Coronavirus disease 2019 (COVID-19) continues to affect a large number of populations in some parts of the world despite the beginning of vaccination drive. In the current situation, drug repurposing is a viable strategy to treat COVID-19 patients. However, assessment of the effectiveness of the existing drugs that have been approved for treating other diseases in context of COVID-19 patients is necessary before prioritizing them for further study. We attempted to shortlist the candidate drugs using an in silico approach. First, we analysed published transcriptomic data sets of COVID-19- and SARS-infected patients compared to healthy individuals to find the key pathways altered after infection. Then, using publicly available drug perturbational data sets in human cell lines from the Broad Institute Connectivity Map (CMAP), we assessed the effects of the approved drugs on the altered pathways. We also used the available pharmacogenomic data sets from the Genomics of Drug Sensitivity in Cancer (GDSC) portal to assess the effects of the altered pathways on resistance or sensitivity to the drugs in human cell lines. Our analysis identified many candidate drugs, some of which are already being investigated for treatment of COVID-19 and can serve as a basis for prioritizing additional viable candidate drugs for COVID-19.
SARS-CoV-2, COVID-19, drug repurposing, patient transcriptomics, pharmacogenomics, in silico
In December 2019, outbreak of a new virus causing severe respiratory disease was reported in Wuhan, China. The disease was attributed to infection by a novel member of the coronavirus family, named SARS-CoV-2 [1]. In contrast with the two other highly pathogenic coronaviruses, SARS-CoV-1 (outbreak in 2002) and MERS-CoV (outbreak in 2012), the novel virus SARS-CoV-2 had a lower fatality rate, but it could spread from person to person more efficiently [2]. The outbreak spread worldwide [3], and by March 2020, the World Health Organization declared the disease, by then named coronavirus disease 2019 (COVID-19), a global pandemic. COVID-19 is currently a leading public health concern in countries worldwide as the hospitalization rate and death toll increase daily (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Apart from mitigation efforts, there is an urgent need for effective treatments for patients showing severe symptoms. After Chinese researchers published the first sequence of SARS-CoV-2 [4], many groups worldwide began working to develop an effective vaccine, resulting in successful launch of several effective vaccines against Covid-19 within an unprecedented time. However, a large population of the world is yet to be vaccinated, which remains a public health concern especially in the wake of growing number of infections from the new strains of coronavirus like the delta and delta-plus strains. Consequently, the best solution in the interim is to look for drugs that have already been approved for humans and that may be repurposed for COVID-19 treatment. The U.S. Food and Drug Administration (FDA) has approved remdesivir, an antiviral drug targeting the viral RNA-dependent RNA polymerase, for COVID-19 treatment [5], and many more re-purposed drugs are now being tested for use against Covid-19 in clinical trials. Apart from drug repurposing, alternative treatment strategies like monoclonal antibodies against SARS-CoV-2 have been approved [6]. However, there is still a persistent need to search for alternative candidate drugs that can be used as single or as combination therapy to benefit patients with severe illness.
Researchers are also exploring targeting the host receptors of SARS-CoV-2 as another potential solution. Inhibitors of the two main receptors for SARS-CoV-2/SARS-CoV-1 entry in human cells, ACE2 and TMPRSS2, are being tested as potential treatment options [7]. A recent study published, other host interacting proteins have been curated from previously published literature [8]. In addition, a list of 332 high-confidence interactions between SARS-CoV-2 and human proteins was recently published by Gordon et al., who used affinity purification mass spectrometry on a human cell line expressing the viral proteins [9].
In this study, we collected the FDA-approved drugs from DrugBank [10] and the Drug Gene Interaction Database (DGIdb) [11] and assessed their effect in COVID-19- and SARS-infected patients in silico using published patient transcriptomic data and drug perturbational data sets. This set of drugs included the drugs targeting the SARS-CoV-2 or SARS-CoV-1-interacting proteins. By combining recently published COVID-19-infected patient transcriptomic data [12] and previous SARS-infected patient transcriptomic data [13], we found key pathways that are altered in infected patients compared to healthy individuals. Next, using the available drug perturbational data sets from the Broad Institute Connectivity map (CMAP) project [14], we assessed how the candidate drugs targeting SARS-CoV-2-interacting proteins affect the pathways that are altered after COVID-19 or SARS infection in a time-dependent manner. Using pharmacogenomic data resource, Genomics of Drug Sensitivity in Cancer (GDSC) [15], we assessed whether the altered pathways affect sensitivity or resistance to our candidate drugs in human cell lines. Finally, we present a ranked list of potential drugs for COVID-19 treatment that affect the COVID-19-altered pathways and incur less resistance due to altered pathways. Some of the drugs we identify from our analysis has been reported in previously published drug screening experiments to have in-vitro effectivity against SARS-CoV, MERS-CoV or other coronaviruses (Supplementary Figure 1).
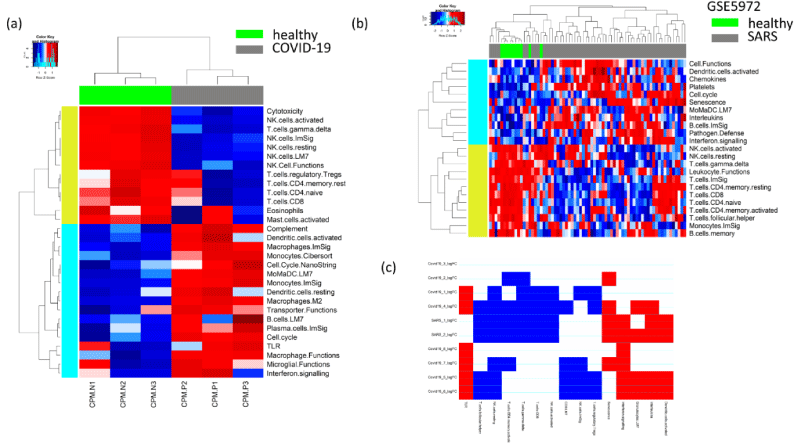
Figure 1: Immunologic signature in SARS and COVID-19 patients. The heatmap showing the immunologic signatures (ssGSEA) between the PBMC of (a) COVID-19 and healthy patients and (b) SARS and healthy patients. (c) The matrix plot shows the enriched pathway signatures common to SARS and COVID-19 vs healthy patients. We chose the pathways that are found to be commonly upregulated or downregulated in at-least 5 out of 10 public datasets used for the analysis. The colors red and blue represent the corresponding pathway is up or downregulated.
Data sets and pre-processing
We collected 4 transcriptomic datasets of SARS-CoV-2 infected patient samples or cell lines vs. healthy controls and 1 transcriptomic dataset of SARS-CoV-1 infected patients vs. healthy controls. The RNA-Seq transcriptomic data of the PBMCs from three COVID-19 infected patients and three healthy individuals were obtained from the Chinese Academy of Science accession number CRA002390 [12]. RNA-Seq Transcriptomic data from the plasma or leukocyte samples of 128 hospitalized patients with or without Covid-19 was collected from GEO accession number GSE157103 [16]. RNA-Seq data from the lung autopsy samples from 3 Covid-19 patients and 3 healthy patients was collected from GEO accession number GSE155241. From this dataset we excluded the hPSC lung organoid samples for our analysis. From another RNA-Seq dataset with GEO accession number GSE14750, we collected SARS-CoV-2 infected vs. healthy controls from multiple human cell lines (NBBE, Calu, A549, A549 expressing ACE2) and human lung biopsies (total 5 SARS-CoV-2 infected vs. healthy comparisons). We excluded the cell lines pre-treated with any drugs and cell lines infected with other respiratory viruses than SARS-CoV-2. The microarray transcriptomic data of the PBMCs from 40 SARS-infected and 10 healthy patients were obtained from the GEO accession number GSE5972 [13]. This dataset included the pre-crisis vs. healthy and post-crisis vs. healthy comparisons for SARS-CoV-1 infected patients. So, in total we included 10 comparisons of SARS-CoV-2 or SARS-CoV-1 infected vs. healthy controls from these 5 transcriptomic datasets (1 comparison from CRA002390, 1 comparison from GSE157103, 1 comparison from GSE155241, 5 comparisons from GSE14750, 2 comparisons from GSE5972). The RNA-Seq data from the COVID-19 patients and healthy controls was normalized to fractions per kilobase per million (FPKM). The microarray data from SARS patients and healthy controls was normalized with locally weighted scatterplot smoothing (LOWESS). Transcriptomic data from both COVID-19 and SARS studies were log2-transformed before further processing.
The list of human proteins interacting with SARS-CoV-2 was collected from the study by Gordon et al. [9]. In addition, the human proteins interacting with SARS-CoV-1 are collected from the article by Zhou et al. [8]. The drugs targeting the SARS-CoV-2-interacting proteins were collected from DrugBank and DGIdb [10,11]. The drug perturbational data set containing dose- and time-dependent transcriptomic profiles upon drug treatments in human cell lines was collected from CMAP [14]. The drug-screening data measuring IC50 upon drug treatments in human cell lines and cell-line transcriptomic profiles are collected from GDSC [15].
Computational analysis and metadata
The general pathway gene sets were obtained from the C2 collection of MSigDB [17]. We curated immunologic signatures specific to immune functions from four published resources: the LM22 immune infiltration signature [18], the LM7 immune infiltration signature [19], the ImSig signature of solid tumor immune infiltration [20], and the NanoString immune signature panel (https://www.nanostring.com). The combined 3,129 curated gene sets and 61 immune gene sets were used for a single-sample gene-set enrichment analysis on the RNA-seq and microarray transcriptomic tumor data sets for COVID-19- and SARS-infected patient transcriptomes as well as for cell-line transcriptomic data for the CMAP drug perturbational studies and GDSC data sets. R-package gene-set variation analysis was used [21]. We checked the differential enrichment of immune function pathways and the other curated pathways between Covid-19 vs. healthy as well as SARS vs. healthy samples in each of the gene expression datasets separately. Differential enrichments of pathways between COVID-19- and SARS-infected patients vs. healthy controls were calculated with limma R package using ssGSEA scores. The pathways with adjusted p-value <0.05 are identified as differentially enriched.
A one-sided student’s t-test was used for calculating the p-value of differences in pathway ssGSEA scores between time-dependent drug treatment responses (from CMAP) at 24 hours vs. 6 hours. For pathways that are differentially regulated due to COVID-19/SARS infection, we checked whether a drug can significantly downregulate the pathway at 24 hours compared to 6 hours of transfection in human cell lines from CMAP by performing t-tests on the pathway ssGSEA scores between these two time points using an alternative hypothesis equal to less. Similarly, to check whether a drug can significantly upregulate the pathway at 24 hours compared to 6 hours of transfection, we performed t-tests on the pathway ssGSEA scores between these two time points using an alternative hypothesis equal to greater. The drug-pathway network was constructed in CytoScape tool, and the topological properties of the network was analyzed with the NetworkAnalyzer plugin in CytoScape and the package iGraph in R.
The pathway-dependent drug sensitivity/resistance for each pathway and drug combination from GDSC is calculated using the following approach. First, ssGSEA scores for the pathways are calculated for the GDSC cell lines. Then, the cell lines are stratified into two sets based on the ssGSEA scores of the pathways. For upregulated pathways, cell lines with pathway ssGSEA z-score>2 have high pathway enrichment, and for downregulated pathways, cell lines with pathway ssGSEA z-score<-2 have low pathway enrichment. The difference between drug IC50 in GDSC cell lines with high ssGSEA scores (z-score>2) for COVID-19/SARS upregulated pathways and all other GDSC cell lines was calculated using a one-sided t-test with an alternative hypothesis equal to greater. The difference between drug IC50 in GDSC cell lines with low ssGSEA scores (z-score<-2) for COVID-19/SARS downregulated pathways and all other GDSC cell lines was calculated using a one-sided t-test with an alternative hypothesis equal to less.
Analysis of transcriptomic data of COVID-19 and SARS-infected patients revealed common altered pathways
To identify transcriptomic alterations in COVID-19-infected patients, we analysed 4 published RNA sequencing (RNA-Seq) data sets of COVID-19 patients and SARS-CoV-2-infected human cell lines compared to healthy controls. One of these datasets (GSE147507) included multiple studies in SARS-CoV-2 infected and control groups from different human cell lines and from human lung biopsies, totalling in 5 different comparison groups (see dataset details in Table 1). We only included SARS-CoV-2 infected cell lines and corresponding healthy controls without drug pre-treatment. Additionally, we included a microarray-based transcriptomic data set of PBMCs of 40 SARS-infected and 10 heathy individuals [13]. This SARS dataset included three conditions: pre-crisis, post-crisis and healthy. In this SARS dataset, we considered pre-crisis vs. healthy and post-crisis vs. healthy as two groups of comparisons. So, in total we considered 10 diseases vs. healthy comparison groups (8 comparisons for Covid vs. healthy and 2 comparisons for SARS vs. healthy). For all of these transcriptomic data sets, we performed single-sample Gene Set Enrichment Analysis (ssGSEA) on a set of curated pathways collected from the Broad Institute Molecular Signatures Database (MSigDB) and selective immune function pathways collected from published resources [18-20]. We checked the differential enrichment of immune function pathways and the other curated pathways between Covid-19 vs. healthy as well as SARS vs. healthy samples in each of the gene expression datasets separately. For the SARS dataset, we performed two comparisons: pre-crisis vs. healthy and post-crisis vs. healthy. So finally, we included 10 datasets (8 studies from Covid-19 and 2 studies from SARS dataset). Figure 1a is showing the differential enrichments of immune functions in Covid-19 vs. healthy patient samples from the dataset CRA002390, Figure 1b is showing the differential enrichment of immune functions in SARS-infected vs. healthy patients from the dataset GSE5972. For downstream analysis, we considered pathways that were commonly altered in at-least five of the 10 datasets listed in Table 1, as shown in Figure 1c. The common immune functions between COVID-19 and SARS infected patients, that were enriched in COVID-19- and/or SARS-infected patients compared to healthy patients, were the Toll-like receptors (TLR), interleukins, and interferon signalling (Figure 1c). We expanded the functional enrichment to include other non-immune specific functions, which included P38-MAPK pathway, Hippo signalling pathway, cardiac EGF pathway, tryptophan metabolism, and NFκB pathway (Figures 2a-2c). Some of the common pathways with less enrichment in the COVID-19- and SARS-infected patients are NK-cell- and T-cell-mediated responses (Figures 1c and 2c). The total list of differentially regulated pathways in the comparison of Covid-19/SARS-infected vs. healthy samples across 10 datasets is included in the Supplementary Table S1.
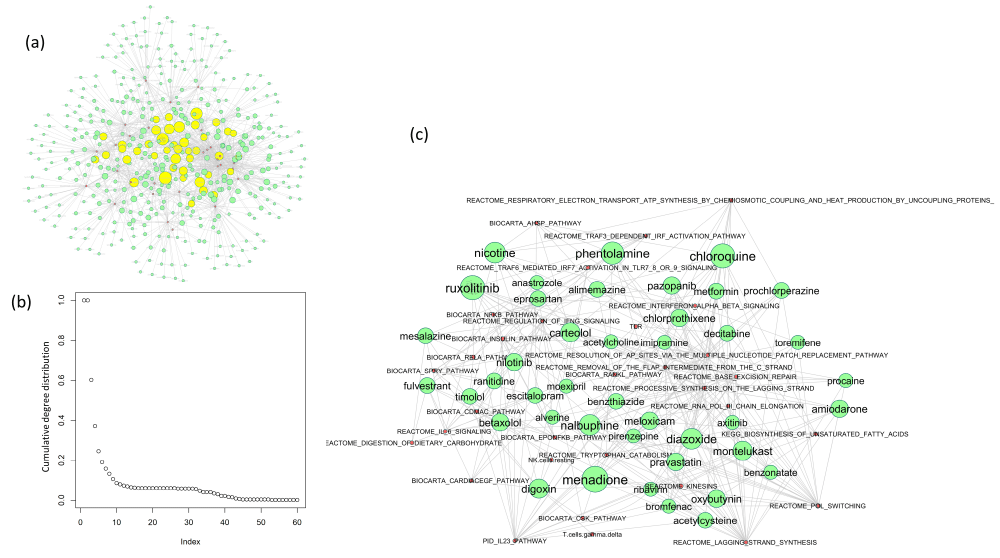
Figure 2: Network of drugs-pathway interactions. (a) The network of drugs that downregulate or upregulate the pathways altered in SARS-Cov-1 or SARS-Cov-2 infection. This network is generated as a directed network where the drugs are the source node and the pathways, they alter are their target nodes. Each connection between a drug and a pathway indicates that the pathway gets significantly upregulated or downregulated by the drug in time dependent manner (measured by differences in pathway ssGSEA score at 24-hour vs 6 hour of drug treatment from CMAP dataset). The node sizes represent the outdegree, bigger nodes are the drugs with high outdegree, i.e., the drugs that can regulate more number of pathways. The central hub nodes are highlighted in yellow. (b) The degree distribution of the nodes in the drug-pathway network in (a) follows the power-law curve, indicating the scale-freeness of the network. (c) The Network shows the hub nodes from (a), consisting of drugs with outdegree >=6 and their connected pathways. Green colored nodes represent drugs and pink colored nodes represent pathways altered by these drugs.
Table 1. Transcriptomic datasets used for comparison of COVID-19/SARS infected vs healthy samples
Dataset Name |
Dataset Accession
/Reference |
Tissue/Cell-Line |
Number of COVID-19
/SARS Infected Samples |
Number of Healthy/Mock
Infected Samples |
COVID-19_1 |
CRA002390
(Chinese Academy of Science) |
Human PBMC |
3 |
3 |
COVID-19_2 |
GSE157103
(Gene Expression Omnibus) |
Human plasma or leukocyte samples from hospitalized patients |
102 |
26 |
COVID-19_3 |
GSE155241
(Gene Expression Omnibus) |
Human lung biopsy |
3 |
3 |
COVID-19_3 |
GSE147507
(Gene Expression Omnibus) |
Calu-3 cell line |
3 |
3 |
COVID-19_4 |
GSE147507
(Gene Expression Omnibus) |
NBBE cell line |
3 |
3 |
COVID-19_5 |
GSE147507
(Gene Expression Omnibus) |
A549 cell line |
3 |
3 |
COVID-19_7 |
GSE147507
(Gene Expression Omnibus) |
A549 cell line transfected with a vector expressing ACE2, without Ruxolitinib pre-treatment |
3 |
3 |
COVID-19_8 |
GSE147507
(Gene Expression Omnibus) |
Human lung biopsy |
2 |
2 |
SARS_1 |
GSE5972
(Gene Expression Omnibus) |
Human PBMC
(SARS pre-crisis) |
26 |
10 |
Assessing the effect of potential drug candidates on the pathways altered in COVID-19 and SARS infection
In an attempt at finding already approved drugs that could be repurposed for COVID-19 treatment, we explored the set of all FDA-approved drugs from DrugBank and DGIDB. As the drugs of potential interest, the list of all human proteins interacting with SARS-CoV-2 were collected from the affinity capture mass-spectrometry study by Gordon et al. [9] and the list of SARS-CoV-1-interacting proteins were curated from previous literature [8]. The list of drugs targeting these host proteins was retrieved from DrugBank and DGIdb databases [10,11]. Additionally, we procured the list of drugs currently in trial for COVID-19 treatment from literature (https://www.excelra.com/COVID-19-drug-repurposing-database/data). The resulting list comprised a total list of 1482 drugs, out of which 244 drugs targeted the proteins that interacted with SARS-CoV-2. Supplementary Table S2a shows the total list of drugs and their targets, while Supplementary Table S2b lists the subset of drugs with available mechanism of action retrieved from CMAP database. We sought to check if the drugs are effective in the context of transcriptomic changes in COVID-19 or SARS patients after infection. We checked the effect of these drugs on the perturbation of the pathways upregulated or downregulated after SARS and COVID-19 infection. In CMAP, gene expression is profiled in cell lines treated with drugs at different dose/time points, and we see how drugs change the expression of a given set of genes. To get a pathway-based estimation instead of individual genes, we calculated ssGSEA scores for the differentially enriched pathways in COVID-19 or SARS infection for all drug-treated cell lines at different dose/time points. Then the ssGSEA scores of a pathway were compared between two time points (24 hours vs. 6 hours) in cells treated with a certain drug using student’s t-test. Among the total list of all FDA-approved drugs, 429 drugs altered the COVID-19/SARS differentially enriched pathways in a time-dependent manner; while among the drugs targeting SARS-CoV-2-interacting proteins, 61 drugs could do that.
The network in Figure 3a (see Supplementary Table S3 for related data), shows the 429 drugs that significantly downregulate or upregulate the common pathways that get altered after COVID-19/SARS infection (described in the previous section and in Figure 1c and Figure 2c). This network is generated as a directed network where the drugs are the source node and the pathways, they alter are their target nodes. Analyzing this drug-pathway network reveals that it has a moderately high degree of modularity (Q= 0.499), and the degree distribution of the nodes follow the power-law which is feature of scale-free networks. This observation is consistent with the properties of other biological networks like metabolic networks and protein-protein interaction networks. These properties indicate the presence of highly connected “hub” nodes in the centre of the network irrespective of the scale of the network. We calculated the indegrees and outdegrees of the pathways and the drugs respectively to find the hub nodes. In order to identify key drugs in the network that serve as hub nodes, regulating (connected with) a large number of pathways in the network, we selected the nodes with outdegree ≥6 (the yellow highlighted nodes in Figure 3a, enlarged visualization in Figure 3b). These drugs can suppress the pathways that were upregulated due to COVID-19/SARS infection (p-value<0.05, t-test with alternative hypothesis = less), while they can also boost the pathways that were downregulated due to COVID-19/SARS infection (p-value<0.05, t-test with alternative hypothesis=greater). We analyzed the community structure of the drug-pathway network based on edge betweenness to find the drugs and pathways that are densely connected between themselves but sparsely connected with the other drugs and pathways. Such analysis helps to find out closely related pathway groups and the common drugs that regulate these pathways. Using clustering of edge betweenness in the network we identified 15 major clusters of pathways and corresponding drugs that can regulate them (Figure 3a). Among these clusters, one cluster of particular interest consisted of the NFκB, RELA and interleukin 6 pathways which are upregulated in Covid-19 and SARS infection and are related to the “cytokine storm” event in severely affected patients. The cluster containing these related pathways and the corresponding drugs are shown zoomed in Figure 3a. The sub-network in Figure 3b shows the connectivity between the pathways and the drugs that regulate them. Here we only included the drugs that have outdegree >4. The drugs chloroquine, ruxolitinib, digoxin, timolol, fulvestrant, anastrazole, pazopanib etc. could regulate NFκB pathway, while nalbuphine, nilotinib, cytarabine, timolol, fulvestrant, tacrolimus etc. could regulate IL-6 pathways.
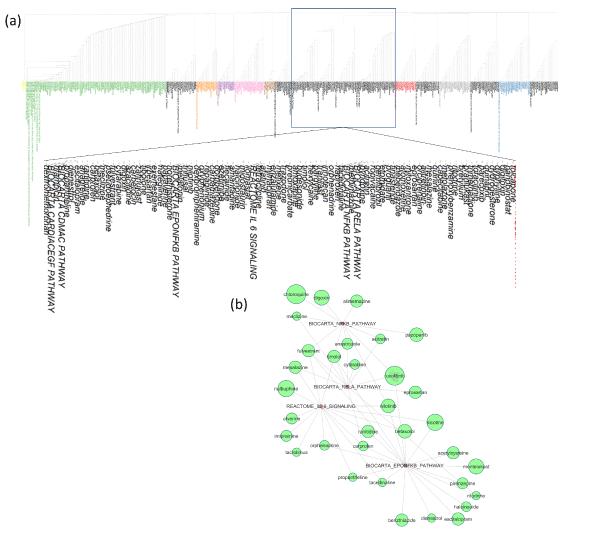
Figure 3: Analyzing the drug-pathway network to identify modules consisting of related pathways and corresponding drugs that can regulate them. (a) The cluster dendrogram of the edge-betweenness of the network in figure 3a. We identified 15 clusters (modules), among which one module consisted of the NFκB and interleukin-6 related pathways and the drugs that regulated them. These pathways are upregulated in Covid-19 and SARS infected patients and are related to the adverse event “cytokine storm” in patients with severe disease. This module is shown zoomed in. (b) The Network shows the connection between the key pathways and drugs from the module shown in (a). Only the drugs that have outdegree > 4 have been included in this network.
We ranked the drugs according to their outdegrees in the drug-pathway network, which signifies the number of pathways they could alter. The drugs ruxolitinib, menadione, chloroquine, phentolamine, nalbuphine, diazoxide, nicotine, carteolol, montelukast and decitabine came out as the top 10 drugs that can reverse the pathways that are altered in Covid-19 infection (Supplementary Table S3). As a validation of the drugs, we identified from our analysis, we have compared the drugs from the above analysis with a published study that evaluated different computational algorithms for Covid-19 drug repurposing against four ground truth datasets from in-vitro drug screening, and completed or ongoing clinical trials. In the in-vitro screening study, they evaluated 918 drugs screened against SARS-CoV-2 in VeroE6 cell line [22] and found only 77 drugs to have a strong or weak effect on SARS-CoV-2 infection in this in-vitro experiment. We matched their in-vitro drug efficacy results with the ranked list of 429 drugs that we generated for Covid-19. From our list, 32 drugs were found to have a strong or weak effect on SARS-CoV-2 (Table 2), while 3 of these drugs were ranked among the top 20 candidates in our analysis (chloroquine, amiodarone and digoxin). We also tested the drugs from our analysis with the drugs in clinical trial for Covid-19 treatment which were curated in the above publication in their supplementary dataset 11 [22]. This ground truth dataset included 37 drugs (out of the 918 drugs tested in-vitro), 19 of which overlapped with our ranked list (Table 2). To see if the drugs in clinical trial are overrepresented in our ranked list, against all the drugs that has been screened in-vitro, we performed a hypergeometric test. Here the population is all the drugs that has been screened in-vitro (918), the number of success in population is the number of drugs in clinical trial (37), sample size is the number of drugs in our ranked list that overlapped with the in-vitro screened drugs (344) and the number of success in the sample is the number of drugs in our ranked list that overlapped with the drugs in clinical trial (19). The p-value from the hypergeometric test came out to be almost significant (p=0.05), which means that the drugs in clinical trial for Covid-19 treatment is overrepresented in our ranked list. When we filtered our drug list to only include the drugs with outdegree ≥4, then the list came down to 70 drugs, among which 7 drugs overlapped with the drugs in clinical trial, namely chloroquine, prednisone, ruxolitinib, ribavirin, iloprost, tacrolimus and sitagliptin (significant overrepresentation of drugs in clinical trial, hypergeometric p-value=0.01). For further validation, we also matched our ranked list of drugs with the previously published drug screening datasets for SARS-CoV, MERS-CoV and other coronaviruses [23,24], as well as the GHDDI database for resources related to Covid-19 drug repurposing (https://ghddi-ailab.github.io/Targeting2019-nCoV/). Among these drugs, chloroquine, hydroxychloroquine, ivermectin, papaverine, ribavirin, loperamide, pevonedistat, imatinib, toremifene, tamoxifen, digitoxin, digoxin were some of the drugs from our analysis that overlapped with these published resources of drugs with in-vitro effectivity against SARS-CoV, MERS-CoV or other coronaviruses (Supplementary Table S4).
Table 2. Evidence of drugs which are effective against SARS-CoV-2 from published in-vitro drug screening results or in clinical trials
Drug Name |
Drug screening outcome [19] |
In clinical trial |
rifabutin |
Weak |
no |
atorvastatin |
Weak |
no |
hydralazine |
Weak |
no |
vincristine |
Weak |
no |
doxazosin |
Weak |
no |
rosiglitazone |
Weak |
no |
tenoxicam |
Weak |
no |
cinacalcet |
Weak |
no |
toremifene |
Weak |
no |
meclizine |
Weak |
no |
candesartan |
Weak |
no |
imipramine |
Weak |
no |
moxifloxacin |
Weak |
no |
zolmitriptan |
Weak |
no |
metixene |
Weak |
no |
trifluoperazine |
Weak |
no |
chloroquine |
Strong |
yes |
methotrexate |
Strong |
yes |
digoxin |
Strong |
no |
hydroxychloroquine |
Strong |
yes |
omeprazole |
Strong |
no |
ivermectin |
Strong |
yes |
quinidine |
Strong |
no |
sertraline |
Strong |
no |
sildenafil |
Strong |
no |
idarubicin |
Strong |
no |
perhexiline |
Strong |
no |
amiodarone |
Strong |
no |
afatinib |
Strong |
no |
amitriptyline |
Strong |
no |
thioridazine |
Strong |
no |
prochlorperazine |
Strong |
no |
prednisone |
No-Effect |
yes |
ribavirin |
No-Effect |
yes |
leflunomide |
No-Effect |
yes |
melatonin |
No-Effect |
yes |
tofacitinib |
No-Effect |
yes |
simvastatin |
No-Effect |
yes |
telmisartan |
No-Effect |
yes |
imatinib |
No-Effect |
yes |
ibrutinib |
No-Effect |
yes |
iloprost |
No-Effect |
yes |
tacrolimus |
No-Effect |
yes |
sitagliptin |
No-Effect |
yes |
ibudilast |
No-Effect |
yes |
naltrexone |
No-Effect |
yes |
Assessing the effect of potential drug candidates on the pathways altered in COVID-19 and SARS infection
Drug treatment sensitivity or resistance also depends on the genomic and transcriptomic features of tissues. The upregulated or downregulated pathway signatures of virus-infected patients can impact the sensitivity or resistance to the drugs that are potentially re-purposable for Covid-19. Considering this, we sought to check whether the pathways that are altered after SARS or COVID-19 infection have any effect on drug resistance. Drug sensitivity is calculated from GDSC [15]. If the upregulated (or downregulated) pathways are associated with reduced half maximal inhibitory concentration (IC50) of a drug, then we can infer that the pathway alteration makes the cell lines more sensitive and less resistant to drug treatment. To remove any potential bias from using only the drugs that target the proteins that interact with SARS-CoV2, we performed the analysis of drug dependent pathway alteration for all drugs from Drugbank and DGIDB. Considering all drugs, we could map 79 drugs to GDSC, while considering only drugs that target SARS-CoV-2 interacting proteins we could map just 16 drugs. However, from the GDSC analysis, we observed that the drugs that targeted SARS-CoV-2 interacting proteins were significantly over-represented in the set of drugs that show increased sensitivity depending on the Covid-19 altered pathways (11 out of 16 drugs targeting SARS-CoV-2 interacting proteins came out to be significant from the GDSC drug sensitivity analysis, while considering all drugs, 28 out of 79 came out to be significant: hypergeometric test p-value=0.0027). Figure 4a (see Supplementary Table S5 for related data) shows the network of drug and pathway relations from this analysis. In this network, a connection between a drug and a pathway exists if the upregulation or downregulation of the pathway is associated with increased sensitivity or reduced resistance to the drug. The association of upregulation or downregulation of a given pathway with the drug sensitivity is assessed using a t-test of the drug IC50 values between the cell lines where the pathway is upregulated (or downregulated) vs all other cell lines (see Methods section). The network includes drugs targeting SARS-CoV-2/SARS-CoV-1-interacting proteins and common pathways altered (upregulated or downregulated) in COVID-19/SARS infection. Figure 4b illustrates that the high enrichment of NFκB pathway (upregulated in COVID-19- and/or SARS-infected patients) is associated with increased sensitivity (reduced resistance, low IC50) to the drug fedratinib (calculated from GDSC data). Similarly, Figures 4c and 4d show reduced resistance to the drugs ruxolitinib and lenalidomide in GDSC cell lines as affected by high enrichment of the interleukin 6 (IL-6) pathway and IGF1-mTOR pathway respectively.
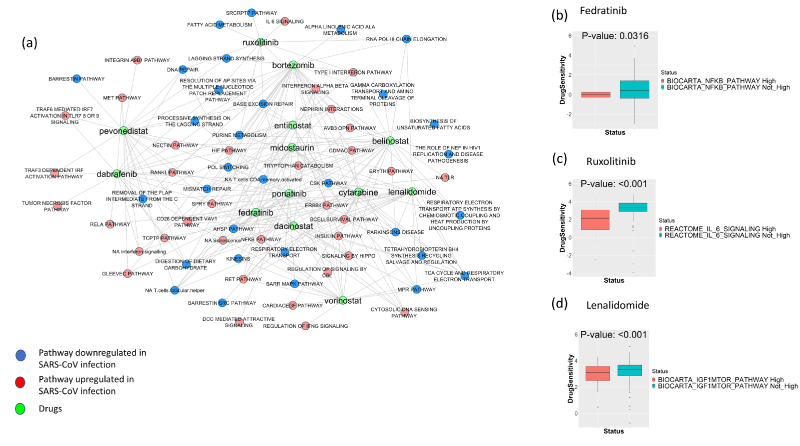
Figure 4: Network of drug-pathway interactions. (a) The network shows the repurposable drugs that are associated with reduced resistance to the pathways up or downregulated in SARS and COVID-19 infection. The drugs targeting the host proteins are collected from DrugBank and DGIDB; and the pathways up or downregulated in COVID-19 infection that makes cell lines less resistant to the drugs targeting SARS-Cov-2 interacting proteins are calculated using cell line drug screening and cell line transcriptomic data from GDSC. (b-d) The boxplots show the differences in resistance (IC50) to the drugs and associated significantly enriched pathways (scaled ssGSEA score > 2 or < -2) compared to when these pathways are not enriched. (b) Fedratinib vs. NFκB pathway (c) Ruxolitinib vs. Il-6 pathway and (d) Lenalidomide vs. IGF1-mTOR pathway.
A feasible way to fight the COVID-19 pandemic is repurposing drugs already approved for clinical use. We tried to address this issue by identifying drugs that can affect the transcriptomic changes incurred after SARS-CoV-2 and/or SARS-CoV-1 infection in patients. We considered that host proteins that interacts with SARS-CoV-2 or SARS-CoV-1 can be possible drug targets for treatment of Covid-19. However, we did not limit our analysis to just the drugs that target these SARS-interacting proteins, rather we included all drugs and assessed their effects on the pathways that gets altered after Covid-19 or SARS infection. We analysed COVID-19 and SARS-infected patients’ and healthy individuals’ transcriptomic data sets from multiple studies available in public repositories to identify the pathways commonly altered due to infection with these viruses. Thereafter, combining the potential human interactome of SARS-CoV-2 from a recently published study [9] and SARS-CoV-1-interacting proteins curated in another publication [8] with drug target databases [10, 11], drug perturbational data sets [14], and drug sensitivity screening data sets [15], we propose a map of the drugs that can be effective in COVID-19 treatment. Our proposed framework for assessing drug efficacy is based on two approaches: 1) whether the drugs can up or downregulate the pathways that get altered due to COVID-19/SARS infection in a time-dependent manner and 2) whether the sensitivity or resistance to the drugs depends on the alteration of these pathways. Based on the number of pathways the drugs can regulate, we derived a ranked list of drugs that can affect the pathways altered in SARS/Covid-19 infections and thus can be explored as a viable treatment option.
Along with recently published transcriptomic profiling studies for Covid-19 infections, we included a previously published data set of SARS-infected patient’s transcriptomes consisting of 40 SARS-infected patients and 10 healthy individuals [13]. We reasoned that the viruses SARS-CoV-1 (causing SARS) and SARS-CoV-2 (causing COVID-19) share many similarities, and the symptoms of the infected patients are also similar, though SARS infection causes more severe symptoms and a higher fatality rate [25]. Some of the drugs we find in our analysis that could affect the pathways altered in SARS-infected compared to healthy patients, have been reported in previously published drug screenings on SARS-CoV-2 or SARS-CoV-1 infected cell lines. Chloroquine, Ruxolitinib, Amiodarone, Hydroxychloroquine, Ivermectin, Ribavirin, Digitoxin, Digoxin, Pevonedistat and Loratadine are such drugs we identified from our study that has been reported to be effective against SARS-CoV-2 or SARS-CoV-1 in previously published screening experiments. Moreover, we have checked the overlap of the drugs we found in our analysis with the experimentally verified drugs against Covid-19 that are in preclinical stage from the GHDDI database and found some additional drugs that have reported in-vitro efficacy against Covid-19. However, in clinical trial with the hospitalized patients, the drug Hydroxychloroquine did not show significant treatment benefit compared to control group of patients [26]. Also, the drugs Chloroquine and Hydroxychloroquine was found to have serious adverse side-effects related to cardiac toxicity [27]. These drugs are weak bases and interfere with lysosomal activity and autophagy. Together with mode of action and drug’s chemical properties, might explain the clinical efficacy and known adverse effects [28]. The unknown dose–response relationships of these drugs and the lack of definitions of the minimum dose needed for clinical efficacy and what doses are toxic pose challenges to clinical practice. Further challenges include patient non-adherence and possible context-dependent variations in blood drug levels. So, it should be noted that the results from the in-silico drug repurposing or in-vitro drug screening studies should be evaluated in clinical trials before they can be considered as treatment options. Nevertheless, the strength of our approach lies in cataloguing a list of drugs that may affect the pathways which are altered after SARS-CoV infection. Knowing which drug can affect which pathway can be a valuable information for researchers in this field.
Our analysis of COVID-19 and SARS-infected patient transcriptomic signatures identified the common pathways upregulated or downregulated after infection with these viruses. Among the common pathways, integrin, NFκB, insulin, P38-MAPK signalling pathways were upregulated due to infection, while fatty acid metabolism and DNA repair related pathways were downregulated. Among the immune pathways, TLR, interleukins, and interferon responses were elevated in patients with COVID-19 and SARS infection, while T-cell and NK cell functions were downregulated. Many of these pathways have been previously implicated in viral infections. Integrins have already been linked to the entry of COVID-19 as possible alternative receptors for SARS-CoV2 [29]. NFκB activation has been shown to exacerbate SARS-CoV-1 infection mediated lung inflammatory reaction [30]. P38-MAPK activation has also been proposed to be associated with SARS-CoV-2 mediated severe inflammatory reaction [31]. Moreover, TLR, interleukins and interferon response activation has been linked to the hyperinflammatory phenotypes in SARS-CoV-1 and SARS-CoV-2 infection that have adverse clinical outcomes. By analysing the drug perturbational data sets from CMAP, we identified drugs that can upregulate or downregulate the pathways that get altered due to COVID-19/SARS infection. Analyzing the drug-pathway network of the drugs that can regulate the pathways altered in Covid-19/SARS infection, we found a network module consisting of the NFκB, RELA and interleukin-6 pathways and the corresponding drugs. The drugs in this network module likely regulate these pathways related to the cytokine storm and may be candidates for treating sever Covid-19 patients. Some drugs that could downregulate the NFκB and inteleukin-6 pathways (upregulated due to COVID-19/SARS infection), which is linked to severe outcomes are: chloroquine, digoxin, ruxolitinib, nalbuphine, nilotinib, tacrolimus, fulvestrant, mesalazine, timolol etc. The drugs that could upregulate the T cells or NK cells, which are important for antiviral immune responses, are: tolcapone, lovastatin, ribavirin, moexipril, decitabine, vorinostat, iloprost, nicotinamide, suramin, lenalidomide, apicidin, haloperidol, and metformin. Many of these drugs have been undergoing clinical trials for COVID-19, including ruxolitinib, ribavirin, iloporst and tacrolimus [22].
Considering that host transcriptomic features can affect the sensitivity or resistance to drugs [15], we checked whether the pathways that get altered after COVID-19/SARS infection can reduce the resistance to any drugs using GDSC cell-line drug sensitivity screening data. We were limited to drugs approved for cancer treatment that have been screened in human cancer cell lines, so we could not test all potential drugs for COVID-19. However, among the drugs we tested, we found that there was reduced drug resistance (increased sensitivity) related to the pathway alteration signature due to COVID-19/SARS infection with the drugs bortezomib, fedratinib, ruxolitinib, lenalidomide, cytarabine, dabrafenib, ponatinib, vorinostat, belinostat, entinostat, dacinostat, pevonedistat and midostaurin. Among these drugs, fedratinib and ruxolitinib are potential treatment options for COVID-19 [32,33]. Fedratinib and ruxolitinib are semi-selective janus kinase inhibitor with anti-inflammatory properties that can be beneficial for treatment of severe COVID-19 patients with hyperinflammatory symptoms that may lead to lung damage and death. Among the other cancer drugs, the histone deacetylase inhibitors vorinostat, belinostat, entinostat, and dacinostat have anti-inflammatory properties, and the drug lenalidomide has immunomodulatory properties that may have treatment benefits in COVID-19 patients with severe symptoms, possibly when treated with antiviral agents. The chemotherapeutic drugs midostaurin, and cytarabine may also have potential antiviral properties [34-36]; however, their suitability for treating virus infection is not well established, and therefore, these drugs should be carefully examined before being considered as treatment options.
We acknowledge that there are some limitations to our study. A limitation of our study is the availability of drug perturbational and drug sensitivity screening data in human cell lines. Many of the drugs targeting the potential SARS-CoV-2- or SARS-CoV-1-interacting proteins did not have drug perturbational data available in human cell lines from CMAP, and only a few drugs have the drug sensitivity screening data available from GDSC, which is limited to drugs commonly used in cancer treatment. Without the available drug perturbational data, we could not assess the effects of antiviral drugs like remdesivir, favipiravir, and oseltamivir in the human transcriptome and were limited to drugs targeting the potential human receptors of the viruses.
Our computational study provides an assessment of the potential effects of possible repurposable drugs targeting SARS-CoV-2- and SARS-CoV-1-interacting proteins in the context of transcriptomic signatures of COVID-19- or SARS-infected patients. Because we have limited understanding of the virus pathogenesis and the safety of targeting specific pathways for treatment of this disease, we have included a complete map of the drug-pathway relationships that can be important in the context of COVID-19 infection and treatment. Due to the urgency of identifying effective drugs for treatment of COVID-19 patients, many groups have been working on repurposing existing drugs that can be used for this disease [8]. Our study may be the first that links potentially repurposable drugs to the pathway signatures of infected patients. The drugs we presented in this study as potentially repurposable for COVID-19 treatment should be clinically tested for efficacy before use.
S.D. and U.S. conceived the study design. S.D. performed all analysis. S.D., U.S. and K.C. performed revision and manuscript writing.
This research was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (NCI).
The transcriptomic datasets used in this study has been collected from publicly available data repositories like GEO and Chinese Academy of Science. The drug screening datasets in human cell lines are publicly available from CMAP and GDSC portals. The analysed data resulting from this study is available in the supplementary tables included.
View Supplementary Data
- Wu F, Zhao S, Yu B, Chen YM, Wang W, et al. (2020) A new coronavirus associated with human respiratory disease in China. Nature 579: 265-269. [Crossref]
- Gates B (2020) Responding to Covid-19 - A Once-in-a-Century Pandemic? N Engl J Med 382: 1677-1679. [Crossref]
- Wang C, Horby PW, Hayden FG, Gao GF (2020) A novel coronavirus outbreak of global health concern. Lancet 395: 470-473. [Crossref]
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, et al. (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9: 221-236. [Crossref]
- Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, et al. (2020) Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med 382: 2327-2336. [Crossref]
- Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, et al. (2021) Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol 21: 382-393. [Crossref]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181: 271-280.e8. [Crossref]
- Zhou Y, Hou Y, Shen J, Huang Y, Martin W, et al. (2020) Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 6: 14. [Crossref]
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, et al. (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583: 459-468. [Crossref]
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, et al. (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46: D1074-D1082. [Crossref]
- Cotto KC, Wagner AH, Feng YY, Kiwala S, Coffman AC, et al. (2018) DGIdb 3.0: a redesign and expansion of the drug-gene interaction database. Nucleic Acids Res 46: D1068-D1073. [Crossref]
- Xiong Y, Liu Y, Cao L, Wang D, Guo M, et al. (2020) Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 9: 761-770. [Crossref]
- Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, et al. (2007) Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol 81: 8692-8706. [Crossref]
- Lamb L, Crawford E, Peck D, Modell JW, Blat IC, et al. (2006) The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313: 1929-1935. [Crossref]
- Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, et al. (2013) Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res 41: D955-D961. [Crossref]
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, et al. (2015) Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12: 453-457. [Crossref]
- Tosolini M, Pont F, Poupot M, Vergez F, Nicolau-Travers ML, et al. (2017) Assessment of tumor-infiltrating TCRVγ9Vδ2 γδ lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology 6: e1284723. [Crossref]
- Nirmal AJ, Regan T, Shih BB, Hume DA, Sims AH, et al. (2018) Immune Cell Gene Signatures for Profiling the Microenvironment of Solid Tumors. Cancer Immunol Res 6: 1388-1400. [Crossref]
- Gysi DM, Do Valle Í, Zitnik M, Ameli A, Gan X, et al. (2020) Network Medicine Framework for Identifying Drug Repurposing Opportunities for COVID-19. ArXiv [Preprint] arXiv: 2004.07229v1. [Crossref]
- Shen L, Niu J, Wang C, Huang B, Wang W, et al. (2019) High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J Virol 93: e00023-19. [Crossref]
- Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, et al. (2014) Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 58: 4885-4893. [Crossref]
- El Zowalaty ME, Järhult JD (2020) From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans - Call for a One Health approach. One Health 9: 100124. [Crossref]
- RECOVERY Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, et al. (2020) Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 383: 2030-2040. [Crossref]
- Tleyjeh IM, Kashour Z, AlDosary O, Riaz M, Tlayjeh H, et al. (2021) Cardiac Toxicity of Chloroquine or Hydroxychloroquine in Patients With COVID-19: A Systematic Review and Meta-regression Analysis. Mayo Clin Proc Innov Qual Outcomes 5: 137-150. [Crossref]
- Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16: 155-166. [Crossref]
- Sigrist CJ, Bridge A, Le Mercier P (2020) A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res 177: 104759. [Crossref]
- DeDiego ML, Nieto-Torres JL, Regla-Nava JA, Jimenez-Guardeño JM, Fernandez-Delgado R, et al. (2014) Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol 88: 913-924. [Crossref]
- Grimes JM, Grimes KV (2020) p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J Mol Cell Cardiol 144: 63-65. [Crossref]
- Wu D, Yang XO (2020) TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53: 368-370. [Crossref]
- La Rosée F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, et al. (2020) The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 34: 1805-1815. [Crossref]
- Ryang J, Yan Y, Song Y, Liu F, Ng TB (2019) Anti-HIV, antitumor and immunomodulatory activities of paclitaxel from fermentation broth using molecular imprinting technique. AMB Express 9: 194. [Crossref]
- Renis HE (1973) Antiviral activity of cytarabine in herpesvirus-infected rats. Antimicrob Agents Chemother 4: 439-444. [Crossref]
- Garcia-Vidal E, Badia R, Pujantell M, Castellví M, Felip E, et al. (2019) Dual effect of the broad-spectrum kinase inhibitor midostaurin in acute and latent HIV-1 infection. Antiviral Res 168: 18-27. [Crossref]
- Overmyer KA, Shishkova E, Miller IJ, Balnis J, Bernstein MN, et al. (2021) Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst 12: 23-40.e7. [Crossref]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102: 15545-15550. [Crossref]
- Hänzelmann S, Castelo R, Guinney J (2013) GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14: 7. [Crossref]
Editorial Information
Editor-in-Chief
Yeun-Hwa Gu
Junshin Gakuen University, Japan
Article type
Research Article
Publication History
Received: October 12, 2021
Accepted: November 15, 2021
Published: November 22, 2021
Copyright
©2021 Das S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Das S, Camphausen K, Shankavaram U (2021) In silico drug repurposing to combat COVID-19 based on pharmacogenomics of patient transcriptomic data. Clin Microbiol Infect Dis 6: DOI: 10.15761/CMID.1000194.




