Background: Aging-related bone and cartilage destructive diseases, such as rheumatoid arthritis, are characterized by excessive bone resorption by osteoclasts. Osteoclastogenesis is mainly regulated by RANKL, which used reactive oxygen species (ROS) for the intracellular signaling. Previously we discovered that RANKL attenuates anti-oxidation via inhibition of transcriptional regulation of Nrf2-mediated anti-oxidant enzymes, and augments intracellular ROS signaling. In this research, we compared several anti-oxidant, such as Nrf2 activators, and non Nrf2-activating molecules, for the inhibitory effects of in vitro osteoclastogenesis and in vivo bone destruction.
Material and methods: RAW 264 cells were stimulated with RANKL in the presence of Catechin, Epigallocatechin-Gallate (EGCG), Sulforaphane, or vitamin C. Nrf2 promoter reporter assay was also performed. Using the calvarial bone destruction mouse model, which induced by repeated LPS injections, we compared inhibitory effect of the intraperitoneal injection of catechin, EGCG, or Sulforaphane on bone destruction using microCT images. Results: RANKL-mediated osteoclastogenesis from RAW 264 cells were blocked by SFN and EGCG. Transcriptional activity of Nrf2 were induced by SFN and EGCG. In vivo bone destruction were also inhibited by SFN and EGCG. Conclusions: In conclusion, the molecule which has Nrf2 activation property successfully inhibited bone destruction, which signifying Nrf2 activator would be therapeutic drug for bone destructive diseases.
oxidative stress, Nrf2, ROS, osteoclast, RANKL, polyphenol
Nrf2: nuclear-factor E2-related factor 2; RANKL: receptor activator of nuclear factor kappa beta ligand; ROS: reactive oxygen species; EGCG: Epigallocatechin-Gallate; LPS: lipopolysaccharide; SFN: Sulforaphane; CT: computed tomography; QOL: quality of life; IL: interleukin
TNF-a: tumor necrosis factor alpha; VC: vitamin C; FBS: fetal bovine serum; TRAP: tartrate resistant acid phosphatase; SD: standard deviation ; ARE: antioxidant response element; HO1: heme oxygenase 1;
GCS: γ-glutamylcysteine synthetase
Japan is in a super-aged society in which 1 in 4 persons in the Japanese population is elderly [1]. Therefore, prevention of an aging-related bone destructive diseases such as rheumatoid arthritis, osteoporosis, and periodontitis is in demand from the point of view of maintaining QOL, social aspects, and medical expenses. As to the pathogenesis of osteoarthritic cartilage destruction, not only cartilage but also bone and synovium play key roles in progression of disease by producing pro-inflammatory cytokines such as IL-1 and TNF-a [2]. Elevated inflammatory cytokines induce matrix metalloproteinase production in site, which resulted in the matrix degradation in articular cartilage [3]. In addition, production of reactive oxygen species (ROS) are induced during articular cartilage destruction. These pro-inflammatory cytokines and ROS favor osteoclastogenesis directly and indirectly, which promote subchondral bone resorption and further destruction of joint tissue [4].
Bone destruction is generally caused by the dysregulated activation of osteoclasts [5], with augmented production of osteoclastogenic cytokines such as RANKL, TNF-a, and interleukins [6]. Among these osteoclastogenic cytokines, RANKL is thought to be an essential factor for osteoclastogenesis [7]. RANKL uses multiple intracellular signaling pathways, which includes reactive oxygen species (ROS) [8]. Not only work as an intracellular signaling molecule, but also ROS exhibits cytotoxicity against cells [9]. Cells have several protective mechanisms against these oxidative stressors, accordingly [10]. One of the major cellular anti-oxidation is the transcriptional induction of anti-oxidant enzymes by nuclear-factor E2-related factor 2 (Nrf2) [11].
Previously we discovered that RANKL attenuates anti-oxidation via inhibition of transcriptional regulation of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated anti-oxidant enzymes, and augments intracellular ROS signaling [12]. Induction of anti-oxidant enzymes by Nrf2 activation subsequently inhibits osteoclastogenesis and in vivo bone destruction by local injection [13-17]. Inversely, augmented osteoclastogenesis and bone destruction was observed with Nrf2 deficiency [18-22]. Taken together, this shows that Nrf2-dependent anti-oxidant enzymes play a critical role in the regulation of bone destruction. More specifically, Nrf2 activation would be therapeutic treatment for bone destructive diseases.
Besides the induction of anti-oxidant enzymes by Nrf2 activation, direct ROS scavenging by anti-oxidant such as vitamin C (VC), also contribute the management of ROS [23]. Though previous report revealed that ROS-scavenging vitamin E, which has no Nrf2-activating property, failed to inhibit bone destruction [24], it still remains unclear whether there is obvious difference of inhibitory effect for bone destruction between Nrf2 activator and ROS scavenger.
In this research, we compared inhibitory effect for bone destruction by systemic administration of Nrf2 activator and ROS scavenger using mice.
Chemicals
Catechin was purchased from Cayman Chemical, Ann Arbor, Michigan, and dissolved in ethanol. Epigallocatechin-Gallate (EGCG) was purchased from Kurita-Kogyo, Tokyo, Japan, and dissolved in ethanol. Sulforaphane (SFN) was purchased from Toronto Research Chemicals, Toronto, ON, and dissolved in dimethylformamide. Vitamin C (ascorbic acid) was purchased from Sigma-Aldrich, St. Louis, MA, USA.
Cells
Mouse monocytic Cell-line RAW264.7 were obtained from the Riken-BioResource Center (Tsukuba, Japan).
Cell culture
RAW264.7 were cultured in α-modified Eagle's medium (Wako-Pure Chemical, Osaka, Japan) containing 10% fetal bovine serum (FBS; Thermo Scientific, South Logan, UT) supplemented with antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). All cells were cultured at 37°C in a 5% CO2 incubator.
Osteoclastogenesis assay
Cells were plated onto 96-well plates (103 cells/well) in triplicated in the presence or absence of recombinant soluble-RANKL (sRANKL; 100 ng/ml; Wako Pure Chemical) with or without catechin (100 mM), EGCG (10 mM), vitamin C (50 mg/mL) and SFN (10 mM). These concentration of factors were maximum inhibitory concentration we tested and no cytotoxicity were confirmed at the concentration (data not shown). After culture, cells were stained for TRAP using an acid-phosphatase kit (Sigma, St. Louis, MO). Dark-red multinucleated cells (≥3 nuclei) were counted as TRAP-positive multinucleated cells.
Nrf2 promoter reporter assay
Cells were transiently transfected with an Nrf2 promoter reporter plasmid (Cignal Antioxidant Response Reporter; Qiagen, Germantown, MD) using the X-tremeGENE HP DNA transfection reagent (Roche Applied Science, Indianapolis, IN, USA). Cells were then stimulated with catechin (100 mM), EGCG (10 mM), Vitamin C (50 mg/mL), and SFN (10 mM) for 24h, and cell lysates were prepared using lysis buffer containing long half-life luciferase substrate (Pikka-gene LT7.5; Toyo B-Net, Tokyo, Japan). Firefly luciferase activities were measured with a Synergy HTX Multi-Mode plate Reader (BioTek Japan, Tokyo, Japan).
In vivo bone destruction model
All animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Tsurumi University (No. 28A087). Animal experiments were performed in compliance with the Regulations for Animal Experiments and Related Activities at Tsurumi University. The calvarial bone destruction mouse model, induced by repeated LPS injections, has been described previously [14]. Twenty five 7-week-old male BALB/c mice (Clea Japan, Tokyo, Japan) were used. Mice were randomly assigned to five groups (n = 5 each): control group, LPS group, LPS and SFN group, LPS and catechin group, and LPS and EGCG group. Control group received repeat calvarial PBS injections and latter four groups received repeat calvarial LPS injections (10 μL of 1 mg/mL LPS) on days 1, 3, 5, 7, and 9. LPS and SFN group, LPS and catechin group, and LPS and EGCG group received repeat intraperitoneal injections of SFN (75 nmol), catechin (3 mmol), and EGCG (0.3 mmol) on days 1, 3, 5, 7, and 9, respectively. On day 11, mice were euthanized by cervical dislocation and cranial tissue samples were fixed overnight with 4% paraformaldehyde in PBS. Then soft tissue were defleshed from calvarial bone, and TRAP staining were performed. The photographs of the stained calvarial bone were then taken, and red channel were separated using Photoshop (Adobe Systems Co., Ltd., Tokyo, Japan). Percent of positive area, (number of pixels of red+ area/ number of pixels of analyzed calvarial bone area) were calculated using Image-J (National Institutes of Health, Bethesda, MD).
Statistical analysis
All data are presented as the mean ± standard deviation (SD). Multiple comparisons (a priori comparisons) were performed using Tukey’s test. P < 0.05 was considered to be statistically significant.
SFN and EGCG strongly, but catechin weakly inhibited osteoclastogenesis in vitro
Firstly, inhibitory effects of SFN, EGCG, catechin, and VC on osteoclastogenesis were examined (Figure 1). SFN almost completely inhibited osteoclastogenesis (Figure 1c). EGCG strongly inhibited osteoclastogenesis (Figure 1d). But catechin weakly inhibited osteoclastogenesis (Figure 1e). VC had marginal inhibitory effects on osteoclastogenesis (Figure 1f). These results suggest that each anti-oxidant has different inhibitory activity on osteoclastogenesis.
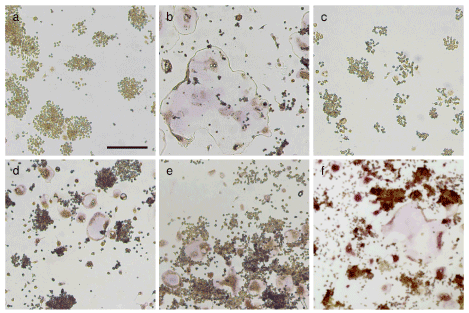
Figure 1. SFN and EGCG strongly, but catechin weakly inhibited osteoclastogenesis in vitro Representative photographs of control (a), RANKL-treated (b), RANKL + SFN (c), RANKL + EGCG (d), RANKL + catechin (e), and RANKL + VC (E) are shown
Nrf2 promoter-luciferase reporter activity
We found that SFN induced antioxidant response element (ARE)-dependent transcription of the reporter gene, indicating increased transcriptional activity of Nrf2 (Figure 2). EGCG also induced reporter gene. However, the induction of the reporter gene by catechin was weak as compared to that of SFN and EGCG. Vitamin C marginally induced the expression of the reporter gene.
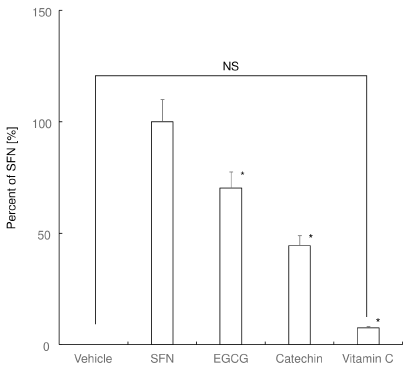
Figure 2. Nrf2 promoter-luciferase reporter activity. Relative activity of the Nrf2-responsive luciferase in RAW 264 cells against SFN-treated cells are shown. *P < 0.05 versus SFN. NS: no significant difference between the groups
These results suggest that SFN and EGCG triggers but catechin and vitamin C cannot trigger Nrf2-mediated transcription of antioxidant enzymes in RAW 264.7 cells.
SFN and EGCG strongly, but catechin weakly induced the expression of anti-oxidant enzymes
We then examined the expression of anti-oxidant enzymes in RAW 264.7 cells. Experiment 1 clearly demonstrated that SFN strongly induced the expression of HO1 (Figure 3a). EGCG induced both HO1 and GCS expression in experiment 1 and 2 (Figures 3a and 3b). Catechin weakly induced GCS expression in experiment 2 (Figure 3b). On the other hand, vitamin C marginally induced both HO1 and GCS (Figures 3a and 3b). These results suggest that SFN and EGCG strongly, but catechin weakly induces Nrf2-mediated transcription of antioxidant enzymes in RAW 264.7 cells.

Figure 3. Realtime RT-PCR analysis for anti-oxidant enzymes. The results from independent experiments for the gene expression of heme oxygenase 1 (HO1) (a) and gamma-glutamylcysteine synthetase (GCS) (b) are shown. (n=3) *: p < 0.05 versus control (vehicle-treated). NS: not significant difference versus control
Systemic administration of SFN and EGCG strongly, but catechin weakly attenuated calvarial bone destruction in vivo
Finally, we examined whether systemic administration of SFN, EGCG, and catechin inhibit LPS-induced bone destruction at calvaria (Figure 4). Repeat LPS injection at calvaria induced extensive bone destruction (G-2; Figure 4b). IP injection of SFN (G-3) and EGCG (G-5) successfully attenuated LPS-induced bone destruction (Figures 4c and 4e). However, IP injection of catechin (G-4) could not inhibit LPS-induced bone destruction (Figure 4d). Calculation of the resorbed area clearly demonstrated statistical difference at G-3 and G-5 against G-2, respectively (Figure 4f). Consistent with the in vitro results, SFN exhibited stronger inhibition of bone destruction as compared to that of EGCG. There was no statistical difference between G-2 and G-4.
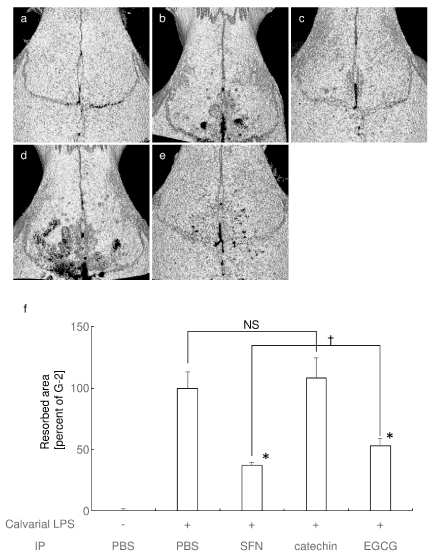
Figure 4. Systemic administration of SFN and EGCG strongly, but catechin weakly attenuated calvarial bone destruction in vivo Representative microCT image of control (a), LPS + PBS IP (b), LPS + SFN IP (c), LPS + catechin IP (d), and LPS + EGCG IP (e) are shown. (f) Percentage of the resorbed area in the cranial bone against G-2 (LPS + PBS IP) are shown. NS: no significant difference between groups. * P < 0.05 versus G-2, †P < 0.05 between the groups
These results suggest that inhibitory effect on osteoclastogenesis and bone destruction closely related to the induction activity of Nrf2-mediated transcription.
In this research, we examined the relationship between the Nrf2 activation and the inhibitory effects on osteoclastogenesis. Our results suggest that the molecule which induces stronger Nrf2 activation exhibited more extensive inhibitory effect on osteoclastogenesis and bone destruction. Exploration of the inductive activity of Nrf2 activation using Nrf2 promoter-luciferase reporter assay would be the novel simple tool for finding the drug for bone destructive diseases.
As to the pathogenesis of osteoarthritic cartilage destruction, not only cartilage but also bone and synovium play key roles in progression of disease by producing pro-inflammatory cytokines such as IL-1 and TNF-a [2]. In addition, synoviocytes in rheumatoid arthritis express RANKL and support osteoclastogenesis [25]. Furthermore, T cells in arthritic joint produce cytokines such as RANKL, interferon g, and IL-17, which regulates osteoclastogenesis [6,26,27]. These elevated inflammatory cytokines induce matrix metalloproteinase production in site, which resulted in the matrix degradation in articular cartilage [3]. In addition, production of reactive oxygen species (ROS) are induced during articular cartilage destruction. These pro-inflammatory cytokines and ROS favor osteoclastogenesis directly and indirectly, which promote subchondral bone resorption and further destruction of joint tissue [4].
Bone destructive diseases are generally caused by the dysregulated activation of osteoclasts [5], we examined the direct effects of the molecules on osteoclast and osteoclast precursors. Our results support our hypothesis that Nrf2 activation more strongly inhibits osteoclastogenesis as compared to the inhibition of osteoclastogenesis by ROS scavenging. Consistent with our results, ROS scavenging vitamin E does not prevent bone loss in rats with ligature-induced periodontitis [24].
Although we focused on the direct effect of the molecules on osteoclast and osteoclast precursors, a possible indirect effect of the molecules on osteoclasts via osteoclastogenesis supporting cells should also be considered. Considering the cartilage/bone destructive site, osteoclastogenesis supporting cells such as osteoblasts, synoviocytes, and immune cells produces osteoclastogenic cytokines including RANKL [2-4,6,25,27]. We did not examine whether RANKL production was attenuated by the molecules or not, but it was reported that EGCG suppresses LPS-induced expression of RANKL in osteoblasts [28]. SFN also suppresses RANKL production in osteocytes [29]. Together, these Nrf2 activators inhibits RANKL production and indirectly attenuates osteoclastogenesis. In conclusion, the molecule which induces stronger Nrf2 activation could be used for the therapeutic drug for bone and cartilage destructive diseases.
The authors acknowledge the Center of Research Instruments, Institute of Development, Aging and Cancer, Tohoku University, for generous permission to use the experimental instruments. Finally, the authors give their heartfelt appreciation to the experimental reagent companies and instrument companies for their various forms of support in the rehabilitation from the damage caused by the Tohoku earthquake on March 11, 2011.
- Sudo K, Kobayashi J, Noda S, Fukuda Y, Takahashi K (2018) Japan's healthcare policy for the elderly through the concepts of self-help (Ji-jo), mutual aid (Go-jo), social solidarity care (Kyo-jo), and governmental care (Ko-jo). Bioscience Trends 12(1): 7-11. [Crossref]
- Samuels J, Krasnokutsky S, Abramson SB (2008) Osteoarthritis: a tale of three tissues. Bulletin of the NYU hospital for joint diseases 66(3): 244-50. [Crossref]
- Loeser RF (2008) Molecular mechanisms of cartilage destruction in osteoarthritis. Journal of musculoskeletal & neuronal interactions 8(4): 303-6.
- Chimenti MS, Triggianese P, Conigliaro P, Candi E, Melino G, et al. (2015) The interplay between inflammation and metabolism in rheumatoid arthritis. Cell death & disease 6: e1887. [Crossref]
- Bartold PM, Marshall RI, Haynes DR (2005) Periodontitis and rheumatoid arthritis: a review. Journal of periodontology 76(11 Suppl): 2066-74.
- Romas E, Gillespie MT, Martin TJ (2002) Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone 30(2): 340-346. [Crossref]
- Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, et al. (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone 25(5): 517-523. [Crossref]
- Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, et al. (2004) Reactive oxygen species mediate RANK signaling in osteoclasts. Experimental cell research 301(2): 119-127. [Crossref]
- Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine 11(1): 81-128.
- Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual review of pharmacology and toxicology 47: 89-116.
- Motohashi H, Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends in molecular medicine 10(11): 549-557. [Crossref]
- Kanzaki H, Shinohara F, Kajiya M, Kodama T (2013) The Keap1/Nrf2 Protein Axis Plays a Role in Osteoclast Differentiation by Regulating Intracellular Reactive Oxygen Species Signaling. Journal of Biological Chemistry 288(32): 23009-23020. [Crossref]
- Kanzaki H, Shinohara F, Kajiya M, Fukaya S, Miyamoto Y, et al. (2014) Nuclear nrf2 induction by protein transduction attenuates osteoclastogenesis. Free radical biology & medicine 77: 239-248.
- Yamaguchi Y, Kanzaki H, Katsumata Y, Itohiya K, Fukaya S, et al. (2018) Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J Cell Mol Med 22(2): 1138-1147. [Crossref]
- Katsumata Y, Kanzaki H, Honda Y, Tanaka T, Yamaguchi Y, et al. (2018) Single local injection of epigallocatechin gallate-modified gelatin attenuates bone resorption and orthodontic tooth movement in mice. Polymers 10(12). [Crossref]
- Kanzaki H, Shinohara F, Itohiya K, Yamaguchi Y, Katsumata Y, et al. (2017) RANKL induces Bach1 nuclear import and attenuates Nrf2-mediated antioxidant enzymes, thereby augmenting intracellular reactive oxygen species signaling and osteoclastogenesis in mice. The FASEB Journal 31(2): 781-792. [Crossref]
- Kanzaki H, Shinohara F, Itohiya-Kasuya K, Ishikawa M, Nakamura Y (2015) Nrf2 activation attenuates both orthodontic tooth movement and relapse. Journal of dental research 94(6): 787-794. [Crossref]
- Hyeon S, Lee H, Yang Y, Jeong W (2013) Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free radical biology & medicine 65: 789-99. [Crossref]
- Rana T, Schultz MA, Freeman ML, Biswas S (2012) Loss of Nrf2 accelerates ionizing radiation-induced bone loss by upregulating RANKL. Free radical biology & medicine 53(12): 2298-2307. [Crossref]
- Sun YX, Li L, Corry KA, Zhang P, Yang Y, et al. (2015) Deletion of Nrf2 reduces skeletal mechanical properties and decreases load-driven bone formation. Bone 74: 1-9. [Crossref]
- Ibanez L, Ferrandiz ML, Brines R, Guede D, Cuadrado A, et al. (2014) Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxidative medicine and cellular longevity 2014: 726590. [Crossref]
- Lippross S, Beckmann R, Streubesand N, Ayub F, Tohidnezhad M, et al. (2014) Nrf2 deficiency impairs fracture healing in mice. Calcified tissue international 95(4): 349-61. [Crossref]
- Blanco-Ayala T, Anderica-Romero AC, Pedraza-Chaverri J (2014) New insights into antioxidant strategies against paraquat toxicity. Free radical research 48(6): 623-640. [Crossref]
- Carvalho Rde S, de Souza CM, Neves JC, Holanda-Pinto SA, Pinto LM, et al. (2013) Vitamin E does not prevent bone loss and induced anxiety in rats with ligature-induced periodontitis. Archives of oral biology 58(1): 50-58.
- Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, et al. (2000) Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis and rheumatism 43(2): 259-269. [Crossref]
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, et al. (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408(6812): 600-605. [Crossref]
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, et al. (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. The Journal of biological chemistry 272(40): 25190-25194.
- Tominari T, Matsumoto C, Watanabe K, Hirata M, Grundler FM, et al. (2015) Epigallocatechin gallate (EGCG) suppresses lipopolysaccharide-induced inflammatory bone resorption, and protects against alveolar bone loss in mice. FEBS open bio 5: 522-527. [Crossref]
- Thaler R, Maurizi A, Roschger P, Sturmlechner I, Khani F, et al. (2016) Anabolic and Antiresorptive Modulation of Bone Homeostasis by the Epigenetic Modulator Sulforaphane, a Naturally Occurring Isothiocyanate. The Journal of biological chemistry 291(13): 6754-6771. [Crossref]




