Multiple sclerosis is an autoimmune disorder that affects the central nervous system, more common in women of childbearing age, so pregnancy can affect the disease. In these patients, the relapse rate decreases during pregnancy, especially in the third trimester, however, there seems to be an increased risk of exacerbations during the first three months postpartum, before returning to the pre-pregnancy rate.
Although the evidence is not conclusive and there is no certainty that pregnancy can protect a genetically susceptible patient, it seems that the risk of disease is lower in women with a previous pregnancy than in nulliparous women, as well as women with exclusively breastfeeding.
Among these patients, there seems to be a greater number of small newborns for gestational age, as well as higher figures for instrumented deliveries or caesarean section, although without other complications, so it is not considered a "high risk" pregnancy. However, the possible interaction between drugs and the course of the disease make essential a good family planning, with proper advice.
Formerly it was recommended to stop the treatment since no drug was approved for its use. Nowadays, although the data is still limited, the therapeutic strategy has evolved remarkably; some drugs may require suspension, even six months before conception, however, others are safe, including during pregnancy. Hence the great importance of preconceptional consultation, in which each case will be assessed individually in order to obtain the best solution for each patient in particular.
multiple sclerosis, pregnancy, disease modifying therapies, breastfeeding
Multiple sclerosis (MS) is a chronic autoimmune and degenerative disease of the Central Nervous System (CNS) in which inflammation, demyelination and axonal loss occur from very early stages of the disease. It mainly afflicts young people between 20 and 40 years of age, with a predominance in the female sex [1]. The average prevalence of MS is 33 out of every 100,000 people, with significant variability between countries. North America and Europe have the highest prevalence (with 140 and 108 cases per 100,000 inhabitants, respectively), while Asia and sub-Saharan Africa have the lowest (with 2.2 and 2.1 out of every 100,000, respectively) [1,2].
Depending on the onset of symptoms and their evolution, different phenotypes have been described. The typical form is presented as an active intermittent disease (relapsing-remitting MS), while about 15% of patients presented a progressive course (primary progressive MS) or a relapsing profile at the beginning, which then becomes constantly aggressive (secondary progressive MS) [1,3]. Today there is still no definitive cure for MS. However, the approval of the first disease-modifying treatments for multiple sclerosis in 1990 changed attitudes toward the condition and triggered 25 years of intense development, which has resulted in multiple therapeutic options [4].
MS afflicts especially to young women in reproductive age, so some concerns arise with regard the possible negative outcomes of pregnancy and vice versa. In addition, unlike a little over 20 years ago, when the only recommendation for these women was to abandon treatment, there are currently a host of new advances in the search for effective treatments, which has led to investigate the effects of the drugs on pregnancy outcomes.
The main objective of the work to investigate about the inter-relations between this condition and the disease, possible complications that may exist, differences with respect to healthy women and therapeutic arsenal that we have in this situation.
Article search
We searched published papers focused on multiple sclerosis and pregnancy in PubMed and EMBASE. The key words used were as follows: pregnancy and multiple sclerosis, disease-modifying drugs (DMT), breastfeeding, and peripartum outcomes. Initially we got 1440 articles. After screening the abstracts selected 76 articles focused on the inter-relations of both conditions.
Data extraction
We retrieved the changes in the annualised relapse rate, disability progression measured with the EDSS comparing the numbers in the pre-pregnancy year with the same figures in pregnancy and postpartum. Peripartum outcomes such as infections, stillbirths, low birth weight, and pre-term deliveries. The influence of breastfeeding on disease activity was also analysed, and the effects of DMT on pregnancy and peripartum outcomes as well.
Therefore, this review has been structured in three main blocks:
• Effects of pregnancy on multiple sclerosis, that is, how this condition influences relapse rate and disability progression inheritance,
• Effects of multiple sclerosis in pregnancy, trying to relate the benefits and disadvantages that the disease can cause to pregnancy and differences with respect to healthy patients in the pre-conception stage with contraception, family planning consultation and related problems with fertility, during pregnancy with care and administration of vaccines, at the time of delivery and in the postpartum period with breastfeeding.
• Currently available treatments for multiple sclerosis in these pregnant patients, mainly the disease-modifying treatments: considerations about withdrawing or not taking drugs, dose modification, and the different indications according to the period and the situation in which they are (pre-pregnancy, pregnancy, breastfeeding, relapses).
Multiple sclerosis (MS) is a demyelinating and neurodegenerative disease that predominantly affects women of childbearing age, so the effect of pregnancy on MS is an important clinical problem [5]. Until the end of the 20th century, patients with MS are advised to avoid pregnancy given concern about the possible exacerbation of neurological symptoms [6-8]. However, a paradigm shift took place in 1998, when Confavreux and his team published the PRIMS study (Study on pregnancy in multiple sclerosis), the first prospective observational multicenter study aimed at understanding the influence of pregnancy on the relapse rate annual and risk of progression of MS [9]. They followed-up 254 women from twelve European countries with recurrent remitting MS (RRMS) during pregnancy and the next twelve months afterwards and compared the relapse rate with those of the year before pregnancy. The study showed a significant decrease in relapse rates during pregnancy, especially during the third trimester compared to the rates of the previous year, however, relapses increased the first three months after the increase in new levels before pregnancy, although Only 28% of the cohort experienced them (Figure 1), Other key findings of the study that deserve to be highlighted were the absence of related differences when comparing the relapse rates of the nine months of pregnancy plus the three months postpartum with the rates of the year prior to pregnancy, and the absence of disability progression during the study period. Posterior cohort studies confirmed the same results with decreased rate of relapses in pregnancy and increased in postpartum [10-28].
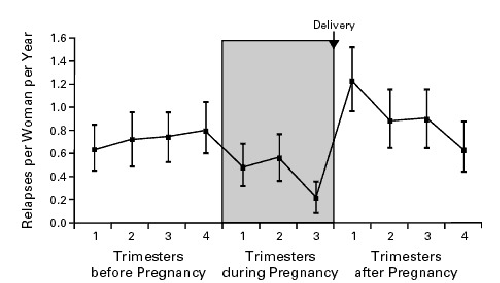
Figure 1. Rate of Relapse per Woman per Year for Each Three-Month Period before, during, and after Pregnancy in 227 Pregnancies Resulting in a Live Birth among Women with Multiple Sclerosis. The values shown are means and 95 percent confidence intervals [9]
From an immunological point of view, pregnancy is considered an immunotolerant state, where the maternal immune system needs to adapt to the foetal allogeneic tissue [29], so it is expected that in pregnant women with autoimmune diseases the severity of their disease will decrease [30]. Foetal tissues are considered semi-allogenic, since they house both maternal and paternal antigens. Therefore, a state of transient tolerance of T cells specific to paternal antigens is necessary to avoid rejection of the foetus [29] This is achieved by a decrease in adhesion molecules, matrix metalloproteinases (MMPs) and a change in cytokine secretion by activated helper T lymphocytes, which are involved in pregnancy and are also crucial in the development of autoimmune diseases [29]. One of the most important changes in the immune response during pregnancy is the decrease in the Th1 immune profile with the reactive increase in the Th2 profile [29]. This balance, with a slight predominance towards Th2 is essential, since the excessive dominance of one or the other could lead to spontaneous abortion [30]. Regulatory T cells also appear to rise during pregnancy; taking into account that they inhibit Th17, the latter are expected to decrease along with Th1 during pregnancy, although alterations in the population of Th17 during pregnancy are not fully clarified. For instance, in multiple sclerosis and other dominant autoimmune diseases of Th1 and Th17, a change towards Th2 is expected to have a favourable outcome in the severity of the disease [29,30]. Also high levels of estrogens during pregnancy and other reproductive hormones such as progesterone, prolactin or corticosteroids could be responsible for the general reduction of MS activity during pregnancy, acting as critical immunomodulators and inducing immune tolerance. These hormones gradually increase during pregnancy, reaching the peak in the third trimester, the moment of greatest protection of the disease. Other hormones, such as cortisol, are also modified during pregnancy, although to a lesser extent [5,31].
In addition to the immunomodulatory property of estrogens, they are also neuroprotective, and are known to play a role in normal cognitive development; so that they favour both the survival of the foetus, as well as the protection of the brain from injuries during maternal insults [5,29,31].
Given the physiological interaction between the reproductive and immune systems in women, it is not surprising that reproductive exposures and modifications such as pregnancy are related with the course of MS [32]. However, few studies have investigated this, and its conflicting results [33,34]. As previously mentioned, pregnancy is a natural modifier of MS, associated with an approximate 70% reduction in the rate of relapse, especially during the third trimester, although with a significant exacerbation after childbirth. The Australian multi-centre study of the environment and immune function (Ausimmune) evaluated in 2012 the relationship between parity and subsequent risk of developing the disease, finding a risk reduction of approximately 50% for each birth, regardless of other well established factors; that is, the possibility of a first demyelinating episode is inversely correlated with the number of offspring, the lowest risk being in multiparous women [35].
However, the evidence of risk reduction in these women is inconclusive since there is no certainty that pregnancy can protect a patient genetically susceptible to developing MS. In addition, even despite obvious association, most studies to date have not addressed the potential impact of selection bias, whereby women with less disabling MS are more likely to get pregnant [5,34,36]. Therefore, much more research is needed to clarify the nature of such an association. Although after several years of research, what can be assured for those patients with a family history of MS is that having several pregnancies will not adversely affect their risk of developing MS. [5,34,36]. There is no evidence of association between MS and the age at first birth, although a small Danish study reported a small protective association with early age at first birth [34].
Other factors that also appear to intervene in the course of the disease would include the possible protective effect of advanced age in menarche or the late onset of symptoms [32-34]. Exclusive breastfeeding seems to act as a protective factor against relapses, a matter not so clear with contraceptives, for which both protective and neutral and risk effects have been shown [34].
According to cohort studies the relapse rate decreases significantly in pregnancy [9-28]. In contrast to the protective effect of pregnancy, the postpartum period carries a higher risk of relapse [10,12-20,23-28], which could be due to the abrupt elimination of the protective factors of pregnancy after childbirth or to unique harmful factors inherent in this period [5,36]. The development of postpartum increase in relapse rates (ARR) has not been associated with breastfeeding, age at onset of the disease, total number of outbreaks prior to pregnancy, number of pregnancies, or sex of the newborn [5,36]. Relapse recurrence does not depend on the type of delivery or the type of anaesthesia used for caesarean section [37]. However, after the evaluation of the clinical predictors of relapse probability by logistic regression analysis in the PRIMS study group, three variables did correlate significantly with the risk of relapse: higher annualised relapse rate (ARR) the year prior to pregnancy, increased number of relapses during pregnancy, and higher score on the Expanded Disability Status Scale (EDSS) at the beginning of pregnancy. On the contrary, exposure to immunomodulatory drugs at the time of conception seems to be a factor associated with a lower number of relapses [30]. When the decrease RR during the nine months of pregnancy and the subsequent increase in RR during the first postpartum trimester are analyzed together, it is observed that the RR of these 12 months does not differ from the usual ARR prior to pregnancy. The Figure 1 depicts the trends of relapse rate in pre-pregnancy year, pregnancy and puerperium.
Patients with MS usually have some insecurity when deciding to get pregnant because of the concern of a possible negative effect of the disease in pregnancy. However, after reviewing several investigations, we can see that the impact of MS on the obstetric course is minimal and pregnancies in patients with MS are generally well tolerated [38-40]. Most studies did not find significant differences in obstetric outcomes compared with healthy women [38-40]. Some studies suggest the possibility of a higher rate of small newborns for gestational age compared to healthy controls [41,42], and a higher probability of instrumented vaginal deliveries (forceps, suction cup), slightly higher caesarean rates, longer labour, and superior hospital stay [38-40,43]. However, it has not been shown that women with MS may have a higher risk of pregnancy complications such as spontaneous abortions, placental abnormalities, ectopic pregnancies, pre-partum haemorrhages [39,43], differences in the Apgar score, or stillbirths [38]. Therefore, patients with MS should be treated in the same way as healthy patients, without special precautions, unless other maternal or foetal issues arise.
Contraception is essential in women with MS of reproductive age since in these patients it is especially important to optimally time the desired pregnancies and prevent unwanted ones. We lack data on the number of unplanned pregnancies in patients with MS. However, it is likely that the population with MS will make a greater effort in family planning, given the impact that the disease has on their social and economic future, the impact on fertility or the possible teratogenicity of the drugs. In 2016, the Center for Disease Control and Prevention (CDC) included MS in the US Medical Eligibility Criteria (MEC) and published evidence-based recommendations for contraceptive use in women with MS for the purpose of help health professionals, including neurologists, to advise on contraception to these women, always taking into account their individual situation [44]. Among the recommendations, there is no contraindication for the use of intrauterine devices (IUDs) or barrier methods such as preservatives, spermicides or diaphragms (Category 1). Within Progestin contraceptives only Etonogestrel implants and Progestin (POP) pills also lack restrictions (category 1). Medotroxyprogesterone Depot (DMPA) acetate, also included in this group, requires careful monitoring since it has been associated with small changes in bone mineral density, which can be harmful in the bone health of these women due to the disease itself, immobility or corticosteroids (Category 2).
Combined hormonal contraceptives (CHC) include oral tablets, patch and vaginal ring. The choice will depend on the patient's state of mobility: for women with MS without movement limitations, there are no restrictions on use (category 1). However, in patients with prolonged immobility, CHCs are not recommended, unless other more appropriate methods are unavailable or unacceptable (category 3), as they may increase the risk of venous thromboembolism in these patients. The DMTs do not seem to decrease the effectiveness of hormonal contraception, although interaction studies are limited. All treatments should be reviewed at each visit, as some therapies for the management of symptoms of MS could affect contraceptive efficacy. Therefore, most contraceptive methods appear to be safe for women with MS according to current evidence, the only exception being the use of combined hormonal contraceptives (CHC) in women with MS and reduced mobility.
MS is mostly diagnosed in young women, many of whom still want to have children. Today it is known that the disease does not preclude pregnancy, however, good family planning is important, with proper advice about the control of the disease at the time of conception, pregnancy, and puerperium, especially when patients are on DMTs [45,46]. Ideally, timing of diagnosis of MS and pregnancy planning should not coincide, since a window period of at least one year is necessary to assess the activity and course of the disease in each patient individually; In addition, the risk of a second attack is much greater after the first event [45,47]. Moreover, since the diagnosis often coincides with the start of a therapy, the therapeutic choice should take into account the woman's desire of pregnancy so as to avoid teratogenic drugs; so the optimal planning begins in the pre-pregnancy phase [45,47]. The most appropriate treatment for a patient with MS who plans a pregnancy should be chosen considering the severity of the disease, the impact of the drugs on the pregnancy and the foetus as well as the risk of relapse in the mother [45]. Some medications may require suspension 6 months before pregnancy or even earlier. However, others are relatively safe for use during pregnancy such as interferons and gratiramer acetate [5].
Pregnancy of a woman with MS is not a “high risk” pregnancy. Prenatal care should follow the general recommendations, with the same scheme of dietary supplements of folic acid, vitamins, minerals and iron required by pregnant women in general, as well as the cessation of tobacco and alcohol [31]. One aspect that may give rise to controversy is vitamin D supplementation in pregnant women with MS. There are studies that relate vitamin D to the slowdown in the development of MS and lower risk of injury and relapse. However, others do not provide significant results, so no arguments were found to recommend pregnant patients with MS to take more vitamin D supplements than healthy women [47]. For all of this, as it was published by the American Academy of Neurology (AAN) in the April 2018 guide, it is important that clinicians are knowledgeable about the reproductive plans of women with MS and offer individualized advice for each patient regarding reproductive risks and birth control during the use of DMT [48].
There is some disagreement about the effect of MS on fertility. Some researchers advocate that MS does not significantly affect fertility or the ability to complete a full-term pregnancy, compared to healthy controls [31,38]. Other studies demonstrated the existence of endocrine and sexual disorders associated with the disease as well as negative effects produced by DMT drugs [31,32,45]. Sexual disorders, caused by significantly reduced levels of antimullerian hormone, known for its correlation with decreased ovarian and endocrine reserves, with a higher prevalence of thyroid autoimmunity, and probably leading to a greater need for assisted reproduction techniques (ART) in patients with this pathology [31,38,39]. Some other reasons why this happens include maternity delay in the context of a diagnosis of MS, high rates of sexual dysfunction, higher rates of hormonal disorders as well as endometriosis [32,38]. To date there are no large studies evaluating the effect of assisted reproduction technique in MS. However, small research suggests the existence of an increased risk of relapse with the use of some assisted reproduction techniques as well as increased activity of nuclear magnetic resonance lesions [49-53], so MS patients should be informed about this risk.
Factors deemed to increase the risk of relapse occur after temporary discontinuation of DMT, and failure in cycles and immune changes induced by the use of gonadotropin-releasing hormone (GnRH) agonists, which may induce an increase in cytokines. proinflammatory and increased migration of immune cells through the blood brain barrier [24,54,55,32,38].
In addition, DMT may affect the patient's ability to conceive, and may have detrimental effects on the foetus, so it is advocated to withdraw treatment before conception [5].
Childbirth and possible complications are generally the same as in other women, and do not need a particular management. Only in a minority of cases with greater disability will special consideration be necessary in order to choose the safest option for both the mother and the foetus [56]. There are no contraindications for natural childbirth or for caesarean section in patients with MS [31,32,39]. There are also no negative consequences with the use of epidural analgesia [31,39].
There are no data that show a higher risk of congenital malformations or abortions in babies born to women with MS [23,43,57]. However, there seems to be a greater proportion of induced, instrumented and caesarean deliveries, which can be explained as a reflection of fatigue, lower limb spasticity, defective pelvic sensation, lack of sensitivity to contraction pain and neuromuscular perineal weakness. for the onset of labour [23]. A greater number of infants with low birth weight or small for gestational age have been registered in two studies [41,42], increased risk of infections [58,59]. These latter complications are not enough to classify pregnancies in women with MS as high-risk pregnancies, although obviously, hospital-assisted delivery is recommended before natural home birth [31].
Making the decision to breastfeed or not may be equally or more challenging for a patient with MS than the decision to get pregnant [38]. Today it is known that breastfeeding has well established benefits for both the health of the mother and the child, which is why breastfeeding is recommended exclusively for the first six months and continue combined with complementary feeding during the two first years [39,60]. However, the evidence on the role of breastfeeding in women with MS seems to be still controversial and generally inconclusive. The risk of incidental exposure of the child to a certain drug is not advised during this period. In addition there are concerns about recurrence of disease relapses in the mother if treatment is not resumed [38,45,60]. It is very unlikely to conduct a randomized trial that definitively solves this problem, so the true effect of breastfeeding on annual relapse rates is still under debate. Although breast feeding may be harmful for the newborn, the existence of a possible protective role against postpartum relapses has not yet been proven and deserves further research [60,61]. For this reason, in women with very active disease and those who do not wish to breastfeed, early restarting of DMT may reduce the relapse risk [39,60,62]. Studies before and in the DMTs era did not find a significant impact of breastfeeding in the course of MS, nor did the PRIMS reference study a link between them [9,13,17,18,25, 63]. Only two smaller studies pointed to a protective role of exclusive breastfeeding; one of the was conducted in North America and showed a reduction in the risk of postpartum relapses of approximately 5 fold during exclusive breastfeeding. He argued that the protective role of breastfeeding is correlated with an increase in interferon-producing CD4 T cells that are expressed due to breastfeeding amenorrhea [64]. A European study showed a modest protective effect [65].
Therefore, pending definitive evidence about the interrelationship between breastfeeding and disease activity, a pragmatic approach is proposed, individualizing each specific case, taking into account the therapeutic history of the patient along with the characteristics of the disease before and during pregnancy [60]. In addition, there are many DMTs with very low probability of reaching breast milk and others are not harmful for the baby [39], so the absolute cessation of breastfeeding will not always be necessary. All available information should be discussed with the mother, whose preference is decisive in the final decision [39,60].
Treatment of MS has evolved greatly in recent decades; Since 1993, a large number of disease-modifying treatments (DMT) have been approved by the United States Food and Drug Administration (FDA), and the European Medicines Agency (EMA) or are still under investigation in clinical trials [66]. These drugs currently represent the first-line treatment, and have demonstrated efficacy in reducing relapse rates and severity, as well as moderating the development of new CNS lesions. They are able to slow the progression of the disease in the long term and delay the onset of neurological disability, however, they do not have the capacity to reverse the disability or restore function [66]. With regard to pregnancy and postpartum, medical management is a challenge, given the risks of exposure to medications for mothers and children.
Until 2015, the FDA classified the risk of the different drugs under a five-letter system (A, B, C, D, X) according to the known effects on pregnancies in animals and humans. A drug A was considered safe because it did not show harmful effects in animal or human pregnancies, while a drug X identified agents totally contraindicated for its clear teratogenic effect in studies. However, for drugs introduced as of June 2015, a narrative approach was included that includes a synopsis of all known effects on animal and human pregnancy, lactation and the reproductive potential of the various approved drugs [33] (Table 1).
There is a large experience with the use of interferons and gratiramer acetate, and both drugs are safe for pregnancy and breastfeeding [67,68]. Teriflunomide has been associated with teratogenic effects and a washout period is required before pregnancy, but in a series of 437 confirmed exposed pregnancies no such association was found [69]. Natalizumab is used in highly active MS patients; although this drug poses no major risks in pregnancy in two published series of exposed pregnancy comprising 369 and 92 pregnancies respectively, it was found an increased rate of birth defects [70] and spontaneous abortions [71]. Cladribine, an immunosuppressant drug, is clearly unsafe in pregnancy because of teratogenicity, and increased risk of lymphopenia and infections [72].
The number of acute relapses in MS is reduced by approximately half during pregnancy, especially in the third trimester. If a relapse occurs, corticosteroid treatment could be used, given its potent anti-inflammatory and immunosuppressive properties, they have been considered the cornerstone in the treatment of acute relapses of the multiple sclerosis [73]. The pattern of choice consists of the intravenous pulse for three days of Methylprednisolone (1gr / day). Prolonged oral administration should be avoided, as well as the use of corticosteroids with placental effects, such as Dexamethasone or Betamethasone. Also, whenever possible, steroids should be avoided during the first trimester, due to an increased risk of orofacial cleft and spontaneous abortion [31,73]. Initially, it was recommended that infants stop breastfeeding when taking steroids. After quantifying the amount of excretion, it was seen that concentrations increased after administration, but decreased rapidly, with a low breast milk concentration (1-1 , 5% of that found in the serum), so breastfeeding can continue while being treated with a short cycle of Methylprednisolone IV two to four hours after each dose. In the context of higher intravenous doses, it may be wise to wait 24 to 48 hours after each corticosteroid infusion before breastfeeding [31,73,74].If Methylprednisolonene were not effective enough, plasma exchange can be considered, since a large number of case reports suggest that it may be a well tolerated procedure in these patients [39,75].
At the beginning of the 21st century, intravenous immunoglobulins (IgG IV) were proposed as relapse prevention during pregnancy and postpartum period. However, its use has generated contradictory results since, despite having a good safety profile, it showed an ineffective cost-benefit profile. This means that the level of evidence for IVIG in preventing relapses of the disease remains too poor to draw conclusions, so that the current recommendation does not include this treatment as management of postnatal MS reactivation [76].
Multiple sclerosis is a demyelinating disease that mainly affects women of childbearing age, which can lead to problems in pregnancy. Its aetiology is not known, probable multi-factorial. However, there is no certainty that pregnancy protect a patient genetically susceptible to developing MS.
There is some controversy about the effects of MS on fertility. Some research shows no involvement, other defends the existence of endocrine and sexual disorders, as well as negative effects produced by the drugs for the disease. The role of breastfeeding in women with MS is also controversial: by the incidental exposure to contraindicated drugs for the foetus in the case of administering them, and in the case of not doing so, for the concern about relapses.
There is evidence that breastfeeding does not modify the clinical course of the disease, but the possible protective role of breastfeeding needs further research. Regarding treatment, the evidence for the use of MSD in pregnancy is still limited. Therefore, here it will also be necessary to individualize depending on the level of disease activity in the patient and the potential risks of using each TME in each individual case both before conception and later during pregnancy and postpartum with breastfeeding.
The physiological interaction between the reproductive and immune systems favours that reproductive exposures and transitions may have an impact on the course of multiple sclerosis, a disease that predominantly appears in women of childbearing age. However, after several years of studies it is known that the impact of the disease on the obstetric course is minimal and pregnancies are generally well tolerated.
Pregnancy is a natural modifier of MS associated with a reduction in the number of relapses, especially during the third trimester, with a significant exacerbation after childbirth, although this does not happen in all patients.
The only recorded harmful effects have been the higher rate of small newborns for gestational age and a higher probability of induced, instrumented and caesarean deliveries, longer labour, and longer hospital stay, so it is not necessary to take special precautions in these patients.
One of the aspects that has contributed the most in the improvement of the disease has been the great evolution of the disease modifying treatments. In pregnant MS women, the drug selection among several available DMT drugs poses a challenge, in terms of the risk of exposure to the drugs for the foetus, and the risk of relapse in the mother in cases of DMT withdrawal.
- The administration of most drugs is not safe during pregnancy, so many of them need to be suspended months before conception, even some of them require an accelerated elimination protocol.
- Gratiramer Acetate is the only drug included in the FDA category B that can be safely administered even during pregnancy provided the benefit outweighs the possible risk.
- Regarding breastfeeding, injectable drugs are the only ones authorized in these patients, yet a pragmatic and individualized approach focused on each patient is proposed.
The authors have no competing interests to declare. No funding has received in relation with this manuscripts.
- Vidal-Jordana A, Montalban X (2017) Multiple Sclerosis: Epidemiologic, Clinical and Therapeutic Aspects. Neuroimaging Clin N Am 27: 195-2042.
- Galetta KM, Bhattacharyya S (2019) Multiple Sclerosis and Autoimmune Neurology of the Central Nervous System. Med Clin North Am 103: 325-363.
- Ropper AH, Samuels MA (2009) Multiple sclerosis and allied demyelinating diseases. In: Ropper AH, Samuels MA (Eds) Adams and Victor'. Principles of Neurology. (9th Edn) McGraw-Hill Companies, Inc. pp: 874-896.
- Tintore M, Vidal-Jordana A, Sastre-Garriga J (2019) Treatment of multiple sclerosis-success from bench to bedside. Nat Rev Neurol 15: 53-85.
- Voskuhl R, Momtazee C (2017) Pregnancy: Effect on Multiple Sclerosis, Treatment Considerations, and Breastfeeding. Neurotherapeutics 14: 974-984.
- Schapira K, Poskanzer D, Neill D, Miller H (1966) Marriage, pregnancy and multiple sclerosis. Brain 89: 419-4287.
- Liebowitz U, Antonovsky A, Kats R Alter M (1967) Does pregnancy increase the risk of multiple sclerosis? J Neurol Neurosurg Psychiatry 30: 354-357.
- Antonovsky A, Liebowitz U, Medalie JM, Smith HA, Halpern L, et al. (1968) Reappraisal of possible etiologic factors in multiple sclerosis. Am J Public Health Nations Health 58: 836-84812.
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 339: 285-291.
- Korn-Lubetzki I, Kahama E, Cooper G, Abramsky O (1984) Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol 16: 229-231.
- Frith JA, McLeod JG (1988) Pregnancy and multiple sclerosis. J Neurol Neurosurg Psychiatry 51: 495-498.
- Bernardi S, Grasso MG, Bertollini R, Orzi F, Fieschi C (1991) The influence of pregnancy on relapses in multiple sclerosis: a cohort study. Acta Neurol Scand 84: 403-406.
- Nelson LM, Franklin GM, Jones MC (1988) Risk of multiple sclerosis exacerbation during pregnancy and breast-feeding. JAMA 259: 3441-3443.
- Roullet E, Verdier-Taillefer MH, Amarenco P, Gharbi G, Alperovitch A, et al. (1993) Pregnancy and multiple sclerosis: a longitudinal study of 125 remittent patients. J Neurol Neurosurg Psychiatry 56: 1062-1065
- Sadovnick AD, Eisen K, Hashimoto SA, Farquhar R, Yee IM, et al. (1994) Pregnancy and multiple sclerosis. A prospective study. Arch Neurol 51: 1120-1124. [Crossref]
- Salemi G, Callari G, Gammino M (2004) The relapse rate of multiple sclerosis changes during pregnancy: a cohort study. Acta Neurol Scand 110: 23-26.
- Airas L, Jalkanen A, Alanen A, Pirttila T, Marttila RJ (2010) Breastfeeding, postpartum and pregnancy disease activity in multiple sclerosis. Neurology 75: 474-476.
- Portaccio E, Ghezzi A, Hakki B (2011) Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology 77: 145-150.
- Keyhanian K, Davoudi V, Etemadifar M, Amin M (2012) Better prognosis of multiple sclerosis in patients who experienced a full-term pregnancy. Eur Neurol 68: 150-155. [Crossref]
- Hughes SE, Spelman T, Gray OM (2014) Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler 20: 739-746
- Karp I, Manganas A, Sylvestre MP, Ho A, Roger E, et al. (2014) Does pregnancy alter the long-term course of multiple sclerosis? Ann Epidemiol 24: 504-508. [Crossref]
- Fares J, Nassar AH, Gebeily S, Kobeissy F, Fares Y (2016) Pregnancy outcomes in lebanese women with multiple sclerosis (the LeMS study): a prospective multicentre study. BMJ Open 6: e011210.
- Benoit A, Durand-Dubief F, Amato MP (2016) History of multiple sclerosis in 2 successive pregnancies: A French and Italian cohort. Neurology 87: 1360-1367.
- Cuello JP, Martinez Ginés ML, Martin Barriga ML, de Andrés C (2017) Multiple sclerosis and pregnacy: a single-centre prospective comparative study. Neurologia 32: 92-98.
- Jesus-Ribeiro J, Correia I, Martins AI (2017) Pregnacy in Multiple Sclerosis: A portuguese cohort study. Mult Scler Relat Disord 17: 63-68.
- Lai W, Kinoshita M, Peng A (2018) Does pregnancy affect women with multiple sclerosis? A prospective study in Western China. J Neuroimmunol 321: 24-28
- Houtchens MK, Edwards NC, Phillips AL (2018) Relapses and disease-modifying drug treatment in pregnancy and live birth in US women with MS. Neurology 91: e1570-1570e1578. [Crossref]
- Bsteh G, Algrang L, Hegen H, Auer M, Wurth S, et al. (2020) Pregnancy and multiple sclerosis in the DMT era: A cohort study in Western Austria. Mult Scler 26: 69-78. [Crossref]
- Avila M, Bansal A, Culberson J, Peiris AN (2018) The Role of Sex Hormones in Multiple Sclerosis. Eur Neurol 80: 93-99. [Crossref]
- Tavakolpour S, Rahimzadeh G (2016) New Insights into the Management of Patients with Autoimmune Diseases or Inflammatory Disorders During Pregnancy. Scand J Immunol 84: 146-149. [Crossref]
- Fragoso YD, Adoni T, Brooks JBB (2018) Practical Evidence-Based Recommendations for Patients with Multiple Sclerosis Who Want to Have children. Neurol Ther 7: 207-232
- Bove R (2016) Women's Issues in Multiple Sclerosis. Semin Neurol 36: 154-162. [Crossref]
- Houtchens MK, Kaplan TB (2017) Reproductive Issues in MS. Semin Neurol 37: 632-642. [Crossref]
- Langer-Gould A, Smith JB, Hellwing K, Gonzales E, Haraszti S, et al. (2017) Breastfeeding, ovulatory years, and risk of multiple sclerosis. Neurology 89: 563-5699.
- Ponsonby AL, Lucas RM, van der Mei IA (2012) Offspring number, pregnancy, and risk of a first clinical demyelinating event: The AusImmune Study. Neurology 78: 867-874.
- Gold SM, Voskuhl RR (2016) Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol 38: 709-718.
- Harazim H, Štourač P, Janků P (2018) Obstetric anesthesia/analgesia does not affect disease course in multiple sclerosis: 10-year retrospective cohort study. Brain Behav 8: 1-9.
- Fabian M (2016) Pregnancy in the Setting of Multiple Sclerosis. Continuum (Minneap Minn) 22: 837-85039.
- Thöne J, Thiel S, Gold R, Hellwig K (2017) Treatment of multiple sclerosis during pregnancy – safety considerations. Expert Opin Drug Saf 16: 523-534.
- Hellwig K (2015) Multiple sclerosis and family planning. Neurodegener Dis Manag 5: 39-42.
- Dahl J, Myhr K, Dalveit AK, Hoff JM, Gilhus NE (2005) Pregnancy, delivery, and birth outcome in women with multiple sclerosis. Neurology 65: 1961-1963.
- 42. Chen YH, Lin HL, Lin HC. Does multiple sclerosis increase risk of adverse pregnancy outcomes: A population-based study. Mult Scler 15: 606-612.
- Fong A, Chau CT, Quant C, Duffy J, Pan D, et al. (2018) Multiple sclerosis in pregnancy: prevalence, sociodemographic features, and obstetrical outcomes. J Matern Fetal Neonatal Med 31: 382-387
- Houtchens MK, Zapata LB, Curtis KM, Whiteman MK (2017) Contraception for women with multiple sclerosis: Guidance for healthcare providers. Mult Scler 23: 757-764.
- Amato MP, Bertolotto A, Brunelli R (2017) Management of pregnancy – related issues in multiple sclerosis patients: the need for an interdisciplinary approach. Neurol Sci 38: 1849-1858.
- Dobson R, Dassan P, Roberts M, Giovannoni G, Nelson-Piercy C, et al. (2019) UK consensus on pregnancy in multiple sclerosis: ‘Association of British Neurologists’ guidelines. Pract Neurol 19: 106-114.
- Runia TF, Neuteboom RF, de Groot CJ, de Rijke YB, Hintzen RQ (2015) The influence of vitamin D on postpartum relapse and quality of life in pregnant multiple sclerosis patients. Eur J Neurol 22: 479-484.
- Rae-Grant A, Day GS, Marrie RA (2018) Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 90: 777-788.
- Laplaud DA, Leray E, Barriere P, Wiertlewski S, Moreau T. Increase in multiple sclerosis relapse rate following in vitro fertilization. Neurology 66: 1280-1281.
- Hellwig K, Beste C, Brune N (2008) Increased MS relapse rate during assisted reproduction technique. J Neurol 255: 592-593.
- Michael L, Foucher Y, Vukusic S (2012) Increased risk of multiple sclerosis relapse after in vitro fertilisation. J Neurol Neurosurg Psychiatry 83: 796-802.
- Correale J, Farez MF, Ysrraelit MC (2012) Increase in multiple sclerosis activity after assisted reproduction technology. Ann Neurol 72: 682-694.
- Bove R, Rankin K, Zhao C (2019) Effect of assisted reproductive technology on multiple sclerosis relapses: Case series and meta-analysis. Mult Scler.
- Alroughani R, Akhtar S, Zeineddine M (2019) Risk of relapses during pregnancy among multiple sclerosis patients. Mult Scler Relat Disord 34: 9-13.
- Berenguer-Ruiz L, Gimenez-Martinez J, Palazon.Bru A, Sempere AP (2019) Relapses and obstetric outcomes in women with multiple sclerosis planing pregnancy. J Neurol 266: 2512-2517.
- Buraga I, Popovici RE (2014) Multiple sclerosis and pregnancy: current considerations. Sci World J pp: 1-6.
- Kelly VM, Nelson LM, Chakravarty E (2009) Obstetric outcomes in women with multiple sclerosis and epilepsy. Neurology 73: 1831.1836.
- MacDonald SC, McElrath TF, Hernandez-Diaz S, Runia TF, Neuteboom RF, et al. (2019) The influence of Pregnancy outcomes in women with multiple sclerosis. Am J Epidemiol 188: 57-66.
- Houtchens MK, Edwards NC, Schneider G, Stern K, Phillips AL (2018) Pregnancy rates and outcomes in women with and without MS in the United States. Neurology 91: e1559-e1569.
- Portaccio E, Amato MP (2019) Breastfeeding and post-partum relapses in multiple sclerosis patients. Mult Scler 25: 1211-1216.
- Krysko KM, Rutatangwa A, Graves J, Lazar A, Waubant E (2019) Association between brestfeeding and postpartum multiple sclerosis relapses: A systematic review and meta-analysis. JAMA Neurol.
- Portaccio E, Ghezzi A, Hakiki B (2014) Postpartum relapses increase the risk of disability progression in multiple sclerosis: the role of disease-modifying drugs. J Neurol Neurosurg 85: 845-850.
- Vukusic S, Confavreux C (2006) Pregnancy and multiple sclerosis: the children of PRIMS. Clin Neurol Neurosurg 108: 266-270. [Crossref]
- Langer-Gould A, Huang SM, Gupta R (2009) Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol 66: 958-963.
- Helwig K, Rockhoff M, Herbstritt S (2015) Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol 72: 1132-1138.
- Gholamzad M, Ebtekar M, Ardestani MS (2019) A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm Res 68: 25-38.
- Thiel S, Langer-Gould A, Rockhoff M (2016) Interferon beta exposure during first trimester is safe in women with multiple sclerosis. A prospective cohort study from the german multiple sclerosis and pregnancy registry. Mult Scler 22: 801-809.
- Herbstritt S, Langer-Gould A, Rockhoff M (2016) Gratiramer acetate during early pregnancy. A prospective cohort study. Mult Scler 22: 810-816.
- Vukusic S, Coyle PK, Jurgensen S (2019) Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide. Clinical study data and 5-years of post-marketing experience. Mult Scler.
- Friend S, Richman S, Bloomgren G, Cristiano LM, Wenten M (2016) Evaluation of pregnancy outcomes from the Tysabri (natalizumab) pregnancy exposure registry: a global, observational, follow-up study. BMC Neurol 16: 150.
- Portaccio E, Annovazzi P, Ghezzi A (2018) Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal riss. Neurology 90: 823-831.
- Robles-Cedeno R, Lamio-Torrenta L (2018) Cladribine in the treatment of relapsing multiple sclerosis. Rev Neurol 67: 343-354.
- Smets I, Van Deun L, Bohyn C, van Pesch V, Vanopdenbosch L, et al. (2017) Corticosteroids in the management of acute multiple sclerosis exacerbations. Acta Neurol Belg 117: 623-633.
- Alroughani R, Altintas A, Al Jumah M (2016) Pregnancy and the Use of Disease-Modifying Therapies in Patients with Multiple Sclerosis: Benefits versus Risks. Mult Scler Int pp. 1-8.
- Cox JL, Koepsell SA, Shunkwiler SM (2017) Therapeutic plasma exchange and pregnancy: A case report and guidelines for performing plasma exchange in a pregnant patient. J Clin Apher 32: 191-195.
- Rosa GR, O`Brien AT, Nogueira EAG, Carvalho VM, Paz SC, et al. (2018) There is no benefit in the use of postnatal intravenous immunoglobulin for the prevention of relapses of multiple sclerosis: findings from a systematic review and meta-analysis. Arq Neuropsiquiatr 76: 361-366.

