling, and increased vascular resistance, and systemic arterial hypertension. ENaC upregulation studies have been carried out mainly in cerebral and renal vessels but not in pulmonary vessels. This is a fertile area for investigation of this canal.
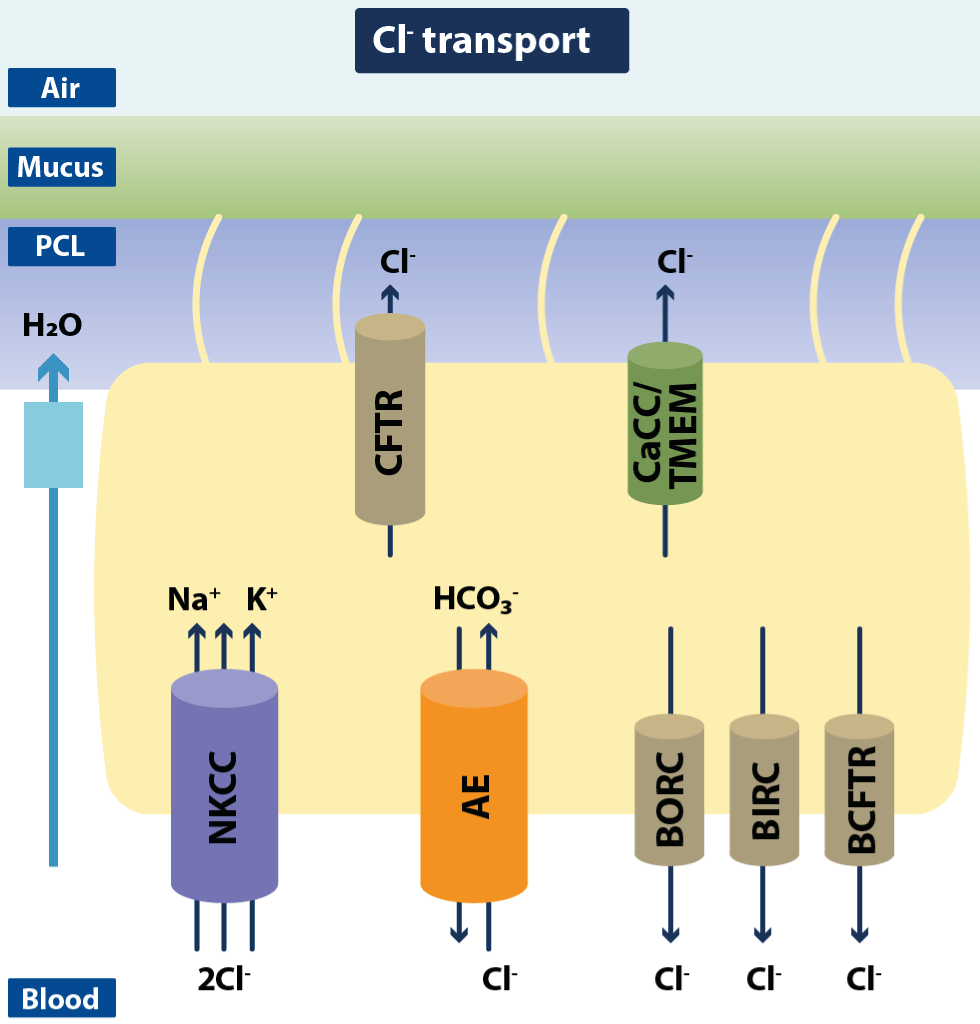
Figure 12.Schematic drawing of ciliated airway epithelial cells with Cl− channels and transporters
In addition to Na+ reabsorption airway, epithelia display a prominent apical Cl− secretion that is mainly mediated by the cystic fibrosis transmembrane conductance regulator (CFTR) in humans and to a lesser extent by Ca2+-dependent Cl− channels (CaCC) such as the TMEM channels. This secretion is kept up by the basolateral Na+/K+/2Cl− cotransporter and the HCO3−/Cl− exchanger (AE). Additionally, three basolateral Cl− channel types have been identified: BORC, BIRC, and BCFTR. These channels have been suggested to be involved in modulation of apical Cl− secretion (modified from Hollenhorst MI).
Besides ENaC, the apical membrane located CNG cation channels may provide another sodium entry pathway in lung epithelial cells and can be activated by micromolar concentrations of cGMP. Because exogenous cGMP was shown to stimulate liquid absorption, the CNG channels have been proposed to play a role in the regulation of ALF volume [68].
NCX (Na+/Ca2+) is a sodium-calcium exchanger (It´s not in the figures). It works in and out of the membrane. If it is inward, it removes a calcium atom and introduces 3 sodium atoms. If it is outwards, it removes 3 atoms of sodium and introduces one atom of calcium. Inward exchange promotes vascular contraction, remodeling, and increased resistance. The reverse (outward) mode has been implicated in the role of calcium in the proliferation of pulmonary artery smooth muscle cells (PASMC) in patients with Idiopathic Pulmonary Arterial Hypertension (IPAH) [69]. NHE (Na+/H+) introduces an atom of Na+ inside the cell and removes an atom of H+, increasing intracellular pH and upregulating and increasing intracellular Ca2+, favoring cell proliferation and vasoconstriction [70].
Probably the most primitive function played by the exquisite control of sodium concentration through ion channels is to prevent edema. If intracellular sodium reabsorption is decreased, ASL reabsorption is decreased. Hydrostatic pulmonary oedema, for example, are caused by an acute elevation of left heart atrial pressure, which results in an increased pressure in the pulmonary vein and, thus, in increased fluid flux from the pulmonary capillaries into the alveolar airspace [71]. Consequently, the gas exchange across the AE is decreased. This leads to local hypoxia, which then results in the decreased expression of ENaC and Na+/K+ ATPase and, thus, insufficient clearance of the fluid from the airspace. Other lung diseases, like acute lung injury (ALI) or its more severe manifestation the acute respiratory distress syndrome (ARDS), are also related to pulmonary oedema formation associated with damages of the alveolar-capillary barrier caused by bacterial sepsis, acid aspiration, smoke inhalation, and reperfusion injury after lung transplantation [72]. Another incident for the formation of pulmonary oedema is represented by artificial ventilation. This ventilator-induced lung injury (VILI) is reasoned by over-distention of the alveoli leading to an increased alveolar-capillary permeability and, thus, influx of oedema fluid into the alveoli. Although modified ventilation strategies proved to be beneficial to avoid VILI, targeting alveolar fluid absorption mechanism is still a major therapeutic option for the resolution of the oedema fluid in these patients [73].
Chloride channels
Cl– is the most abundant anion in vivo, and its extracellular concentration is higher than that of intracellular fluid. Cl– accounts for about 70% and 65% of the total anions in tissue fluid and plasma, respectively. Cl– is involved in a variety of physiological functions, including cell volume regulation, proliferation, membrane potential changes, signal transduction, acid basis balance, blood pressure regulation, etc. [74]. The apical secretion of intracellular chloride towards the outside of the membrane occurs mainly by CFTR, but this concentration of intracellular chloride is maintained at the expense of the basolateral Na+/K+/2Cl-(NKCC) and HCO3-/Cl- cotransporters. The first cotransporter introduces chlorine along with sodium and potassium and the second introduces chlorine and removes HCO3- regulating intracellular pH. Just as apical sodium influx depends on basolateral Na+/K+/ATPase (which removes sodium and introduces potassium), so apical Cl- extrusion by CFTR depends on basolateral channels that feed intracellular Cl- stores [56]. At the apical level, CaCCs (calcium-activated chloride channels) also participate in chlorine extrusion, as do BIRC, BORC, and BCFTR at the basolateral level (Figure 12). CFTR is cAMP-dependent and releases chloride and bicarbonate into the lumen and is primarily responsible for chloride secretion in humans [75]. Both ENaC and CFTR function apically, one introducing sodium and the other removing chlorine, both critical functions for PLC homeostasis. It is therefore not surprising that the function of one channel influences that of the other. For example, In CF, the reduced function of the channel and the decreased excretion of chloride (with the consequent increase in it at the intracellular level) will force the increased activity of ENaC to reabsorb more sodium and balance the ionic charge. Consequently, the absorption of water from the lumen will be increased, and the mucus will become dehydrated, thick, infected, and impacted on the airway walls [56].
In 1989 the gene encoding cystic fibrosis was identified on the long arm of chromosome 7. It´s a large gene that contains more the 250.000 bases with 27 exons, and that encodes a 1.480 amino acid protein, called CFTR. This protein is clearly a chlorine channel activated by cAMP. It´s the genetic disease that leads to higher mortality in white people. The most frequent mutation is the Delta-Phe 508 deletion, but more than 2000 mutations of the gene has been identified [76]. So, the correction of the channel isn´t easy. The normal channel is responsible for production sweat, digestive juices, and various mucus fluids [77]. Patients with pulmonary cystic fibrosis show severe airway mucus hypersecretion, and many suffer recurrent pulmonary infections, which can accelerate decline in the lung function. Patient sputum is characterized by obvious neutrophils infiltration, cellular DNA released from injured cells, and abundant Pseudomonas aeruginosa [78]. Patients also show substantial epithelial goblet cell metaplasia in the airway in contrast to healthy individuals, as well as significantly higher MUC5AC (mucin) expression [78]. Airway mucus hypersecretion in patients with pulmonary cystic fibrosis is associated with persistent cough, expectoration, and dyspnea. Laboratory evidence suggests that cystic fibrosis can be triggered by viral infections that lead to unrestrained liquid absorption, mucus hyper concentration, and the formation of mucus plaques and plugs [79]. Sputum measurements revealed higher total mucin concentrations and a higher percentage of solids in patients with cystic fibrosis than in healthy persons [80,81]. Aspirated oral anaerobes appear to be the first bacterial pathogens in the lungs of patients with cystic fibrosis, followed by the classic gram-negative pathogens that probably accelerate the vicious cycle depicted in and loss of lung function [82,83]. Ivacaftor (VX-770) is a potentiator of residual CFTR function that has been approved for patients with cystic fibrosis with gating and some splicing CFTR mutations [84]. For patients with cystic fibrosis who are homozygous for a deletion of phenylalanine 508 (Phe508del) in CFTR, ivacaftor–lumacaftor and tezacaftor–ivacaftor are available but offer more modest clinical benefit. Three-drug combinations of correctors and potentiators appear to be highly effective in patients with at least one Phe508del mutation (approximately 90% of patients). Very recently, Barry,et al.demonstrated in a phase 3 study that the Elexacaftor – tezacaftor – ivacaftor combination was efficacious and safe in patients with Phe508del –gating or Phe508del – residual function genotypes and conferred additional benefit relative to previous CFTR modulators [85]. The basic idea is to increase the activity of the channels with the drugs.
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death worldwide, with a responsible for 3.23 million deaths and it is estimated that the increased prevalence of smoking in LMICs (low- and middle-income countries) coupled with aging populations in high-income countries will result in over 5.4 million annual deaths from COPD and related conditions by 2060 [86]. COPD largely results from a chronic inflammatory response to environmental irritants, for example, noxious particles or gases, and is characterized by progressive lung function decline and airflow limitation [87,88]. Dysfunction of ion channels, in particular CFTR, play several roles in COPD and may be a major contributing factor in inducing muco-obstructive lung disease [89-90]. A mounting body of evidence suggests that acquired CFTR dysfunction is an important driver of COPD pathophysiology [91]. So, CFTR will be a treatment target in COPD by reduction inflammation and hypersecretion. It is becoming clear that mucus is associated with luminal occlusion (mucus plugging) and airflow limitation. Mucus hypersecretion is a driver of the accelerated decline in FEV1, which is characteristic of COPD, leading to a poor quality of life, increased exacerbations, and increased risk for death [92]. Chronic inflammation is also associated with dysregulation of ion channel expression and function of the airway epithelium, leading to reduced mucus hydration, a crucial covariate of mucus viscoelasticity and transportability, as manifest by reduced ASL and PCL depth [93]. The impact of smoke and/or pollution on CFTR function includes decreased CFTR protein and mRNA expression, CFTR degradation by protease activity (increased neutrophil elastase activity), reduced CFTR cell surface expression, and direct modifications to the CFTR channel [94]. Other ion channels have roles in the mucus hypersecretion and accumulation in COPD. ENaC, voltage-gated K+ channels, and TRP (transient receptor potential) channels have also been implicated in COPD pathogenesis. Direct modulation of mucus properties via ion channel targeting could be a viable strategy in treating patients with COPD. Studies in CF have shown that CFTR potentiators can reverse mucus obstruction in both large and small airways [84], leading to reduced airway obstruction and pronounced clinical benefit. Icenticaftor (NCT04072887) and ivacaftor (NCT03085485 and NCT04066751) are small-molecule channel potentiators of CFTR [95]. Although some features of COPD are currently not reversible, smoke-induced changes to CFTR are at least partially reversible after smoking cessation. Roflumilast, a phosphodiesterase-4 inhibitor that is currently approved for treatment of COPD, is effective in reducing exacerbations and bronchitis symptoms, specifically in patients with mucus hypersecretion. In vivo, ex vivo, and in vitro studies have shown that it may exert its effect mainly via CFTR potentiation [96].
Another chlorine channels are CLCN2 and CLCN5 (chloride voltage-gated channel. They aren´t in the figure). Inhibiting this channel, CaCC, and modulating Na+/K+/2Cl- could lead to smooth muscle relaxation and be used as a therapeutic strategy in bronchial asthma and provide alternatives in cystic fibrosis [97]. Cl- swell channels are regulated by cell volume and are activated when the cell expands and the volume increases. Other chlorine channels are P64-related chloride channels and ligand-gated chloride channels.
Potassium channels
The wide variety of diverse potassium channels (over 40) that are apically and basolaterally located in airway epithelia maintain the electrochemical gradient and thus support lung ion and fluid homeostasis (Figure13) [98]. Changes in activity of airway epithelial basolaterally located potassium channels (including KCNQ voltage-activated Na+–Kv7channels) were shown to impact apical cAMP-dependent chloride secretion and Ca2+-dependent chloride secretion [99].To date, the presence of all three classes of Ca2+-dependent potassium channels (big-conductance potassium [BK], intermediate-conductance potassium [IK]/KCa3.1, and small-conductance potassium [SK]) was confirmed at the basolateral membrane of airway epithelial cells. Furthermore, some apically located Ca2+-dependent potassium channels, including SK4-like channels and BKCa, have a significant influence on chloride secretion and thus are important for MCC and ASL volume regulation [100]. The fundamental role of potassium channels in anion exchange is to maintain sodium reabsorption. When K+ channels open, K+ moves out of cells due to the large chemical gradient (intracellular K+ 140 mM, extracellular K+∼5 mM). This generates a negative or hyperpolarized cell membrane potential. Equilibrium is reached when negative intracellular charge counteracts the outward chemical driving force for K+. For K+ ions, this equilibrium sets the cell membrane potential to about −80 mV [101]. Potassium channels act in alveolar epithelium as oxygen sensors and thus adjust lung function to environmental changes in O2levels [102]. Alveolar potassium channels, BKCa, that have been shown to reduce their opening time during hypoxia are able to detect O2 variation and modulate their activity to adjust ion transport and fluid clearance [103]. Although the molecular mechanism(s) governing oxygen sensing by alveolar potassium channels remains unclear, their properties make them potential therapeutic targets for lung diseases associated with hypoxia that include pulmonary hypertension.
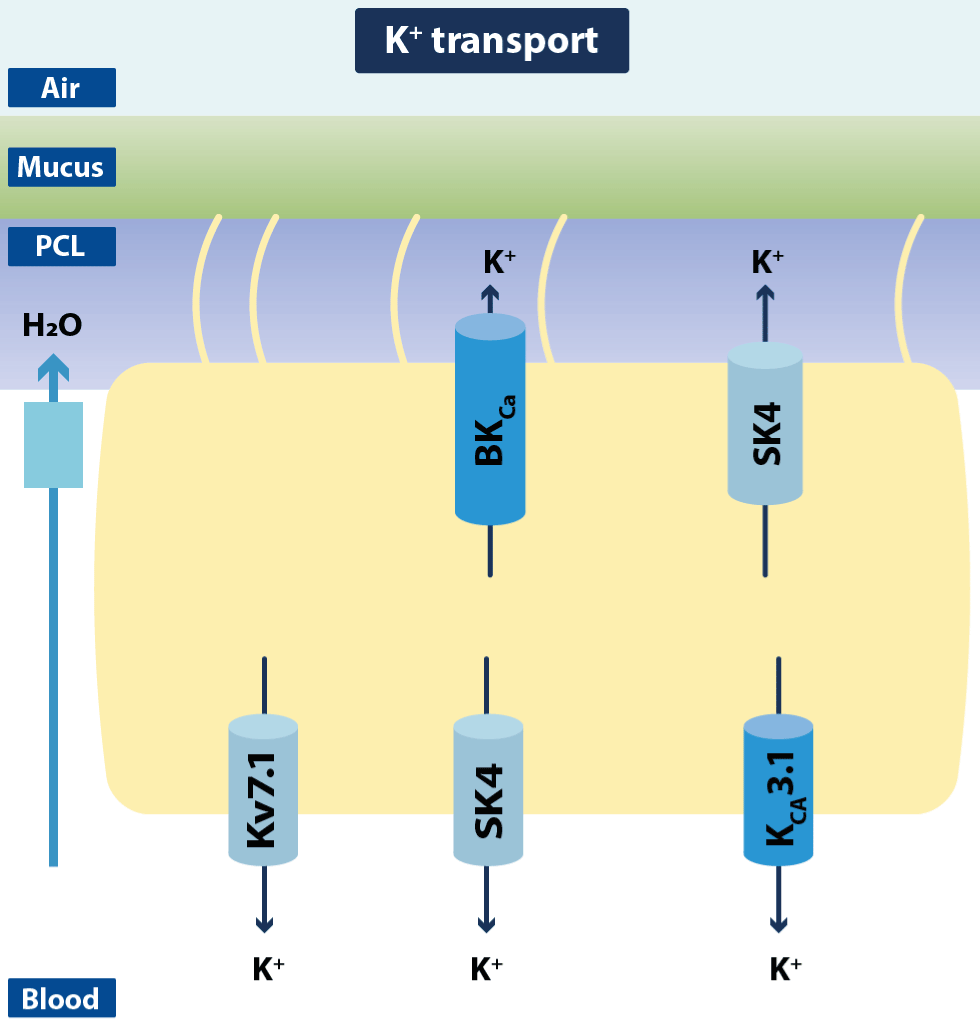
Figure 13.Schematic drawing of ciliated airway epithelial cells with K+ channels and transporters
The cell depicts the K+ channels so far identified in airway epithelium that are supposed to modulate apical Cl− secretion. In the basolateral membrane, several voltage-dependent K+ channels have been identified (Kv7.1–Kv7.5). Ca2+-dependent K+ channels have been characterized in the apical and the basolateral membrane (SK4, BKCa, KCa3.1) (Modified from Hollenhosrt).
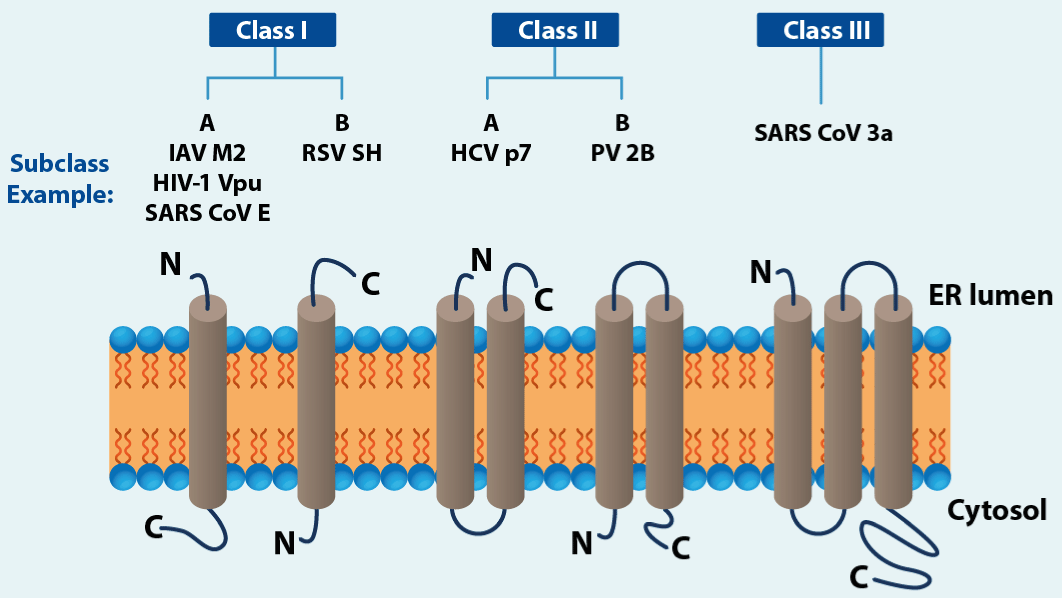
Figure 14.Viroporins and transmembrane topology
Schemes represent one subunit only, while in vivo all viroporin assemble into dimers up to pentamers (heptamers have been reported for HCV p7 channels) to form the ion channel. IAV (Influenza A virus), HIV-1 (human immunodeficiency virus type 1), RSV (respiratory syncytial virus), SH (small hydrophobic), HCV (hepatitis C virus), PV (poliovirus). (Modified from Rossman,et al.).
The involvement of potassium channels has been proposed in respiratory conditions such as asthma and COPD [104]. Pulmonary hypertension can be caused by a defect in the function of potassium channels or by alveolar hypoxia. Importantly, hypoxia selectively inhibits the function and expression of voltage-gated potassium (KV) channels in pulmonary arterial smooth muscle cells (SMCs). The activity of potassium channels regulates the membrane potential (Em) of SMCs, which in turn regulates the cytoplasmic free Ca2+ concentration ([Ca2+]cyt). Depolarization of the Em leads to an elevated [Ca2+]cyt by opening voltage-dependentCa2+ channels. Elevated [Ca2+]cyt is implicated in stimulating vascular SMC proliferation and inducing vasomotor tone and, hence, vasoconstriction. Vasoconstriction causes elevation of intravascular pressure and elastic stretch of the SMCs, both of which have been shown to play a role in pulmonary arterial cellular growth and synthetic activity, creating a vicious cycle of cellular hypertrophy, proliferation, and vascular remodeling[105].
Since potassium is the main intracellular cation, this cation plays an important role in many aspects of human physiology such as pulmonary circulation, endocrine function, inflammation, immunity, neurological function, etc. Some researchers functionally divide potassium channels into 5 large groups. Kv (voltage-gated potassium channel) is a family of 12 members and its function is inhibited in primary and secondary pulmonary hypertension (hypoxia). As potassium does not leave, the membrane potential becomes less negative (it depolarizes), the Ca2+ cation enters inducing proliferation and vasoconstriction of PASMCs[106].Kcawasoriginally described in red blood cells. The BK subtype has been studied in vascular diseases. When intracellular calcium increases, the BKCa2+ sensor senses it and opens, inducing the release of potassium, hyperpolarizing the membrane, and generating vasodilation. Since there are several subtypes with different functions, the choice of specific drugs for pulmonary hypertension is difficult. K2p(double-pore domain K+) has 5 families and 15 members. The electrophysiological characteristics are different from those of other potassium channels. They do not have voltage sensors and are not subject to changes in membrane potential. they produce a kind of spontaneous inactive current called "K+ leakage current". They play a role in pulmonary hypertension. The gene encoding the channel (K2p3.1) is mutated and downregulated in hereditary and non-hereditary pulmonary hypertension. Upregulation of the gene (KCNK3) inhibits vasoconstriction, proliferation, and improves right ventricular hypertrophy and dysfunction in pulmonary hypertension [107]. KATP are channels that close when intracellular ATP increases, potassium does not come out, the membrane depolarizes and the change in voltage opens the Ca2+ channels (Figure1). The influx of calcium releases eg insulin. There is thus a connection between KATP, metabolism and electrical excitation. By controlling the influx of calcium into the cell, they induce vasoconstriction, thus having a major role in pulmonary hypertension. There are a few mediators that control vascular tension through their effects on these channels [108]. Keeping the KATP channels open would decrease the influx of calcium into the vessels, produce vasodilation, and reduce the pressure in the pulmonary artery. It is also known that genes encoding subunits of these channels on chromosome 12 are mutated and lose their function in children with IPAH (idiopathic pulmonary arterial hypertension) [109]. Kir (inward rectifier potassium channels) can control calcium influx through VDCCs (voltage-dependent calcium channel), and it may regulate extracellular potassium concentration to regulate PASMC membrane potential to mediate vasoconstriction or relaxation [110].
KCa3.1 is an intermediate conductance channel activated by free calcium in the cytosol, and widely expressed in lung cells, and involved in inflammation, remodeling, fibrosis of the lung parenchyma and post-transplant bronchiolitis. Its blockade could reduce the progression of pulmonary fibrosis.
Calcium channels
Calcium is fundamentally an extracellular ion with a small intracellular fraction which is 90% in calcium stores (ER and mitochondrion). Ca2+ participates in muscle contraction and relaxation, cell proliferation, stabilization of the potential inside and outside the cell membrane, neuromuscular signal transduction, coagulation, enzyme regulation, etc. Many these cellular processes rely on signals that depend for their "delivery" on changes in intracellular Ca2+ concentrations, and the influx of calcium from extracellular fluid occurs through calcium-specific ion channels. This influx of calcium is, to some extent, dependent on the plasma membrane potential, controlled in turn by the transfer of ions such as K+ and Cl, through their own channels. In turn, the transport of K+ and Cl- in airway epithelial cells depends on the activity of Ca2+ channels (egCaCC and BKCa). This evidences how the activity belongs to the channels, although there is specific selectivity, it is interdependent whether in series, in parallel or in concert. Finally, the functioning of calcium channels depends on the main intracellular reservoir [101]. The activity of these channels in the airway epithelium can be affected by environmental insults such as hypoxia, oxidative stress, and inflammation, so they could be targets for drugs in asthma, lung injury, and CF. For example, TRPV4 (transient receptor potential vanilloid-4 channel) is a calcium channel that responds to a variety of stimuli that release inflammatory mediators in the airways. Inhibition of said receptor has been proposed as a therapeutic objective in ALI, ARDS, and CF [111,112]. There are different channels, for example, along the pulmonary vessels. In regular vasoconstriction and pulmonary vascular remodeling there are at least three well-identified channel types. SOCCs (store-operated calcium channels) are channels that regulate calcium operated by intracellular stores. Blocking SOCCs inhibits the intracellular increase in Ca2+ induced by endothelin 1 (ET-1) and angiotensin II (Ang-II) reducing systemic blood pressure and vascular smooth muscle cell proliferation. CRAC (Ca2+ release active Ca2+ channel) it is the major component of SOCC and is made up of a single pore that forms a subunit, Orai (cationic channels consist of the CRAC) and STIM (stromal interaction molecule), are the membrane´s Ca2+ sensors. STIM is scattered in the ER while Orai is in plasmatic membranes. For example, the increase in Ca2+ favors the translocation of NFAT (nuclear factor of activated T cells) to the nucleus and the initiation of a new transcription that results in cytokine secretion and proliferation of T cells. CRAC opens, in response to Ca2+, depletion of the ER via a process known as SOCE (store-operated calcium entry). CRAC inhibitors could be therapeutic options for autoimmunity in inflammatory conditions. Modulating this channel has the potential to reduce responses T-cell undesirables such as in Sarcoidosis, and reduce allergen-induced, IG-E-dependent activation of mast cells [113]. The CM4620-IE CRAC channel blocker (Calci-Medica) has entered phase II of clinical work to prevent cytokine storm in COVID-19. In pulmonary hypertension. STIM can upregulate intracellular Ca2+ and promote proliferation of pulmonary artery smooth muscle cells in response to molecular signals from mediators.
ROCCs are receptor-operated calcium channels. IP3R is distributed in the ER and releases Ca2+ from intracellular stores in an oscillatory manner and is the main Ca2+ binder of vascular smooth muscle cells and of the 3 phenotypes, IP3R1 is the main regulator in PH. When activated by some stimulus (hypoxia, inflammation, Ang II, ET-1, or norepinephrine), it is upregulated, calcium release increases, and vasoconstriction and smooth muscle cell remodeling are promoted. The receptor is sensitive to several vasoactive substances [114]. TRPC (transient receptor potential channel) has a wide range of functions. In blood vessels, it can participate in the mechanical stimulation of blood vessels and GPCR-related (G-protein coupled receptor) signal pathways, EC-related vasodilation, vascular permeability, neovascularization, vasoconstriction, remodeling and so on. The above functions are mainly realized by regulating intracellular Ca2+. It plays an important role in the genesis of cardiovascular disease. TRPC regulates Ca2+ mainly throughROCCs and SOCCs [115]. It had been found that in the model of PH, activation of TRPC not only regulated the contraction and proliferation of PASMC, but also mediated the proliferation and migration of pulmonary adventitia fibroblasts. TRPC mediated the synthesis of extracellular matrix proteins by up regulating the expression of type I collagen and fibronectin, thus participating in pulmonary adventitia remodeling [116]. Cough is the most common reason for a visit to the doctor in the UK, and chronic cough affects up to 12% of the population and yet there are no safe and effective therapies. TRPC is a family of ion channels are expressed on sensory nerve terminals, and when activated can evoke cough. They are so named from their original discovery in Drosophilla fly, which showed a transient response to bright light. Five channels have a role in chronic cough: 4 TRPCs (TRP Vannilloid 1 [TRPV1], TRP Ankyrin 1 [TRPA1], TRP Vannilloid 4 [TRPV4] and TRP Melastatin 8 [TRPM8])and the purinergic P2X3 receptor. They may represent excellent therapeutic targets for the treatment of respiratory symptoms in chronic lung disease [117]. For example, the P2X3 antagonist AF-219/MK-7264 (gefapixant) suppresses idiopathic cough [118].
VDCCs (voltage-dependent calcium channels). LTCC (L-type calcium channels). LTCC is the primary channel involved in the influx of extracellular calcium ions into cells. Cav1.2 is the main subtype of LTCC channels to regulate vascular smooth muscle contraction. When endovascular pressure increases, vascular smooth muscle membrane depolarizes, activates LTCC channels, and allows a large amount of calcium ions to flow into the cytoplasm, causing vasoconstriction [119]. It has been found that the β subunit of LTCC structure plays an important role in the up regulation of LTCC activity in VSMCs and participates in the increase of blood pressure induced by Ang II. The use of inhibitors of LTCCs can significantly reduce the absolute contraction of pulmonary artery and the interaction with other channels to regulate vascular tension. The increase of intracellular Ca2+ induced by opening LTCC can activate the potassium channel, which leads to the outflow of K+, and then the cell membrane is hyperpolarized. Hyperpolarization of cell membrane leads to the decrease of LTCC activity, then the decrease of Ca2+ influx and the decrease of intracellular Ca2+, and finally leads to vasodilation. Ca2+ plays an important role in the occurrence and development of PH. Many signal molecules and vasoactive substances eventually affect the concentration of Ca2+, resulting in vasoconstriction and remodeling. At the same time, Ca2+ can also activate other ion channels. Therefore, it is necessary to clarify the differences and develop highly selective targeted drugs to achieve personalized treatment [107].
Hydrogen channels
The H+channel is activated by strong membrane depolarization, intracellular acidity, or extracellular alkalinity. In type ATII cells it was empirically determined that the threshold potential (V threshold) at which the H+conductance activates is linearly related to the transmembrane inside-to-outside H+gradient (Δ pH) and the membrane potential. Voltage-dependent H+conductance has a role in epithelial acid secretion and occurs in the apical part of the membrane toward the lumen. It is activated in parallel by NOX2 NADPH oxidase (nicotine amide dinucleotide phosphate oxidase). NADPH oxidases are cell membrane proteins that transfer electrons from NADPH to form extracellular superoxide anion and kill microorganisms. This process generates intracellular H+. The conductance of H+from the membrane leads to a massive release of H+from the cell and then to a repolarization of the membrane. The membrane potential is a significant determinant of the conductance of H+[60].
Porins
Porins are ionic protein channels in the outer membrane, with 50-120 AA, virus-encoded, not very selective, with high conductance to ions and permit the passage of small molecules. Viroporins and other’ ion channels to be emerged and they are involucres in viral infections and respiratory disorders. Viroporins are hydrophobic ion channels essential in the life cycle of DNA and RNA viruses. They perform multiple functions such as entry into virus host cells, particle production, and virus dissemination. They are therefore potential therapeutic targets to block viral replication and dissemination [120]. Their presence is required for the activity of viral proteins. For example, the M2 proton channel of the influenza virus A (IVA) is vital for the activity of the M2 protein (M2 as a virus-coded proton channel). Thus, viroporins are a family of proteins that include proteins encoded by many human pathogens and that are essential for the life cycle of viruses. They include one, two, or three TMDs (transmembrane domains) and must oligomerize to form the pore. They unusually behave as highly selective and voltage or ligand-gated, like conventional channels. They can be activated by electrochemical gradients. In addition to ions, they are transited by small molecules and solutes (channel-pore dualism) such as antibiotics, fluorescent dyes, etc. (viroporin plasticity?). Maintaining the membrane gradient and ion reclusion within the cell organelles is vital for cellular homeostasis. Perturbations of these systems, through viroporins, can have a profound impact on trafficking, signaling, and induction of cell death by apoptosis or other mechanisms [121]. For example, the NSp4 protein of rotaviruses releases calcium from intracellular stores (ER and mitochondria) during infection and this promotes viroplasm formation and expedited virus release, but they can also be secreted by an independent mechanism through the Golgi apparatus (managed by microtubules) which acts as an enterotoxin producing diarrhea. Inhibition of NSp4 can make a reduction of viral replication and of the endotoxic effects in the intestinal epithelium. This also occurs with the 2β channel of picornaviruses. Disproportionate ionic gradient by viroporins activates the NLPR3 inflammasome which generates IL-1B and IL-18 [122].
RNA virus-encoded viroporins
The most studied virus is that affect the human species are enterovirus (polio, coxsackie, E71 enterovirus [EV 71]) and rhinovirus. IVA M2. The M2 ORF channel is in segment 7 of the IVA genome. The channel is selective for protons. M2 is a 97 AA protein and a single TMD (class 1) and protonation opens the channel. HIV-1 Vpc has an uncertain role in the life cycle of HIV-1. It is a small protein that is not a component of the virion and plays a pivotal role in the release of infectious virions. It promotes CD+4 degradation. It has greater potential target for antiviral therapy is unclear (Figure14) [123]. HCV p7 It is a highly hydrophobic protein that constitutes a proton channel, with 63 AA and 2 TMD (class 2). It has protein-protein interactions and impacts the life cycle of the HCV virus, since it influences the acid stability of certain physiological parameters necessary for the reproduction of the virus. It is found in the membranes of mitochondria, ER and in cell membranes [124]. The cooperation between VP1 and VP4 in human RNA viruses (they favor viral passage to the cytosol) represents a pore-channel dualism and could be therapeutic targets with profound impact from eradicating polio to treating common colds. Protein E, 3a and others form channels in coronaviruses (CoV). Protein E (envelope) was the first CoV protein that showed channel activity, being that it is a class 1 viroporin with cation selectivity. 3a is a K+ selective channel that mediates infectious viral progeny and is linked to cellular trafficking of CoV´S protein. It is pro-apoptotic and is linked to the structural component of infectious virions. Two other CoV proteins have shown channel activity. They are ORF4a and ORF8a [125]. SHP (small hydrophobic protein) from type 1 of human metapneumovirus (one of three genus of paramyxoviridae family) model the membrane permeability to virions and the F protein activity (fusion protein). Alphavirus 6K (insect arbovirus protein, transmitted to humans by mosquitoes such as Chikungunya), induce increased membrane permeability to bacteria and Na+, Ca2+ and Cl-channel activity. Protein M of flavivirus induce selectivity activity of cationic cannels.
DNA virus-encoded viroporins
Most of the viroporins have been identified in RNA viruses, although there are some proteins by DNA viruses that have "viroporins-like" characteristics. Polyomavirus and papillomavirus have proteins that fulfill channel functions.
Other channels
VRAC (volume-regulated anion channel) transport Cl- and another organic ion (taurine or glutamate) and water through the plasmatic membranes. They are formed by LRRC (8 heterometers with 5 variations). LRRC 8/VRAC has a crucial role in viral immunity. It´s conducted by cGAMP and is activated by viral inflammatory factors, playing a vital role in host defense against DNA virus as herpes simplex virus (HSV-1). cAMP-STING (stimulator of interferon genes) contributes to the host defense INF via. Really cGMP-cAMP synthase (cGAS) sense and catalytic the production of 2´-3´cGMP-cAMP, and it trigger the INF production STING via. The enzyme sense cytosolic DNA in neoplastic and infected cells. STING active TBK1 (kinase that phosphorylates the transcriptional factor of IRF3) to induce an INF robust response. VRAC is also involucres in insulin secretion, apoptosis and antineoplastics resistance drugs [126].
TPC (two pore channel) is in the cellular endosomes, and it is activated by second messengers (for example 3´-5´IP2) and conducts Ca2+ and Na+. It plays a role in Ebola infection and could be a therapeutic target against this and other filoviruses [127].
Transporter SLC 6A19 in SARS-CoV-2. Bo AT1 (an AA transporter Na+-dependent) is a chaperone protein that encodes the SLC A19 gen, that mediates the membrane epithelial reabsorption of neutral AA through the apical section of them, in intestinal and renal tissue. ACE-2 and BoAT1 produces complex that encodes the SARS-CoV-2, playing a role in recognition and infection of the virus [128].
TMEM 16A is a Cl- channel, Ca2+ gating expressed in the epithelia surface of airway, submucosa glands, and goblet cells. It´s upregulated by inflammation. Their positive modulation can to higher the anion fluid secretion in cystic fibrosis. So, it can be a therapeutic target [129].
Conclusions
Ion transport accomplished by the bronchopulmonary epithelial cells is imperative for proper lung function. The mechanisms that control ion homeostasis in airways and lungs rely on the coordinated action of an entire network of channels, and this is reflected in the complex pathological features of most bronchopulmonary diseases. The situation becomes even more complicated when considering that each epithelium (airway and alveolar epithelium) consists of different cell types and that these different cells are differentially equipped with ion transporting proteins. The ions do not function independently, but interact with each other, mediating the opening or closing of the ion channels, thus regulating the ion concentration on both sides of the membrane. In addition to the interaction between ions, ion channels can be regulated with different chemicals, such as vasoactive substances, inflammatory mediators, transcription-inducing factors, apoptosis factors, translation modification factors, etc. Hence, understanding the rules that govern these channel interactions within this network of airway ion regulation remains an important challenge to overcome for achieving successful interventions in various lung airway diseases.
Source of economic support
No.
Conflict of interest
No.
Authorship
This work was only carried out by the author. Author AA contributed on the planning, data collection, data analysis, writing and critical review. AA read and approved the final manuscript.
References
- Aidley DJ, Stanfield PR (1998) Ion channels. Molecules in Action. (2ndEd), Cambridge University, UK.In Press.
- Kunzelmann K, Kathöfer S, Greger R (1995)Na+ and Cl- conductance in airway epithelial cells: increased Na+ conductance in cystic fibrosis. Pflugers Arch431:1-9.[Crossref]
- Boucher RC (2004)Relationship of airway epithelial ion transport to chronic bronchitis. Proc Am Thorac Soc1:66-70.[Crossref]
- Standen NB(1992)Potassium channels, metabolism, and muscle. Experiment Physiol77:1-25.
- Hodgkin AL, Huxley AF (1952) Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol116:449-472.[Crossref]
- Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol117:500-544.[Crossref]
- Hodgkin AL, Keynes RD (1955) The potassium permeability of a giant nerve fibre. J Physiol128:61-88.[Crossref]
- Neher E (1992) Ion channels for communication between and within cells. Science 256:498-502.[Crossref]
- Neher E, Sakmann B (1976) Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature260:799-802.[Crossref]
- Neher E, Stevens CF (1977) Conductance fluctuations and ionic pores in membranes. Ann Rev Biophys6:345-381.[Crossref]
- Numa S (1986) Evolution of ionic channels. Chemica Scripta26B:173-178.
- Changeux JP, Galzi JL, Devillers-Thiéry A, Bertrand D (1992) The functional architecture of the acetylcholine nicotinic receptor explored by affinity labeling and site-directed mutagenesis. Quart Rev Biophys 25:395-432.[Crossref]
- Hadiatullah H, Zhang Y, Samurkas A, Xie Y, Sendarri R, et al (2022)Recent progress in the structural study of ion channels as insecticide targets. Insect Sci29:1522-1551.[Crossref]
- Zhang T, Liu Q, Li Z, Tang S, Am Q, et al (2023) The role of ion channels in immune-related disease. Prog Biophys Mol Biol177:129-140.[Crossref]
- Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, et al. (1984) Primary structure of Electrophorus electrics sodium channel deduced from cDNA sequence. Nature 312:121-127.[Crossref]
- Bezanilla F (2008) Ion Channels: From Conductance to Structure. Neuron 60:456-468.[Crossref]
- Freifelder D (1985) Principles of Physical Chemistry with applications to the Biological Sciences. (1stEd) Jones and Bartlett, Boston MA.
- Pauling L (1960) The Nature of the Chemical Bond. (3rd Ed) Cornell University Press, Ithaca NY.
- Einstein A (1926) Investigation on the Theory of the Brownian Movement. R Fürth (Ed), Transl. AD Cowper. London: Methuen. Republished in 1956 by Dover Publications, Inc.
- Hill EP, Power GG, Longo LD (1977) Kinetics of O2 and CO2 exchange. In West JB. Bioengineering aspects of the Lung. (1stEd) Dekker, New York.
- Saumon G, Basset G (1985) Electrolyte and fluid transport across the mature alveolar epithelium. J Appl Physiol74:1-15.[Crossref]
- West JW, Numman R, Murphy BJ, Scheuer T, Catterall WA (1991) A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science 254:866-868.[Crossref]
- West JW, Patton DE, Scheuer T, Wang Y, Golding AI, et al (1992) A cluster of hydrophobic amino acid residues required for fast Na+ channel inactivation. Proc Nat Acad Sci USA89:10910-10914.[Crossref]
- Skou JC (1989) Sodium-potassium pump. In membrane transport: People and Ideas. (1st Ed) DC Tosteson, Bethesda MD (Eds) American Physiology Society pp.155-185.
- Dryer SE, Fujii JT, Martin AR (1989) A Na+ -activated K+ current in cultured brain stem neurons from chicks. J Physiol410:283-296.
- Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. JMol Biol157:105-132.[Crossref]
- Catterall WA (1992) Cellular and molecular biology of voltage-gated sodium channels. Physiologic Rev72:S15-S48.[Crossref]
- Catterall WA (1993) Structure and function of the voltage-gated ion channels. Trends Neurosci16:500-506.[Crossref]
- Catterall WA, Striessnig J (1992) Receptors sites for Ca2+ channel antagonists. Trends Pharmocol Sci13:256-262.[Crossref]
- Latorre R (1994) Molecular working of large conductance (maxi) Ca2+-activated K+ channels. In: handbook of membrane Channels, (1st Ed)Peracchia CC (Eds) San Diego, CA. Academic Press. Pp. 79-102.
- Kubo Y, Baldwin TJ, Jan YN, Jan LY (1993) Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362:127-133.[Crossref]
- Bormann J, Hamill OP, Sakmman B (1987) Mechanism of anion permeation through channel gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurons. J Physiol 385:243-286.[Crossref]
- Chiu SW, Subramanian S, Jakobsson E (1993) The nature of ion and water barrier crossing in a simulated ion channel. Biophysic J56:253-261.
- Alvarado A (2017) Dual bronchodilator therapy: A review. Clin Res Trials3:1-12.
- Ashrafuzzaman MD (2021) Artificial Intelligence, Machine Learning and Deep Learning in Ion Channel Bioinformatics. Membranes (Basel)11:672.[Crossref]
- Govindaraj RG, Subramaniyam S, Manavalan B (2023)Extremely-randomized-tree-based Prediction of N6-Methyladenosine Sites in Saccharomyces cerevisiae. CurrGenom21:26-33. [Crossref]
- Zhang D, Xu ZC, Su W, Yang YH, Lv H, et al (2021)iCarPS: A computational tool for identifying protein carbonylation sites by novel encoded features. Bioinformatics 37:171-177.[Crossref]
- Zhengdan Z, Deng Z, Wang Q, Wang Y, Zhang D, et al.(2022) Simulation and Machine Learning Methods for Ion-Channel Structure Determination, Mechanistic Studies, and Drug Design. Front Pharmacol13:939555.[Crossref]
- de Oliveira TM, van Beek L, Shilliday F, Debreczeni JÉ, Phillips C (2021) Cryo-EM: The Resolution Revolution and Drug Discovery. SLAS Discov26:17-31.[Crossref]
- Lees JA, Dias JM, Han S (2021)Applications of Cryo-EM in Small Molecule and Biologics Drug Design. Biochem Soc Trans49:2627-2638.[Crossref]
- Robertson MJ, Meyerowitz JG, Skiniotis G. (2022) Drug Discovery in the Era of Cryo-Electron Microscopy. Trends Biochem Sci47:124-135.[Crossref]
- Zhao R, Liang X, Zhao M, Liu SL, Huang Y, et al (2014)Correlation of apical fluid-regulating channel proteins with lung function in human COPD lungs. PLoS One9: e109725.[Crossref]
- Alvarado A (2022)Differences, Similarities, and Controversies between Bronchial asthma and Chronic Obstructive Pulmonary Disease: A review. In: Inflammatory and Immunological Profiles of Chronic Obstructive Pulmonary Disease. Basic Research and Clinical Application. (1st Ed). India, United Kingdom. PB International. Pp. 141-149.
- Matthay MA, Landolt CC, Staub NC (1982)Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol Respir Environ ExercPhysiol53:96-104.[Crossref]
- Breitinger U, Farag NS, Sticht H, Breitingera HG (2022)Viroporins: Structure, function, and their role in the life cycle of SARS-CoV-2. Int J Biochem Cell Biol 145:106185.[Crossref]
- Spina D (1998) Epithelium smooth muscle regulation and interactions. Am J Respir Crit Care Med158. Suppl 2: S141-S145.[Crossref]
- Livraghi A, Randell SH (2007) Cystic fibrosis and other respiratory diseases of impaired mucus clearance. ToxicolPathol35:116-129.[Crossref]
- Matalon S, Bartoszewski R, Collawn JF (2015)Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol309: L1229-L1238.[Crossref]
- Zeitlin PL (2008)Cystic fibrosis and estrogens: a perfect storm. J Clin Invest 118: 3841-3844.[Crossref]
- Choi HC, Kim CS, Tarran R (2015) Automated acquisition, and analysis of airway surface liquid height by confocal microscopy. Am J Physiol Lung Cell Mol Physiol309: L109-L118.[Crossref]
- Gallos G, Remy KE, Danielsson J, Funayama H, Fu XW, et al (2013) Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol305: L625-L634.[Crossref]
- Frizzell RA, Hanrahan JW (2012) Physiology of epithelial chloride and fluid secretion. Cold Spring HarbPerspect Med2: a009563.[Crossref]
- Hollenhorst MI, Richter K, Fronius M (2011)Ion transport by pulmonary epithelia. J Biomed Biotechnol2011:174306.[Crossref]
- Button B, Goodell HP, Atieh E, Chen YC, William R, et al (2018) Roles of mucus adhesion and cohesion in cough clearance. Proc Natl Acad Sci USA115:12501-12506.
- Boson B, Legros V, Zhou S, Siret E, Mathieu C (2021) The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J Biol Chem296:100111.[Crossref]
- Matthay MA, Clerici C, Saumon G (2002)Active fluid clearance from the distal air spaces of the lung. J Appl Physiol 93:1533-1541.[Crossref]
- Deneka D, Sawicka M, Lam AKM, Paulino C, and Dutzler R (2018) Structure of a volume-regulated anion channel of the LRRC8 family. Nature 558:254-259. [Crossref]
- Gaunsbaek MQ, Kjeldsen AD, Svane-Knudsen V, Henriksen ML, Hansen S (2014) Surfactant proteins A, B, C and D in the human nasal airway: associated with mucosal glands and ciliated epithelium but absent in fluid-phase secretions and mucus. ORL J OtorhinolaryngolRelat Spec76:288-301.[Crossref]
- Fischer H (2012)Function of proton channels in lung epithelia. Wiley Interdiscip Rev MembrTransp Signal1:247-258.[Crossref]
- Feraille E, Dizin E (2016)Coordinated control of ENaC and Na+,K+-ATPase in renal collecting duct. J Am Soc Nephrol27:2554-2563.[Crossref]
- Lakunina VA, Burnysheva KM, Mitkevich VA, Makarov AA, Petrushanko IY (2017) Changes in the receptor function of Na+, K+-ATPase during hypoxia and ischemia. Mol Biol (Mosk)51:172-179.[Crossref]
- Zhuang J, Zhao L, Zang N, Xu F (2015)Prenatal nicotinic exposure augments cardiorespiratory responses to activation of bronchopulmonary C-fibers. Am J Physiol Lung Cell Mol Physiol308: L922-L930.[Crossref]
- Cater RJ, Ryan RM, Vandenberg RJ (2016) The split personality of glutamate transporters: a chloride channel and a transporter. Neurochem Res41:593-599. [Crossref]
- Kleyman TR, Carattino MD, Hughey RP (2009) ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem284:2045-2047.[Crossref]
- Tarran R, Sabater JR, Clarke TC, Tan CD, Davies CM, et al (2013)Nonantibiotic macrolides prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion. Am J Physiol Lung Cell Mol Physiol304: L746-L756.[Crossref]
- Downs CA, Kreiner L, Zhao XM, Trac P, Johnson NM (2015) Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 308: L943-L952.[Crossref]
- Wilkinson WJ, Benjamin AR, De Proost I, Orogo-Wenn MC, Yamazaki Y (2011) Alveolar epithelial CNGA1 channels mediate cGMP-stimulated, amiloride-insensitive, lung liquid absorption. Pflugers Arch462:267-279.
- Liu B, Zhang B, Huang S, Yang L, Ross CM, et al (2018) Ca2+ entry through reverse mode Na+/Ca2+ exchanger contributes to store operated channel-mediated neointima formation after arterial injury. Can J Cardiol 34:791-799.[Crossref]
- Huetsch JC, Jiang H, Larrain C, Shimoda LA (2016)The Na+/H+ exchanger contributes to increased smooth muscle proliferation and migration in a rat model of pulmonary arterial hypertension. Physiol Rep4: e12729.[Crossref]
- Mutlu GM, Sznajder JI (2005)Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol289: L685-L695.[Crossref]
- Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol295: L379-L399. [Crossref]
- Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, et al (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:637-645.[Crossref]
- Okada Y, Okada T, Sato-Numata K, Islam MDR, Ando-Akatsuka Y, et al (2019)Cell volume-activated and volume-correlated anion channels in mammalian cells: Their biophysical, molecular, and pharmacological properties. Pharmacol Rev71:49-88.[Crossref]
- Quinton PM (2008) Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372:415-417.[Crossref]
- Sabuscap CM, Wang W, McNicholas CM, Chung WJ, Fu L, et al (2016) Analysis of cystic fibrosis associated P67LCFTR illustrates barriers to personalized therapeutics for orphan disease. JCl Insight1: e86581.[Crossref]
- Shen Y, Huang S, Kang J, Lin J, Lai K, et al (2018) Management of airway mucus by hypersecretion in chronic airway inflammation disease: Chinese expert consensus (English edition)Int J Chron Obstruct Dis13:399-402.[Crossref]
- Kreda SM, Davis CW, Rode MC (2012) CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring HarbPerspect Med2: a009589.[Crossref]
- Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, et al (2005) Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem280:35751-35759.[Crossref]
- Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, et al (2014) Cystic fibrosis airway secretions exhibit mucin hyper concentration and increased osmotic pressure. J Clin Invest124:3047-3060.[Crossref]
- Esther CR, Muhlebach MS, Ehre C, Hill DB, Wofgang MC, et al (2019) Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med11: eaav3488. [Crossref]
- Abdullah LH, Coakley R, Webster MJ, Shu Y, Tarran R, et al (2018) Mucin production and hydration responses to mucopurulent materials in normal versus cystic fibrosis airway epithelia. Am J Respir Crit Care Med197:481-491.[Crossref]
- Muhlebach MS, Zorn BT, Esther CR, Hatch JE, Murray CP, et al (2018) Initial acquisition and succession of the fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoSPathog14: e1006798.[Crossref]
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, et al (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med365:1663-1672.[Crossref]
- Barry PJ, Mall MA, Alvarez A, Colombo C, de Winter de Groot KM, et al (2021) Triple therapy for cystic fibrosis Phe508 del-gating and-residual function genotypes. N Engl J Med 385:815-825.[Crossref]
- WHO (2023) Chronic obstructive pulmonary disease, World Health Organization.
- Global Initiative for Chronic Obstructive Lung Disease (2023)Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2023 report.
- Fernandez-Fernandez E, De Santi C, De Rose V, Greene CM (2018) CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease. Expert Rev Respir Med12:483-492.[Crossref]
- De Rose V, Molloy K, Gohy S, Pilette C, Greene CM (2018)Airway epithelium dysfunction in cystic fibrosis and COPD. Mediators Inflamm2018:1309746.[Crossref]
- Raju SV, Solomon GM, Dransfield MT, Rowe SM (2016)Acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis and other diseases of mucus clearance. Clin Chest Med 37:147-158.[Crossref]
- Lin VY, Kaza N, Birket SE, Kim H, Edwards LJ, et al (2020)Excess mucus viscosity and airway dehydration impact COPD airway clearance. Eur Respir J55:1900419.[Crossref]
- Hartman JE, Prinzen J, van Lummel RC, Ten Hacken NH (2015)Frequent sputum production is associated with disturbed night’s rest and impaired sleep quality in patients with COPD. Sleep Breath19:1125-1133.[Crossref]
- Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA (2017)Airway mucus, inflammation, and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res367:537-550.[Crossref]
- Dransfield M, Rowe S, Vogelmeier CF, Wedzicha J, Criner GJ, et al (2021) Cystic Fibrosis Transmembrane Conductance Regulator: Roles in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 205:631-640.[Crossref]
- Rowe SM, Jones I, Dransfield MT, Haque N, Gleason S, et al (2020)Efficacy and safety of the CFTR potentiator icenticaftor (QBW251) in COPD: results from a phase 2 randomized trial. Int J Chron Obstruct Pulmon Dis15:2399-2409.[Crossref]
- Lambert JA, Raju SV, Tang LP, McNicholas CM, Li Y, et al (2014)Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol50:549-558.[Crossref]
- Hendrick SM, Mroz MS, Greene CM, Keely SJ, Harvey BJ (2014)Bile acids stimulate chloride secretion through CFTR and calcium-activated Cl− channels in Calu-3 airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 307:L407-L418.[Crossref]
- Bardou O, Trinh NT, Brochiero E (2009)Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol296: L145-L155.[Crossref]
- Brueggemann LI, Haick JM, Neuburg S, Tate S, Randhawa D (2014) KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol306: L476-L486.[Crossref]
- Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, et al (2014) IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am J Physiol Lung Cell Mol Physiol306: L453-L462. [Crossref]
- Bradding P, Wulff H (2013) Ion channels. Thorax 68:974-977.
- Sedivy V, Joshi S, Ghaly Y, Mizera R, Zaloudikova M (2015)Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. Am J Physiol Lung Cell Mol Physiol308: L48-L57.[Crossref]
- Lv Y, Tang LL, Wei JK, Xu XF, Gu W, et al (2013)Decreased Kv1.5 expression in intrauterine growth retardation rats with exaggerated pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol305: L856-L865.[Crossref]
- Malerba M, Radaeli A, Mancuso S, Polosa R (2010)The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. J Biol RegulHomeost Agents24:123-130.[Crossref]
- Liu G, Fu D, Tian H, Dai A (2021) The mechanism of ions in pulmonary hypertension. PulmCircul11:1-20.[Crossref]
- Brachiero E (2009) Molecular diversity and function of K+ channels in airway and alveolar epithelia cells. Am J Physiol Lung Cell Mol Physiol296: L145-L155.[Crossref]
- Zamponi GW, Striessnig J, Koschak A, Dolphin AC (2015) The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev67:821-870.[Crossref]
- McClenaghan C, Woo KV, Nichols CG (2019)Pulmonary hypertension and ATP-sensitive potassium channels. Hypertension 74:14-22.[Crossref]
- Aziz Q, Li Y, Tinker A (2018)Endothelial biology, and ATP-sensitive potassium channels. Channels (Austin)12:45-46.[Crossref]
- Lambert M, Capuano V, Olschewski A, Sabourin J, Nagaraj C, et al(2018)Ion channels in pulmonary hypertension: A therapeutic interest? Int J Mol Sci19:3162.[Crossref]
- Suresh K, Servinsky L, Reyes J, Bakgh S, Undem C (2015) Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves. Am J Physiol Lung Cell Mol Physiol309: L1467-L1477. [Crossref]
- Henry CO, Dalloveaur C, Pérez-Berezo MT, Plata C, Wu Y, et al (2016)In vitro and in vivo evidence for an inflammatory role of the calcium channel TRPV4 in lung epithelium: potential involvement in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol311: L664-L675.[Crossref]
- Standerman KA (2018) CRAC channels as target for drug discovery and development. Cell Calcium74:147-159.[Crossref]
- Mikoshiba K (2015)Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul57:217-227.[Crossref]
- Bavencoffe A, Zhu MX, Tian JB (2017)New aspects of the contribution of ER to SOCE regulation: TRPC proteins as a link between plasma membrane ion transport and intracellular Ca2+ stores. Adv Exp Med Biol993:239-255.[Crossref]
- Cussac LA, Cardouat G, Tiruchellvam PN, Campagnac M, Robillard P, et al. (2020) TRPV4 channel mediates adventitial fibroblast activation and adventitial remodeling in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 318: L135-L146.[Crossref]
- Bonvini SJ, Belvisi MG (2017) Cough and airway disease: the role of ion channels. PulmPharamacolTher47:21-28.[Crossref]
- Abdulqawi R, Docckry R, Holt K, Laytons G, McCarthy BG, et al (2015) P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomized, double-blind, placebo-controlled phase 2 study. Lancet 385: 1198-1205.[Crossref]
- Ávila-Medina J, Calderón-Sánchez E, González-Rodríguez P, Monge-Quiroga M, Rosado JA, et al (2016)Orai1 and TRPC1 proteins co-localize with CaV1.2 channels to form a signal complex in vascular smooth muscle cells. J Biol Chem291:21148-21159.[Crossref]
- Scott C, Griffin S (2015)Viroporins: structural, function and potential as antiviral target. J Gen Virol96:2000-2027.[Crossref]
- Nieva JL, Madan V, Carrasco L (2012)Viroporins: structure and biological functions. Nat Rev Microbiol10:563-574.[Crossref]
- Dansako H, Yamane D, Welsch C, McGivern D.R, Hu F, et al (2013)Class A scavenger receptor 1 (MSR1) restricts hepatitis C virus replication by mediating toll-like receptor 3 recognition of viral RNAs produced in neighboring cells. PLoSPathog9: e1003345. [Crossref]
- Boson B, Legros V, Zhou B, Siret E, Mathieu C, et al (2021)The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J Biol Chem296: 100111. [Crossref]
- Farag NS, Breitinger U, Breitinger HG, El Azizi MA (2020)Viroporins and inflammasomes: A key to understand virus-induced inflammation. Int J Biochem Cell Biol122:105738.[Crossref]
- Madan V, Castelló A, Carrasco L (2008)Viroporins from RNA viruses induce caspase‐dependent apoptosis. Cell Microbiol10:437-451.[Crossref]
- Xu H, Akinyemi IA, Chitre SA, Loeb JC, Lednicky JA, et al (2022) SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology 568:13-22.[Crossref]
- Zhen C, Chen X, Planells-Cares R, Chu C, Wang L, et al (2020) Transfer of c-GAMP into Bystander Cells via LRRC 8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity5:767-781. [Crossref]
- Castonuvay J, Orth JHC, Müller T, Sleman F, Grimm C, et al (2017) The two pores channel (TPC1) is required for efficient protein processing through early and recycling endosomes. Scient Reports7:10.1038. [Crossref]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, et al (2020)Structural basis for the recognition of SARS-CoV-2 by full-length human ACE-2. Science 367:1444-1448. [Crossref]
- Danahy DHL, Lilley S, Fox R, Charlton H, Sabater J, et al (2019) TMEM 16A potentiation: a novel therapeutic approach for the treatment of cystic fibrosis. Am J Respir Crit Care Med201:946-954.[Crossref]














