Abstract
Developing stem cells require specific culture media for each cell culture. Unexpected chemical reactions may occur between the components of the culture medium and the products secreted by the stem cells.
In this study we used two human bone fragments: mandibular alveolar bone and tuberosity bone. Mesenchymal stem cells derived from the bone tissues were isolated using the explant method and cultured in specific culture medium DMEM/F-12 (Dulbecco's modified Eagle's medium / Nutrient mixture F-12). Cell cultures were maintained at a humidity of 99%, a temperature of 37 °C and 5% CO2. The bone stem cells were examined by optical inverted microscopy and scanning electron microscopy with energy-dispersive X-ray spectroscopy (EDAX) qualitative and quantitative elemental analysis. In primary cultures, the first stem cells emerged from the explants after 7 days (from tuberosity bone) and after 11 days (for the mandibular alveolar bone). The light microscopy examination revealed, in the developing stem cells cultures, spherical-oval masses, of varying size, with amorphous pearly white aspect and hyperchromatic centre. The scanning electron microscopy and EDAX qualitative and quantitative elemental analysis showed that these masses consisted of natrium chloride (NaCl) crystals precipitated around organic material. The masses of precipitated crystals progressively increased in size and number within the cell cultures, limiting the cell proliferation and development to reach confluence.
Stem cells derived from human maxillary and mandibular bones promoted the precipitation of NaCl crystals in culture medium, which influenced the cells’ development.
Keywords
culture medium, mandibular alveolar bone, mesenchymal stem cells, NaCl precipitates, tuberosity bone
Introduction
Nowadays, stem cells are considered for multiple uses in regenerative medicine and tissue engineering applications [1-3]. In order to be used for these purposes, extensive research regarding their properties and ability to replace the tissue at the implantation site need to be conducted [2,4,5]. It is particularly challenging to isolate and culture functional tissue-specific cells that would provide effective results. Another challenge is to determine whether the cells used for regenerative therapy will function at the site of implantation as they were in the native tissues. Many methods previously used for tissue engineering failed to assess the structural, mechanical and biochemical properties that enabled the cells’ and tissues’ functions. This applies in particular to the bone tissue.
The ultimate goal of tissue engineering used in bone defects is to promote bone formation. In the body, bone develops through a complex process based on a three-dimensional matrix. Even though, when grown under mineralizing conditions, mesenchymal stem cells (MSCs) are capable to form a mineralized extracellular matrix similar to the bone matrix [6,7], it is still unclear if the mineralized material formed in vitro is similar to the native bone matrix [8].
Several studies demonstrated that under appropriate culture conditions, MSCs are capable to differentiate towards an osteoblastic lineage and to form mineralized material in vitro [1,9-11]. There are multiple sources of MSCs that can be harvested from the oral cavity [12-15]. Teeth and the surrounding periodontal tissues are accessible for isolating cells with MSCs-like properties when cultured in vitro. The oral cavity can be the source of many types of stem cells: (i) soft tissues, such as dental pulp, dental papilla, periodontal ligament, remnant gingival tissue or (ii) hard tissues, such as mandibular alveolar bone, tuberosity bone, deciduous or adult teeth [13,16-18].
Previous studies confirmed the capacity of neonatal calvarial osteoblasts (OB), adult bone-marrow derived mesenchymal cells (MSCs) and embryonic stem cells (ESC) to form mineralized nodules in vitro [1,19,20]. However, little attention has been given to the composition and the origin of this extracellular matrix.
A detailed understanding of the biomolecular processes, mechanical properties, and growing conditions is required in order to conduct further successful applications of the tissue engineering strategies [21,22].
Developing stem cells require specific culture media for each cell culture, to enable transplantation, preservation or further differentiation [23-26]. Since the development of the culture medium in the early 1900s, regenerative medicine and culture methods have been significantly improved. However, there was no substantial change in the composition of commercial cell culture mediums [27]. Therefore, there is a debate over whether all of the culture medium components are safe for application in the transplantation of cells and if they do not interact with the seeded cells [28-30]. Unexpected chemical reactions between culture medium and secretory products of stem cells may occur, changing dramatically the outcome of the stem cells transplantation into the human body.
Thus, a detailed analysis of the mineralized nodules formed in cell culture medium should be performed, in order to assess the interaction between different types of cells and the components in the culture medium [31-34].
In this study, we used scanning electron microscopy and energy-dispersive X-ray spectroscopy (EDAX) qualitative and quantitative elemental analysis to examine the composition of mineralized nodules formed in vitro in association with two types of mesenchymal stem cells isolated from bone tissues from the oral cavity.
Materials and Methods
Cell harvesting and isolation
In order to reduce the risk of contamination, the tissues were harvested from clinically healthy subjects, without any oral inflammatory processes and untreated caries or periodontal diseases. The study was approved by the Ethics Committee from the "Iuliu Hatieganu" University of Medicine and Pharmacy, Cluj-Napoca, no. 343 / 02.10.2014. Patients have expressed their informed consent to participate in the study.
After harvesting, the tissues were immediately immersed in a specific medium and refrigerated at 4 °C for 24 hours. The samples were washed three times with Phosphate-buffered saline (PBS) and disinfected with a 70% alcohol solution during 15 seconds, then washed again three times with PBS (in order to neutralize the harmful effect of alcohol on the cells and to maintain the viability of the MSCs in the deep layers of the tissues).
During the primary processing, the samples were immersed in a DMEM/F-12 medium with 15% of fetal bovine serum (FBS) (Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12) enriched with 2 mM L-Glutamine, 1% antibiotics (Penicillin and Streptomycin), 1% non-essential amino acids (NEA). The F-12 is a complex medium, including various microelements and can be used with or without bovine serum. The proportion of the two components of the medium is 1:1.
The isolation of the MSCs was performed using the explant method, consisting in the fragmentation of the harvested tissue in small pieces with “bone shaver” type pliers. This method is indicated only for the hard tissues harvested from the oral cavity. The samples were placed on a Petri dish and a drop of FBS was added on each bone fragment. Then, the samples were incubated for one hour, at 37 °C temperature and 5% CO2 atmosphere; afterwards, 2.5 ml of DMEM/F-12 medium with 15% FBS were added by dripping, in order to prevent the detachment of the bone fragments from their initial positions (Figure 1).
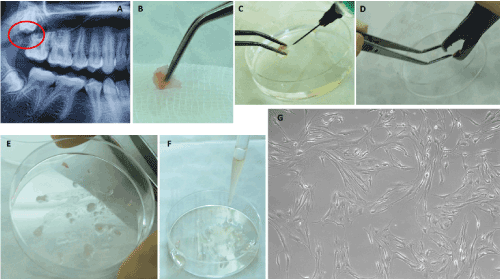
Figure 1. Isolation of mesenchymal stem cells derived from the tuberosity bone. A – Radiographic image of the impacted upper right third molar with the highlight of the cortical bone covering the occlusal surface of the tooth (circular red marking); B – The harvested bone fragment; C – Disinfection and PBS washing of the harvested sample; D – Mechanical processing using a bone shaver; E – The obtained bone fragments; F – Covering of the bone fragments with fetal bovine serum; G – primary culture of the MSCs derived from the tuberosity bone, on the 9th day (100X).
Isolation of MSCs derived from the tuberosity bone : The tuberosity bone tissue was harvested from the impacted upper right third molar. After the initial chemical and mechanical processing, the tuberosity bone samples were incubated at 37 °C temperature and 5% CO2 atmosphere until the first stem cells emerged from the bone explants, on the 9th day (Figures 1A-1G).
Isolation of MSCs derived from mandibular alveolar bone: The mandibular alveolar bone tissue was obtained after the extraction of an inferior left third molar, erupted in the oral cavity. The tooth was extracted for orthodontic reasons. After the extraction, the fragment of inter-radicular bone fractured from the buccal aspect of the alveolar bone and remained attached between the mesial and distal roots, was harvested. The bone fragment was processed using the same procedure as for the tuberosity bone (Figure 2).
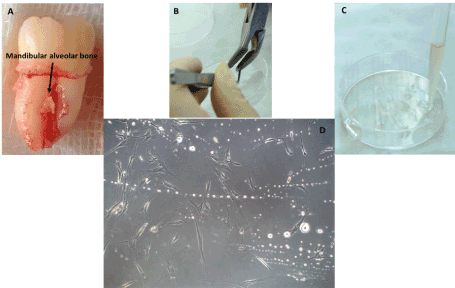
Figure 2. Isolation of mesenchymal stem cells harvested from the mandibular alveolar bone. A – The bone fragment between the mesial and distal roots of the inferior left third molar, detached from the buccal aspect of the alveolar bone; B – Mechanical processing of the sample using the bone shaver; C – Covering of the bone fragments with fetal bovine serum; D – Primary culture of MSCs harvested from the mandibular alveolar bone, on the 11th day (100X).
Assessment of cells’ morphology and medium characteristics: The morphology and phenotype of the stem cells derived from the oral cavity bone were assessed by optical inverted microscopy (Zeiss) and scanning electron microscopy (SEM) FEI Quanta 3D FEG 200/600 system with EDAX qualitative and quantitative elemental analysis.
Results and Discussion
Primary stem cell cultures derived from the bone tissue emerged at different intervals of time after isolation.
On the 9th day there was a significant proliferation of MSCs derived from the tuberosity bone tissue in the primary culture, even though some stem cells could be noticed from the 7th day around an explant at the periphery of the Petri dish. Given the fact that the MSCs’ isolation was performed in Petri dishes, which are not specific recipients for cell cultures, the medium conditions at the periphery of the dish were superior to the ones in the center, probably due to the evaporation of the culture medium, which could not be controlled in these dishes.
On the 11th day, the first MSCs derived from the mandibular alveolar bone were seen in the primary culture.
In light microscopy, during the development of the MSCs, we observed in the primary culture of both oral cavity bone derived stem cells, spherical-oval masses, of varying sizes, with an amorphous pearly white aspect and hyperchromic center. The number and size of these amorphous masses grew progressively, showing a curve-like shape of an arc of circle. These aspects were not observed in the further passages of the stem cells harvested from the bone tissues (Figure 3).
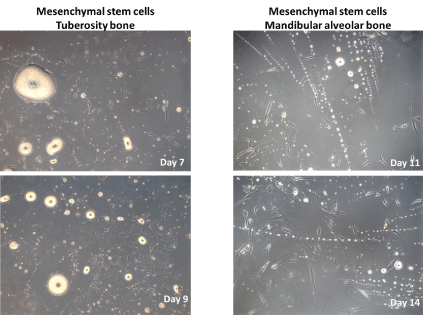
Figure 3. Primary culture of mesenchymal stem cells harvested from tuberosity and mandibular alveolar bone tissues. The presence of amorphous, pearly-white masses between the stem cells could be noticed (100X).
In optical microscopy, under 200X, and 1000X magnification, the amorphous masses exhibited a crystallin structure (Figure 4).
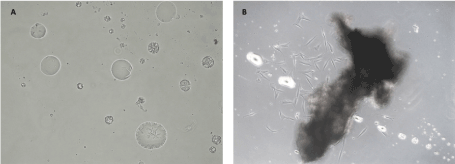
Figure 4. Primary culture of mesenchymal stem cells harvested from the tuberosity bone A – The presence of amorphous structures, with an internal crystal shape, (200X); B – Amorphous, pearly-white structures located within the mesenchymal stem cells culture, interfering with the cells’ growth and migration from the explant (100X).
SEM and EDAX analyses demonstrated that the masses consisted of natrium chloride (NaCl) crystals precipitated around some organic substrates, apparently the proteins from the bone matrix (Figure 5).
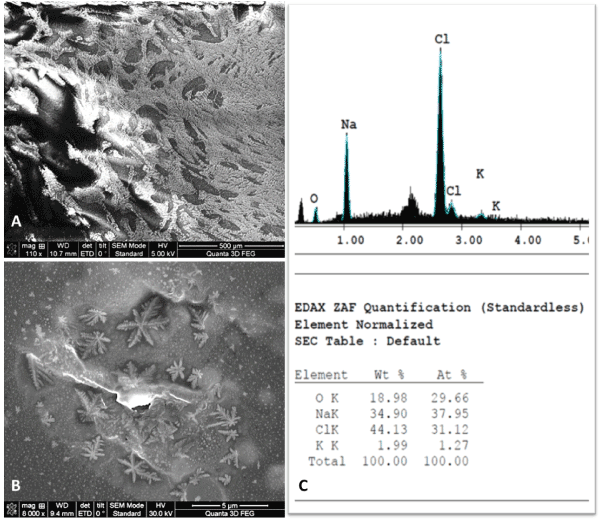
Figure 5. SEM and EDAX analyses of the amorphous precipitate from the primary cultures of bone mesenchymal stem cells. A – SEM image – aspect of a precipitated amorphous material (500 µm); B – SEM image – suggestive aspect for NaCl precipitated crystals (5 µm); C – EDAX showing the elemental analysis, confirming the NaCl composition of the crystals.
These precipitated NaCl crystals increased in size and number during the development of bone stem cells. Thus, these precipitates limited the stem cells’ proliferation and prevented cells’ conglomeration towards confluence.
The NaCl crystal precipitates grew in number at the same time with the development of the MSCs derived from the bone tissues and interfered with the cellular growth and proliferation. These aspects were not noticed in the primary cultures of other types of stem cells isolated from the oral cavity tissues [35].
The growth and proliferation of the MSCs is essential for the tissue engineering strategies. The outcome of seeding MSCs on specific cell culture mediums can be predicted by knowing the possible interactions between each type of stem cell and the components of the culture medium [36,37].
It has been shown that cells’ development depends on the composition of the culture medium, as well as on the substrate onto which they are seeded [38]. Several in vitro studies evaluated the effects of cell-seeding density, cell source, culture follow-up, scaffold composition and architecture on matrix deposition and mineralization [39-42].
The results of Meinel, et al. suggest that osteogenesis in MSCs cultures can be modulated by scaffold properties and flow environment [43]. They conducted a study on MSCs isolated from human bone marrow. In static culture, calcium deposition was similar for MSCs grown on collagen scaffolds and films. Under medium flow, MSCs on collagen scaffolds deposited more calcium and had a higher alkaline phosphatase (AP) activity than MSCs on collagen films.
Osteogenic differentiation of the cells may also be influenced by the material on which they are seeded. Arpornmaeklong, et al. studied the osteoconductive effect of beta-tricalcium phosphate (ß-TCP) scaffolds. It was concluded that these scaffolds created an environment in which cells could function to express a rich extracellular matrix, promoting survival and differentiation of human embryonic stem cells (hESCs)-derived neural crest stem cells and osteoprogenitor cells [39].
In our study, spherical or oval mineral masses, of varying sizes, and a pearly white color with hyperchromic center and amorphous appearance were formed while cultivating MSCs isolated from the oral cavity bone on a complex culture medium.
The composition of the mineral masses may vary, depending on the reaction between the cells and the medium. While in our case, these minerals were NaCl crystal precipitates, Volponi et al. showed that different types of stem cell populations isolated from dental and other tissues produced materials with different mineral composition [44]. In their in vitro study, Volponi, et al. obtained a highly mineralized matrix from the BCMP (bone chip mass population), SCAP (stem cells from apical papilla), and SHED (stem cells from human-exfoliated deciduous teeth) cells when compared to that produced by PDL (periodontal ligament), DPA (dental pulp adult), and GF (gingival fibroblast) cells. These results are similar to ours, even though the interaction between the cells and the medium was not invoked as a particular reason of the more carbonate-substituted mineral from the DPA cells and a less crystalline material from the SCAP, SHED, and GF cells, compared to the hard tissue cells and the native tissue.
Another approach in underlining the factors that influence the cell growth and proliferation is to study the culture medium used in cell transplantation and the culture surface [45-48].
While we used a classical stem cell medium, Lee et al. proposed a modified culture medium – medical fluid-based culture medium (FCM), designed by using various injectable drugs and fluids that are already permitted for use in clinical medicine. It was adjusted in order to be similar to the commercial Dulbecco’s modified Eagle’s medium (DMEM). The FCM medium was able to induce the growth of FS-MSC and H9c2 cells, showing no difference between the results obtained when seeding the cells on a DMEM medium. Moreover, it was more cost-efficient. Despite its benefits, however, there are issues that have not been resolved when using this new medium, concerning the efficiency of FCM in culturing other types of cells, such as MSCs, embryonic stem cells, inducible pluripotent stem cells, particularly for longer periods. Nonetheless, this approach could be beneficial in the future, while studying different types of cell culture mediums, capable to promote optimal cell proliferation, cell morphology, passage conditions and physiology characteristics. Ahmadian Baghbaderani et al. described the development of a robust, defined and xeno-free human pluripotent stem cells (hPSC) medium that supports reliable propagation of hPSCs and generation of human induced pluripotent stem cells (hiPSCs) from multiple somatic cell types, proving its capacity to sustain an every-other-day (EOD) schedule of medium replacement for both the generation and long-term cultivation of hPSCs. The reliability of this defined medium formulation allowed hPSCs to be reproducibly propagated under conditions where critical supporting supplements and factors were not limiting. Importantly, the medium played an integral role in establishing a good manufacturing practice (cGMP)-compliant process for the manufacturing of hiPSCs that can be used for generating clinically relevant cell types for cell replacement therapy applications [49]. Multiple various modified mediums were proposed, in order to provide a reliable, reproducible and controllable cell culture systems and promote the proliferation and growth of the seeded cells [50-52].
Another study conducted by Zanicotti et al. examined the development of adipose-derived stem cells (ADSC) under serum-free conditions, which affected the proliferative and differentiating potential of ADSC [53]. Similar results were obtained by Zhu, et al. while studying cancer stem cells [54]. This may represent an alternative approach to developing an appropriate culture medium.
Conclusion
That there is a valid hypothesis that the bone matrix from the maxillary and mandible bones contributes to the precipitation on NaCl crystals in the complex culture medium specific for the isolation of the mesenchymal stem cells. Salts precipitates altered the growth, proliferation and confluation of the seeded cells. Our results showed the importance of the interaction between different types of cell populations and the components of the culture medium.
Understanding the process and the potential factors that influence the in vitro biochemistry involving the formation of various mineralized substrates could enable the use of the mesenchymal stem cells derived from the oral cavity for regenerative tissue engineering applications.
Acknowledgements
This research was supported by the internal grant No. 4995/2/08.03.2016 within the "Iuliu Hațieganu" University of Medicine and Pharmacy, Cluj-Napoca and Doctoral Research Projects (PCD 2016) of “Iuliu Haţieganu” University of Medicine and Pharmacy Cluj-Napoca, No. 7690/15.04.2016 and the COFUND-ERA-HDHL ERANET Project, European and International Cooperation Subprogram 3.2 Horizon 2020, PNCDI III Program Biomarkers for Nutrition and Health “Innovative technological approaches for validation of salivary AGEs as novel biomarkers in evaluation of risk factors in diet-related diseases”, grant no 25/1.09.2017.
Author contributions
AI, DT and OS conceived the idea. OS, BNP and AMB were involved in study conception and design. AI, OS, AV, BAB and EP performed the experiment and acquisition of data. Analysis and interpretation of data were performed by AI, OS, AV, BNP and AMB. AI, DT and BAB were drafting of manuscript. Critical revision was made by OS, RSC, ASȘ and AI.
Conflicts of Interest
There are no conflicts of interest to declare
References
- Evans ND, Swain RJ, Gentleman E, Gentleman MM, Stevens MM (2012) Embryonic stem cells undergoing osteogenic differentiation produce mineral non-specifically compared to marrow stromal cells or calvarial osteoblasts. Eur Cell Mater 24: 211-223.
- Francis E, Kearney L, Clover J (2019) The effects of stem cells on burn wounds: a review. Int J Burns Trauma 9: 1-12. [Crossref]
- Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726-736. [Crossref]
- James A, Péault B (2019) Perivascular Mesenchymal Progenitors for Bone Regeneration. J Orthop Res. [Crossref]
- Kosaric N, Kiwanuka H, Gurtner GC (2019) Stem cell therapies for wound healing. Expert Opin Biol Ther. [Crossref]
- Song D, Xu P, Liu S, Wu S (2019) Dental pulp stem cells expressing SIRT1 improve new bone formation during distraction osteogenesis. Am J Transl Res 11: 832-843.
- Lee C, Bishop E, Dumanian Z, Zhao C, Song D (2019) Bone Morphogenetic Protein-9–Stimulated Adipocyte-Derived Mesenchymal Progenitors Entrapped in a Thermoresponsive Nanocomposite Scaffold Facilitate Cranial Defect Repair. J Craniofac Surg.
- Gentleman E, Swain RJ, Evans ND, Boonrungsiman S, Jell G (2009) Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat Mater 8: 763-770.
- Lewallen EA, Jones DL, Dudakovic A, Thaler R, Paradise CR, et al. (2016) Osteogenic potential of human adipose-tissue derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene 581: 95–106.
- Strauß S, Dudziak S, Hagemann R, Barcikowski S, Fliess M, et al. (2012) Induction of osteogenic differentiation of adipose derived stem cells by microstructured nitinol actuator-mediated mechanical stress. PloS One 7: e51264.
- Kanczler JM, Mirmalek-Sani S-H, Hanley NA, Ivanov AL, Barry JJA, et al. (2009) Biocompatibility and osteogenic potential of human fetal femur-derived cells on surface selective laser sintered scaffolds. Acta Biomater 5: 2063–2071.
- Morsczeck C1, Reichert TE1 (2018) Dental stem cells in tooth regeneration and repair in the future. Expert Opin Biol Ther 18: 187-196. [Crossref]
- Hu L, Liu Y, Wang S (2018) Stem cell-based tooth and periodontal regeneration. Oral Dis 24: 696-705. [Crossref]
- Yu T, Volponi AA, Babb R, An Z, Sharpe PT (2015) Stem Cells in Tooth Development, Growth, Repair, and Regeneration. Curr Top Dev Biol 115: 187-212. [Crossref]
- Botelho J, Cavacas MA, Machado V, Mendes JJ (2017) Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann Med 49: 644-651.
- Han J, Menicanin D, Gronthos S, Bartold PM (2014) Stem cells, tissue engineering and periodontal regeneration. Aust Dent J 59 Suppl 1: 117-130. Crossref]
- Shakoori P, Zhang Q, Le AD (2017) Applications of Mesenchymal Stem Cells in Oral and Craniofacial Regeneration. Oral Maxillofac Surg Clin North Am 29: 19-25. [Crossref]
- Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88: 792-806. [Crossref]
- Abdel Meguid E, Ke Y, Ji J, El-Hashash AHK (2018) Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J Cell Physiol 233: 1825-1835.
- Walmsley GG, Ransom RC, Zielins ER (2016) Stem Cells in Bone Regeneration. Stem Cell Rev 12: 524-529. [Crossref]
- Wilems T, Vardhan S, Wu S, Sakiyama-Elbert S (2019) The influence of microenvironment and extracellular matrix molecules in driving neural stem cell fate within biomaterials. Brain Res Bull 148: 25-33.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017) Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci 18: 1852.
- Mushahary D1, Spittler A2, Kasper C1, Weber V3, Charwat V1 (2018) Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 93: 19-31. [Crossref]
- Brehm JL1, Ludwig TE2 (2017) Culture, Adaptation, and Expansion of Pluripotent Stem Cells. Methods Mol Biol 1590: 139-150. [Crossref]
- Monge C, DiStasio N, Rossi T (2017) Quiescence of human muscle stem cells is favored by culture on natural biopolymeric films. Stem Cell Res Ther 8: 104. [Crossref]
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126: 677-689.
- Pawitan JA (2014) Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014: 965849. [Crossref]
- Lee M-S, Youn C, Kim JH, Park BJ, Ahn J (2017) Enhanced Cell Growth of Adipocyte-Derived Mesenchymal Stem Cells Using Chemically-Defined Serum-Free Media. Int J Mol Sci 18: 1779.
- Huber B, Volz A-C, Kluger PJ (2015) How do culture media influence in vitro perivascular cell behavior?: Medium influence on pericytes in vitro. Cell Biol Int 39: 1395-1407.
- Sidney LE, Branch MJ, Dua HS, Hopkinson A (2015) Effect of culture medium on propagation and phenotype of corneal stroma–derived stem cells. Cytotherapy 17: 1706-1722.
- Moll CWI, Schmiedinger T, Moll MA, Seppi T, Pfaller K (2017) Extracellular matrix mimicking scaffold promotes osteogenic stem cell differentiation: A new approach in osteoporosis research. Biomed Mater Eng 28: 87-103.
- Sadowska JM, Guillem-Marti J, Montufar EB, Espanol M, Ginebra MP (2017) Biomimetic Versus Sintered Calcium Phosphates: The Behavior of Osteoblasts and Mesenchymal Stem Cells. Tissue Eng Part A 23: 1297-1309.
- Bhuiyan DB, Middleton JC, Tannenbaum R, Wick TM (2017) Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds. Biomed Mater Eng 28: 671-685.
- Tirkkonen L, Haimi S, Huttunen S, Wolff J, Pirhonen E, et al. (2013) Osteogenic medium is superior to growth factors in differentiation of human adipose stem cells towards bone-forming cells in 3D culture. Eur Cell Mater 25: 144-158.
- Moga M, Boșca AB, Sorițău O, Băciuț M, Lucaciu O, et al. (2016) Nicotine cytotoxicity on the mesenchymal stem cells derived from human periodontium. Rom Biotechnol Lett 21: 11763-11772.
- Alves da Silva ML, Costa-Pinto AR, Martins A, Correlo VM, Sol P, et al. (2015) Conditioned medium as a strategy for human stem cells chondrogenic differentiation: Conditioned medium for MSCs chondrogenic differentiation. J Tissue Eng Regen Med 9: 714-723.
- Lian RL, Guo XL, Chen JS, Guo YL, Zheng JF, et al. (2016) Effects of induced pluripotent stem cells-derived conditioned medium on the proliferation and anti-apoptosis of human adipose-derived stem cells. Mol Cell Biochem 413:69-85.
- Reyes JMG, Fermanian S, Yang F, Zhou SY, Herretes S, et al. (2006) Metabolic Changes in Mesenchymal Stem Cells in Osteogenic Medium Measured by Autofluorescence Spectroscopy. Stem Cells 24: 1213-1217.
- Hillsley MV1, Frangos JA (1994) Bone tissue engineering: the role of interstitial fluid flow. Biotechnol Bioeng 43: 573-581. [Crossref]
- Goldstein SA1, Patil PV, Moalli MR (1999) Perspectives on tissue engineering of bone. Clin Orthop Relat Res 419-423. Crossref]
- Arpornmaeklong P1, Pressler MJ2 (2018) Effects of ß-TCP scaffolds on neurogenic and osteogenic differentiation of human embryonic stem cells. Ann Anat 215: 52-62. [Crossref]
- Akkouch A, Zhang Z, Rouabhia M (2014) Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly ( l -lactide-co-?-caprolactone) scaffold. J Biomater Appl 28: 922-936.
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, et al. (2004) Bone Tissue Engineering Using Human Mesenchymal Stem Cells: Effects of Scaffold Material and Medium Flow. Ann Biomed Eng 32: 112-122.
- Volponi AA1, Gentleman E1, Fatscher R2, Pang YW1, Gentleman MM2, et al. (2015) Composition of Mineral Produced by Dental Mesenchymal Stem Cells. J Dent Res 94: 1568-1574. [Crossref]
- Qian L, Saltzman WM (2004) Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials 25: 1331-1337.
- Burridge PW, Holmström A, Wu JC (2015) Chemically Defined Culture and Cardiomyocyte Differentiation of Human Pluripotent Stem Cells. Curr Protoc Hum Genet 87: 1-15.
- Heng BC, Lim LW, Wu W, Zhang C (2016) An Overview of Protocols for the Neural Induction of Dental and Oral Stem Cells In Vitro. Tissue Eng Part B Rev 22: 220-250. [Crossref]
- Jayaraman P, Nathan P, Vasanthan P, Musa S, Govindasamy V (2013) Stem cells conditioned medium: a new approach to skin wound healing management: Stem cells conditioned medium. Cell Biol Int 37: 1122-1128.
- Baghbaderani BA, Tian X, Scotty Cadet J, Shah K, Walde A, et al. (2016) A Newly Defined and Xeno-Free Culture Medium Supports Every-Other-Day Medium Replacement in the Generation and Long-Term Cultivation of Human Pluripotent Stem Cells. PLoS ONE 11: e0161229.
- Badenes SM, Fernandes TG, Cordeiro CSM, Boucher S, Kuninger D, et al. (2016) Defined Essential 8TM Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS ONE 11: e0151264.
- Takenaka C, Miyajima H, Yoda Y, Imazato H, Yamamoto T, et al. (2015) Controlled Growth and the Maintenance of Human Pluripotent Stem Cells by Cultivation with Defined Medium on Extracellular Matrix-Coated Micropatterned Dishes. PLoS ONE 10: e0129855.
- Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, et al. (2017) Differentiation of human induced pluripotent stem cells in William’s E initiation medium supplemented with 3-bromopyruvate and 2-deoxy-d-glucose. Mol Med Rep 15: 3719-3723.
- 53. Zanicotti DG, Coates DE (2017) Growing Adipose-Derived Stem Cells Under Serum-Free Conditions. Methods Mol Biol 1537: 439-446.
- Zhu YT, Wang CY, Pang SY, Lei CY, Luo Y, et al. (2018) A modified method by differential adhesion and serum-free culture medium for enrichment of cancer stem cells. J Cancer Res Ther 14: 421-426.





