Background: Carotid atherosclerotic disease is a chronic inflammatory disorder that produces an intrinsic immune response and promotes the formation of plaque in the vascular endothelium, leading to clinical outcomes.
Aim: To assess the presence of inflammation in patients with carotid atherosclerosis and calcified aortic valve disease (AVD) based on serum markers, endothelial function and carotid intima-media thickness (CIMT).
Method: This is a cohort observational study. Patients with clinical suspicion of AVD were assessed by echocardiography and vascular ultrasound for measuring the endothelial function and carotid arteries plaque. Blood samples were taken for determination of inflammatory markers. A multivariate analysis was used to determine the most important variable for determine carotid artery disease (CAD) in patients with AVD.
Results: Seventy-two patients with a mean age of 62 ± 11.94 years were included. Forty six percent were men. Forty percent of them had CAD. The patients with CAD were older (p=0.001), had more percentage of systemic hypertension (p=0.049) and coronary calcium score> 400 HU (p=0.045).
Multivariate analysis showed that the strongest predictor of CAD in patients with calcified AVD was age greater than 65 years; Beta 3.57, p= 0.001. In this prediction, area under curve was 0.728 (CI95% 0.612-0.844), p=0.006.
Conclusions: In patients with AVD the best predictive marker for CAD was the age over 65 years, which is associated with the appearance of carotid atherosclerotic plaques.
endothelial dysfunction, carotid atherosclerosis, aortic valve disease, metalloproteinases
The prevalence of carotid atherosclerotic disease increases as the population gets older. Epidemiological studies demonstrate that carotid atherosclerosis is present in 5% of women and up to 12% of men over 80 years [1,2]. The presence of carotid atherosclerotic disease contributes to the risk of cerebrovascular events, with annual reports ranging from 0.35-1.3% in asymptomatic patients with moderate stenosis to 0.5-5% in patients with severe atherosclerotic disease [2].
Ultrasound measurements of carotid intima media thickness (CIMT) is a non-invasive method that allows the identification of patients at high risk of cardiovascular events [2,3]. Increased CIMT had been associated with progression of coronary artery disease. It can predict the occurrence of acute myocardial infarction in addition to cerebrovascular events, and it is a variable used in the subclinical evaluation of organ lesion and in the stratification of cardiovascular risk of the hypertensive population [3]. For every 0.1mm of increase in the CIMT, the relative risk of cardiovascular disease increases up to 15%. An unstable atherosclerotic plaque has a higher risk of causing an atherothrombotic or atheroembolic event including a cerebrovascular event and a myocardial infarction. These plaques have a thinner fibrous layer, and a large necrotic core or a highly inflammatory reaction within them [3]. The presence of matrix metalloproteinase and interleukins have been identified as markers of plaque instability and its association with clinical events, because they cause matrix degradation and induce an inflammatory response that leads to rupture and cerebrovascular events [4,5].
The objective of this study was to assess the importance of age and inflammation markers and matrix metalloproteinase in patients with both carotid atherosclerosis and calcified aortic valve disease.
This is a transversal study, in patients who sought medical attention in the out-patient care department of our institution, because of a clinical suspicion of aortic valve disease.
Inclusion criteria: a) Patients of both sexes; b) >18 years of age, c) systolic murmur on the aortic valve focus; d) patients with suspicion of aortic valve disease; d) agreement to participate in the study.
Exclusion criteria: Patients with known aortic valve stenosis; patients in treatment for hypertension, diabetes and hyperlipidemia.
Clinical suspicion was defined when the patient had risk factors for atherosclerotic disease and a heart murmur, suggesting sclerosis or aortic stenosis during the first evaluation. Blood samples were taken for a complete blood count, blood chemistry, electrolytes, and markers of inflammation (for determination of MMPs, 1, 2, and 9, and of TIMP-1, as well as IL 1, 6, CRP, TNF-⍺) as well as a general urinalysis. A resting electrocardiogram was performed. A transthoracic echocardiogram was performed by a single expert, who was blinded to the results of other studies. Endothelial function was determined by high resolution vascular ultrasound. All studies were conducted on the same day.
With the patient in sitting position, 5 ml of blood were taken from the antecubital vein with collector tubes. The blood was transported on ice and then centrifuged at 600g for 15 min at 4°C. The serum was stored in aliquots of 500mL at -70°C until processing. Concentrations of the following C-reactive protein (CPR) inflammatory markers (MMPs 1, 2, 9 and TIMP-1) were determined by immunostaining tests (Sandwich ELISA), using commercial kits (R & D systems, Minneapolis, USA) according to the instructions of manufacturer. Samples were processed in an ELISA reader at a wavelength of 450nm. High sensitivity CRP was measured by the nephelometry method (Beckman, Colter, La Brea CA, USA).
Evaluation of endothelial function
An endothelial function study was performed on the patient after at least 4 hours of fasting. The patient was placed in the supine position for 10 minutes before beginning the study. The diameter of the brachial artery was measured by high resolution vascular ultrasound at rest, during reactive hyperemia, and after administration of sublingual nitrate. The brachial artery was scanned in longitudinal section in the dominant arm 2 to 15cm proximal to the elbow. The distance was set with an anatomical marker. After the baseline measurements, a cuff was placed on the arm and inflated to 200mmHg for 5min and the arterial diameter was measured one minute after deflation. Three measurements of the brachial artery diameter were taken during reactive hyperemia and then 4 minutes later after sublingual nitrate. The endothelium-independent vasodilation was expressed as the percentage of brachial artery diameter in comparison to baseline measurements, using the formula: arterial diameter in hyperemia- basal artery diameter x100. Endothelial dysfunction was defined when the difference between the hyperemic diameter and the basal diameter was less than 10% [6].
Carotid intima media thickness
The carotid intima media thickness (CIMT) was measured by high resolution vascular ultrasound with a 13 mHz linear transducer. Longitudinal plane images of left and right carotid arteries were acquired, and the intima media thickness was automatically measured in the Qlab. The normal intima media thickness values vary from 0.59mm in individuals younger than 25 years of age to 0.95mm in those older than 65 years of age, [2,3,7].
Transthoracic Echocardiogram (TTE)
The TTE was performed with a Philips IE33 equipment, with M mode, two-dimensional and Doppler mode, using a 5 mHz transducer. The aortic valve was evaluated in the parasternal long and short axis view. The peak and medium velocity and gradient were measured from the apical 3 and 5 chambers with pulsed and continuous Doppler. The left ventricular mass was measured according to ASE guidelines. The left ventricular ejection fraction was calculated from apical 4 and 2 chambers images by the modified Simpson method [8].
Aortic sclerosis was defined as a peak aortic valve velocity of < 2 m/s. Moderate aortic stenosis was defined as a valve area of 1.0–1.5 cm2 or a mean aortic gradient of 25–40 mmHg, and severe aortic stenosis as a valve area of less than 1.0 cm2 or a mean gradient of > than 40 mmHg under normal flow conditions [9].
Coronary computed tomography (CCTA)
Employing 256-slice dual helical tomography, images of the heart and aorta were acquired in 10 seconds. The cardiac examination area extended from the carina to the diaphragm. Using a low radiation protocol (120 Kv, 50 to 80 mAs) and 3 mm thick images with an increment of 1.5 mm. Simple images were acquired to quantify the coronary calcium index, which was reported in Agatston units. Images were contrasted using 70 to 90 ml. Iopamidol (Iopamiron 370, 370 mg l / ml: BayerShering Pharma AG, Berlin Germany) using the bolus or test bolus tracking technique. The images were acquired in the skull-caudal direction with collimation of 64 x 0.6, rotation time of 330 msec. Step of 0.24, a voltage of 120 kV and current of 500 to 750 mAs and reconstructed in retrospective synchronization with the electrocardiogram in diastolic phases from 10% to 90% of the RR interval with increments of 10% for each of the patients, with a thickness of 0.7 mm and an increment of 0.4 mm, using a middle filter (Kernel B30f) with a mediastinum window. All the images were transferred and reconstructed on a workstation dedicated to a cardiac study (Leonardo Siemens). The modified 16-segment system (16 is the branch) of the American Heart Association was used. The minimum diameter of the coronary vessel to be assessed was 1.5 mm. Reconstructed images of all evaluable segments were made in multiple longitudinal and transverse axes. It was defined as significant coronary stenosis when the obstruction was greater than 50%.
All patients signed the informed consent. The protocol has been approved by the ethics and research committee of our institute.
Frequency and proportions were used to describe the categorical data and the differences were analyzed using the Chi square test. Continuous variables were expressed as mean and standard deviation using the U Mann-Whitney. The Kruskall-Wallis test with the Dunn´s multiple comparison test was used for analyzing more than two groups. The MMPs/TIMP-1 index was calculated for each patient and the arithmetic mean of the whole group was obtained from the individual data. This mean was used to express the global index. Multivariate analysis was used to determine the association between endothelial function, carotid intima-media thickness, inflammatory markers and matrix metalloproteinase with carotid atherosclerosis. All of the analyses were performed in two phases, and statistical significance was stablished with a p value of < 0.05. The GraphPad Prism version 4.02 (graphPAd INC, San Diego CA, USA) and the statistical analysis software SPSS 22 were used.
Seventy-two patients with a mean age of 62 ± 11.94 years were included. Forty six percent of the patients were men. Forty percent of the studied patients had carotid artery disease (CAD). The demographic data in patients with and without CAD are shown in table 1. The patients with CAD were older (p=0.001), had more percentage of systemic hypertension (p=0.049) and coronary calcium score> 400 HU (p=0.045). The echocardiographic and vascular ultrasound findings did not have significant differences between both groups, Table 1. Also, the inflammatory markers (hs-CRP, TNF-⍺, IFN-ϒ, IL-6, IL-10, MMPs-1,2,9 and TIMP-1) have no statistically significant differences, Table 2.
Table 1. Demographic, vascular and echocardiographic data in patients with AVD & CAD
AVD =aortic valve disease; CAD = carotid artery disease; hs-CRP= C Reactive Protein; IFN-ϒ= interferon-gamma; TNF-α = Tumoral; necrosis factor alfa; IL= interleukin; MMP = matrix metalloproteinases; TIMP-1= tissue inhibitor of metalloproteinase 1.
X2 and t Student. α = 0.05.
Multivariate analysis showed that the strongest predictor of CAD in patients with calcified AVD was age greater than 65 years; Beta 3.57, p= 0.001. In this prediction, area under curve was 0.728 (CI95% 0.612-0.844), p=0.006, Table 3, figure 1.
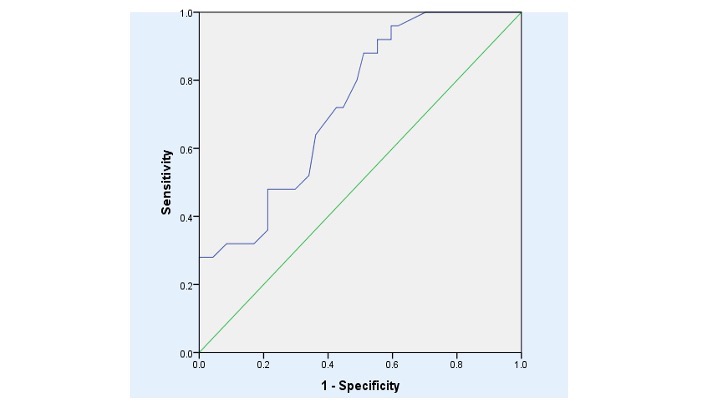
Figure 1. ROC (Relative operating characteristic) curve of age in carotid artery disease in patients with calcified aortic valve disease
Table 3. Prediction of CAD in patients with AVD
AVD= aortic valve disease; CAD= carotid artery disease; hs-CRP= C Reactive Protein; IFN-ϒ= interferon-gamma; TNF-α=tumoral necrosis factor alfa; IL= interleukin; MMP= metalloproteinases; TIMP-1= tissue inhibitor metalloproteinase 1.
X2 and t Student. α = 0.05.
In the literature there are studies that have demonstrated the association between carotid plaque vulnerability and cardiovascular events. Some studies have evaluated the relationship between different biochemical markers as predictors of such events, and the relationship between the pathophysiology of the formation of atherosclerotic plaque and aortic valve disease [10-12]. For these reasons we designed this study. Based on our results, a direct relationship with aging was found, and the carotid ultrasound showed that there was an association between CAD and calcified AVD.
MPMs, as well as their inhibitors, have been identified in association with the development of carotid atherosclerosis [5,13]. MMP 9 was detected in advanced carotid plaques [5]. In our study, which only included patients with AVD, metalloproteinases 1, 2 and 9 and their inhibitor did not show statistical significance between the group with and without CAD.
TNF-⍺ stimulates the production of other inflammatory factors such as IL-6 and IL-1β. In our study, its association with CAD could not be demonstrated [5,13]. The CIMT has a relationship with cardiovascular risk factors: in the study reported by Carrizo, et al. [3] its association with cardiovascular events in early stages of aortic valve disease was established. Increased CIMT and left ventricular hypertrophy- a risk factor for extracardiac diseases- was found to be related to the progression of aortic valve disease [13]. The presence of endothelial dysfunction associated with increased CIMT, is an important early marker of the disease, which leads to adverse long-term clinical outcomes [14], but we did not find any difference in patients with and without CAD.
In the future these parameters may be an indicator of early intervention in the progression of the disease, and also in the early prevention of adverse clinical events and in the reduction of mortality [14,15].
It has been demonstrated for decades that age is a risk factor for cardiovascular events and that mortality from these causes is linearly related to age and the presence of atrial fibrillation [16,17]. In our study, the multivariate analysis showed that the single strongest predictor for CAD was age over 65 years.
In patients with AVD the best predictive marker for CAD was the age over 65 years, which is associated with the appearance of carotid atherosclerotic plaques. CAD and AVD are probably manifestations of systemic atherosclerosis mediated by inflammatory markers.
None.
None.
None.
- Woo S, Joh J, Han S, Park H (2017). Prevalence and risk factors for atherosclerotic carotid stenosis and plaque. Medicine 96: e5999. [Crossref]
- Mannami T, Konishi M, Baba S, Nishi N, Terao A, et al. (1997) Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japa-nese city: the Suita study. Stroke 28: 518-525. [Crossref]
- Carrizo A, Tazar J, Mendía A (2013) Correlación del espesor íntima-media de arterias carótidas con parámetros ecocardiográficos, factores de riesgos y eventos cardiovasculares. Insuf Card 8: 112-118.
- Ammirati E, Moroni F, Danilo Norata GD, Magnoni M, Camici PG, et al. (2015) Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm 2015: 1-15. [Crossref]
- Moreno-Ajona D, Irimia P, Rodríguez J, García-Velloso M, López-Fidalgo J, et al. (2020) Elevated circulating metalloproteinase 7 predicts recurrent cardiovascular events in patients with carotid stenosis: a prospective cohort study. BMC Cardiovasc Disord 20: 93. [Crossref]
- Storch A, Mattos J, Alves R, Galdino I, Rocha H (2017) Methods of Endothelial Function Assessment: Descrip-tion and Applications. Intern J Cardiovasc Sci 30: 262-273.
- von Reutern G, Goertler M, Bornstein N, Sette M, Evans D, et al. (2012) Grading Carotid Stenosis Using Ultrasonic Methods. Stroke 43: 916-921. [Crossref]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015) Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiog-raphy and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1-39. [Crossref]
- Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, et al. (2017) Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 30: 372-392. [Crossref]
- Joshi FR, Rajani NK, Abt M, Woodward M, Bucerius J, et al. (2016) Does vascular calcification accelerate inflammation? A substudy of the dal-PLAQUE Trial. J Am Coll 67: 69-78. [Crossref]
- Nsaibia MJ, Boulanger MC, Bouchareb R, Mkannez G, Quang KL, et al. (2017) OxLDL-derived lisophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-kB pathway. Cardiovasc Res 113: 1351-1363. [Crossref]
- Skeoch S, Williams H, Cristinacce P, et al, Evaluation of carotid plaque inflammation in patients with active rheumatoid arthritis using 18F-fluorodeoxiglucose PET-CT and MRI: a pilot study. Lancet 385 Suppl 1: S91. [Crossref]
- Hénaut L, Sanchez-Nino, Aldamiz-Echevarría Castillo G, Sanz a, Ortiz A (2015) Targeting local vascular and systemic consequences of inflammation on vascular and cardiac valve calcification. Expert Opin Ther Targets 20: 89-105. [Crossref]
- Yoon H, Jeong M, Cho S, Kim K, Lee M, et al. (2012) Endothelial Dysfunction and Increased Carotid Intima-Media Thickness in the Patients with Slow Coronary Flow. J Korean Med Sci 27: 614-618. [Crossref]
- Alvarez B, Ruiz C, Chacón, Alvarez-Sabin J, Matas M (2004) Serum values of metalloproteinase-2 and metalloproteinase-9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J Vasc Surg 40: 469-475. [Crossref]
- Aulin J, Siegbahn A, Hijazi Z, Michael D Ezekowitz, Ulrika Andersson, et al. (2015) Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am Heart J 170: 1151-1160. [Crossref]
- Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, et al. (2016) Biomarkers of inflammation and risk of cardiovascular events in antico-agulated patients with atrial fibrillation. Heart 102: 508-517 [Crossref]

