Abstract
Background: Acute pain is a common, short-term condition that may result from tissue or nerve injury or from temporary functional disorders such as dysmenorrhea or tension-type headache. Although typically self-limiting, it can substantially affect daily functioning. Numerous over-the-counter analgesics are widely available in different oral forms and commonly used for self-medication, a key aspect of modern self-care. Ibuprofen has been widely used for decades as an effective and well-tolerated over-the-counter treatment for mild-to-moderate pain and fever of different origins.
E-Pharma Trento S.p.A. has developed an alternative to conventional pharmaceutical forms, in the form of granules in sachet containing 400 mg of ibuprofen, designed to ensure quick and easier administration of the drug. This formulation can be placed on the tongue and swallowed as such, allowing more rapid use without the need of liquids. Thus, a study was carried out to define the pharmacokinetic profile of this formulation. The primary objective of the study was to evaluate the pharmacokinetic parameters of the 400-mg ibuprofen granules formulation in healthy volunteers following single-dose administration.
Methods: A phase 1, single dose, pharmacokinetic study was conducted. Subjects were assigned to receive 1 sachet of granules (racemic ibuprofen 400 mg), applying the granules directly on the tongue and swallowing them without water, under fasting conditions. The main pharmacokinetic parameters were evaluated; safety and tolerability were also monitored throughout the trial.
Results: The developed formulation showed good gastrointestinal absorption reaching the maximum concentration of the analyte within 1.5 hours. The mean maximum plasma concentration observed was 37.22 ± 6.26 µg /ml. Overall, the safety and tolerability of the study medication resulted well.
Conclusions: The newly developed formulation of ibuprofen granules swallowed directly from the tongue without the use of water was able to provide a pharmacokinetic profile substantially consistent to that of other similar oral formulations.
Keywords
granules, ibuprofen, oral administration, pharmacokinetics
Introduction
Acute pain is a common experience affecting nearly everyone at some point in life and is generally defined as pain lasting less than 12 weeks. It may result from tissue or nerve injury, or from temporary dysfunction of a body system, such as dysmenorrhea, constipation, or certain types of headaches (e.g., tension headache). Acute pain often reflects an inflammatory process, sometimes accompanied by swelling in muscles or joints. Although it is not expected to persist indefinitely, it can significantly impair normal daily functioning [1].
To treat these conditions, a wide variety of non-prescription analgesics are available worldwide, including paracetamol (acetaminophen), nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen, and, in some countries, low-dose opioids like codeine. These medications are marketed in different formulations - tablets, capsules, effervescent powders, oral suspensions - and are frequently combined with each other, or with other products [1,2]. Self-medication has become a cornerstone of modern self-care, with NSAIDs among the most widely used medicines [3].
In this contest, across a half-century of evidence, ibuprofen (2-(4-isobutylphenyl) propionic acid), discovered by Adams and Nicholson at Boots and launched in 1969, became a widely used OTC analgesic/antipyretic in the early 1980s [4,5]. Pharmacologically, it’s a racemic propionic-acid NSAID whose clinical effects derive from reversible COX-1/COX-2 inhibition of prostaglandin synthesis. As an OTC medicine (≤1200 mg/day, short courses) it is effective for mild-to-moderate pain and fever (e.g., dysmenorrhea, headache/migraine, dental and musculoskeletal pain, URTI symptoms) [5-7] and shows a good safety profile, gastrointestinal tolerability comparable to paracetamol and no increase cardiovascular risk when administered at OTC doses, with dose-related risks primarily emerging at higher or long-term prescription doses [6,8,9].
Oral formulations of ibuprofen 400 mg have been approved and used for decades worldwide to relieve pain, inflammation, and fever in both adults and adolescents, with a well-established and accepted risk/benefit profile. A new product of ibuprofen 400 mg has been developed in the form of granules to be placed on the tongue swallowed as such, allowing rapid use without water. This formulation allows, in fact, the patient to have an extremely easy and practical product to take without the need for liquids, guaranteeing rapid administration a greater possibility of adherence to the therapy.
A phase 1 study was conducted to characterize its pharmacokinetics. The main objective of the study was to assess the pharmacokinetic profile in healthy subjects of this 400 mg ibuprofen formulation administered after single dose a under fasting conditions. The secondary objective was to evaluate the product’s safety and tolerability based on clinical and laboratory safety assessments, as well as the recording of adverse events and/or adverse drug reactions.
Materials and methods
Subjects
A total of 36 healthy male and female volunteers aged 18 to 55 years, with a body mass index (BMI) between 18.5 and 30 kg/m2, were considered eligible for inclusion based on medical history, physical examination, and laboratory evaluations.
Main exclusion criteria included history of drug abuse or use of illegal drugs; alcohol abuse; regular consumption of beverages or food containing methylxanthines; pregnancy; allergic diathesis or any clinically significant allergic disease; any history of drug hypersensitivity (especially to the active and inactive ingredients of the ibuprofen preparation) or intolerance to any sugar; known hypersensitivity or intolerance to NSAIDs; presence or a history of clinically significant cardiovascular, renal, hepatic, pulmonary, metabolic, endocrine, hematological, gastrointestinal neurological, psychiatric or other diseases; major surgery of the gastrointestinal tract except for appendectomy; any chronic disease which might interfere with resorption, distribution, metabolism or excretion of the drug; history of difficulty in swallowing; positive serologic findings for HIV antibodies, HBsAg, and/or HCV antibodies, vaccination within 14 days prior to screening visit. All volunteers provided written informed consent.
Ethics
The trial was performed in accordance with the Declaration of Helsinki and its last amended [10] and met the ethical requirements set in the Directive 2001/20/EC of the European Parliament and of the Council of 04-Apr-2001 and the Clinical Trial Regulation (EU) No. 536/2014 [11]. The trial was also performed in accordance with ICH Topic E8. Note for Guidance on General Considerations for Clinical Trials [12] and ICH Topic E6. Guideline For Good Clinical Practice E6(R2) Step 5 [13].
Before the start of the study, the clinical trial Protocol and other relevant documents (e.g. Case Report Form, Information for Volunteers and Informed Consent Form were submitted to the Ethics Committee for Clinical Trials in accordance with local and European legal requirements.
Study design
This was a phase 1 single-dose, pharmacokinetic trial conducted in healthy volunteers. Each subject received a single dose of granules (1 sachet =400 mg of ibuprofen). Before administration the subjects moistened their mouth by swallowing 20 ml of water, subsequently applied directly the granules on the tongue and swallowed them without water under fasting conditions.
Volunteers abstained from consuming any food or beverages other than water, starting at 9:00 PM the evening before administration and continuing until approximately 4 hours after administration, coinciding with lunchtime the following day. Water was provided ad libitum until 1 hour before and from 1 h after the drug administration on day 1. The total intake of water on day 1 of dosing later than 1 hour after drug administration was at least 1.5 liters. Concomitant medication (except from paracetamol) was strictly forbidden for the whole trial period i.e. from the start of trial medication until final laboratory safety test.
Criteria for evaluation
The primary endpoint of the trial was the assessment of main pharmacokinetic parameters considering AUC(0-t) and Cmax of ibuprofen (racemic), while the evaluation of tmax and AUC(0-∞) was the secondary endpoint. AUCres, MRT, and t½ of the analyte were also calculated as additional endpoints.
Determination of plasma concentrations
Blood samples for analysis of ibuprofen in plasma (total number of 18 blood samples, 8 ml each) were drawn at the following times: 0:00 (pre-dose), 0:10, 0:20, 0:30, 0:40, 0:50, 1:00, 1:15, 1:30, 1:45, 2:00, 2:15, 2:30, 3:00, 4:00, 6:00, 8:00, 12:00. All blood samples were collected into tubes using EDTA K2 as anticoagulation agent. After the end of the trial the plasma samples were transported frozen to the bioanalytical center for the bioanalytical procedures. Plasma concentrations were analyzed using liquid chromatography by tandem mass spectrometry (LC-MS/MS). The calibration range at on-line validation for ibuprofen (racemic) was 149.91-59962.00 ng/ml. The lowest calibrator (and thus the limit of quantification) was 149.91 ng/ml. The on-line validation on the basis of quality control samples at 4 concentration levels (450.18, 6002.20, 30012.00, 45016.00 ng/ml) measured twice per analytical run showed an inter-assay precision of 4.56, 1.87, 1.14, 2.34% CV. All plasma samples were stored for at least 3 months.
Pharmacokinetic analysis
Pharmacokinetic parameters were derived from plasma concentration–time data using a noncompartmental approach and summarized by descriptive statistics, namely arithmetic and geometric means, standard deviation (SD), coefficient of variation (CV), median and ranges (lower and upper). These parameters included AUC(0-t), AUC(0-∞), Cmax, tmax, AUCres, MRT and t½. The descriptive statistic was used for the evaluation of secondary and additional endpoints.
Safety
Safety clinical and laboratory examinations, assessed at the start (screening visit) and at the end of the trial (final visit), and registration of adverse events and/or adverse drug reactions were recorded during the trial. Descriptive statistics were used to summarize all safety data: clinical (physical) examination, standard laboratory examinations (clinical blood chemistry, hematology, urinalysis), monitoring of vital signs (pulse rate, blood pressure), ECG monitoring, and registration of adverse events and/or adverse drug reactions during the trial. The pharmacokinetic and statistical evaluation were carried out by means of the validated statistical software package SAS for Windows, version 9.4.
Results
Subject disposition and characteristics
A total of 36 subjects (15 female and 21 male) were randomized and completed the trial according to the protocol. The statistical evaluation was based on the data of all 36 subjects. The baseline demographic characteristics of the study population are summarized in Table 1. The mean ± SD age was 39.3 ± 8.6 years, body weight was 69.2 ± 10.4 kg, and body mass index was 23.2 ± 1.68 kg/m2. All subjects were Caucasian.
Pharmacokinetic parameters
The plasma concentration time profiles and all pharmacokinetic parameters of ibuprofen (racemic) are summarized in Figure 1 and in Table 2. The developed formulation showed good gastrointestinal absorption reaching the maximum concentration of the analyte within 1.4 h (median). The mean maximum plasma concentration observed was 37.22 ± 6.26 µg /ml with a mean AUC(0-∞) of 126.17 ± 22.41 µg.h/ml.
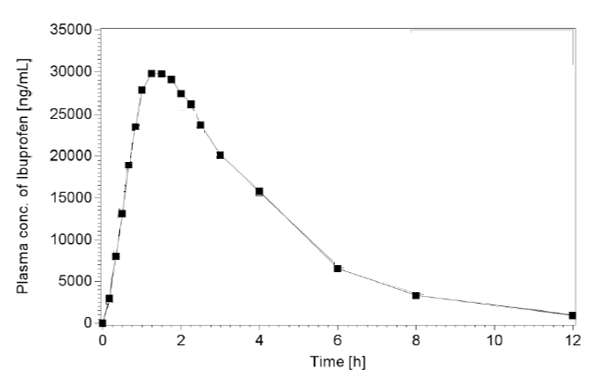
Figure 1. Mean ibuprofen plasma concentration-time profile
Safety
Thirty-six healthy volunteers were exposed to an oral single dose of ibuprofen 400 mg corresponding to 1 sachet of granules. During the trial, one non-serious adverse event (AE) was registered in one subject (Table 3): leukocytosis. This AE was considered with not related causal relationship to product administration. No serious adverse events (SAEs) were registered. All laboratory and clinical screening revealed no indications for adverse events or poor tolerability.
Discussion
The present study was designed to assess the pharmacokinetic profile of a formulation of ibuprofen (racemic) formulated as granules to be swallowed directly on the mouth as such, without liquid (1 sachet = 400 mg) in male and female healthy volunteers. The active ingredient is well known, and it has been used for many years both as prescription and as OTC drug. Its pharmacokinetic profile has been well characterized.
Following oral administration, ibuprofen is rapidly and almost completely absorbed from the gastrointestinal tract [14,15]. Peak plasma concentrations are typically achieved within approximately 50 minutes for fast-acting formulations and around 90 minutes for standard formulations [16]. However, food intake can influence absorption kinetics, mainly through alterations in gastric pH, emptying rate, and motility [7,15].
Published pharmacokinetic data indicate that a single oral dose of a very known conventional 400 mg ibuprofen tablet (e.g., Nurofen®, Motrin®, Brufen®) administered to healthy fasting volunteers results in mean plasma Cmax values ranging between 30.6 and 38.4 µg/ml at approximately 1.5 hours post-dose, with corresponding AUC(0-∞) values between 99.2 and 145.6 µg·h/ml [17-23]. Despite the time required to reach peak concentration, the onset of analgesic effect generally occurs earlier - approximately 20 minutes after a single 400 mg dose - when plasma concentrations reach about 8.4 µg/ml [24].
Ibuprofen exhibits extensive plasma protein binding, with approximately 99% of the circulating drug bound primarily to albumin [7,14,15]. Metabolic clearance occurs predominantly via oxidative biotransformation to hydroxylated and carboxylated metabolites, which constitute the major circulating and excreted forms. Cytochrome P450 isoenzymes CYP2C9 and CYP2C8 are principally involved in ibuprofen metabolism [7,14,15]. Excretion occurs mainly through the renal route in the form of metabolites and conjugates, with less than 1% of the administered dose recovered unchanged in urine [7,14].
As reported, the proposed ibuprofen granules formulation demonstrated rapid gastrointestinal absorption, achieving peak plasma concentrations within approximately 90 minutes. This absorption profile is therefore consistent with the data published for other conventional solid oral formulations of ibuprofen [17-23].
Similarly, the absorption kinetics of the test product—both in terms of rate and extent of absorption—were in line with established pharmacokinetic parameters for conventional formulations, showing a mean Cmax of 37.22 µg/ml and an AUC(0-∞) of 126.17 µg·h/ml. Accordingly, in healthy volunteers, the developed ibuprofen granules formulation provides a pharmacokinetic profile that is substantially comparable to those of other well-characterized and widely marketed oral ibuprofen formulations. Safety results confirmed that the product was well tolerated. Only one not serious treatment emergent adverse event in one patient (2.8%) was observed and no clinically important laboratory changes or trends was evidenced.
Conclusion
The developed formulation of ibuprofen 400 mg granules can provide a pharmacokinetic profile substantially consistent to that of other similar formulations.
Authors’ contributions
Matteo Mezzena, Leila Tosi and Marco Anelli contributed equally to the preparation of the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, et al. (2015) Non‐prescription (OTC) oral analgesics for acute pain‐an overview of Cochrane reviews. Cochrane Database Syst Rev 2015: CD010794. [Crossref]
- Mobasheri A, Morlion B, Sethi VS, Cardosa M, Della Pasqua O, et al. (2025) Combination vs. single‐drug nonprescription analgesics for acute pain management: A narrative review. Br J Clin Pharmacol 91: 2796-2816. [Crossref]
- Amirimoghadam P, Zihayat B, Dabaghzadeh F, Kiani K, Ebrahimi J, et al. (2017) Evaluation and awareness of over the counter use of non-steroidal anti-inflammatory drugs. J Appl Pharma Sci 7: 154-159.
- Connelly D (2017) A brief history of ibuprofen. The Pharmaceutical J.
- Rainsford KD (2013) Ibuprofen: From invention to an OTC therapeutic mainstay. Int J Clin Pract 178: 9-20. [Crossref]
- Rainsford KD (2009) Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 17: 275-342. [Crossref]
- Ngo VTH, Bajaj T (2024) Ibuprofen. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2025.
- Michels SL, Collins J, Reynolds MW, Abramsky S, Paredes-Diaz A, et al. (2012) Over-the-counter ibuprofen and risk of gastrointestinal bleeding complications: A systematic literature review. Curr Med Res Opin 28: 89-99. [Crossref]
- Moore N, Salvo F, Duong M, Blin P, Pariente A (2014) Cardiovascular risks associated with low-dose ibuprofen and diclofenac as used OTC. Expert Opin Drug Saf 13: 167-179. [Crossref]
- World Medical Association (2013) World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310: 2191-2194.
- Regulation (EU) No 536/2014 of the European parliament and of the council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC (Text with EEA relevance). Official Journal of the European Union 27. May 2014;L158: 1-76.
- ICH Topic E 8. General Considerations for Clinical Trials. Step 5. Note for Guidance on General Considerations for Clinical Trials (CPMP/ICH/291/95). March 1998.
- Guideline for Good Clinical Practice E6(R2), Step 5 (EMA/CHMP/ICH/135/1995). Adopted by CHMP on 15 December 2016. Effective since: 14 June 2017.
- Martindale on line 2025. Ibuprofen.
- PubChem 2025 Ibuprofen.
- Moore RA, Derry S, Straube S, Ireson-Paine J, Wiffen PJ, et al. (2014) Faster, higher, stronger? Evidence for formulation and efficacy for ibuprofen in acute pain. Pain 155: 14-21. [Crossref]
- Bramlage P, Goldis A (2008) Bioequivalence study of three ibuprofen formulations after single dose administration in healthy volunteers. BMC Pharmacol 8: 18. [Crossref]
- Dawood MY (1984) Ibuprofen and dysmenorrhea. Am J Med 77: 87-94. [Crossref]
- Legg TJ, Laurent AL, Leyva R, Kellstein D (2014) Ibuprofen sodium is absorbed faster than standard ibuprofen tablets: Results of two open-label, randomized, crossover pharmacokinetic studies. Drugs R D 14: 283-290. [Crossref]
- Sörgel F, Fuhr U, Minic M, Siegmund M, Maares J, et al. (2005) Pharmacokinetics of ibuprofen sodium dihydrate and gastrointestinal tolerability of short-term treatment with a novel, rapidly absorbed formulation. Int J Clin Pharmacol Ther 43: 140-149. [Crossref]
- Sugár D, Francombe D, da Silva T, Hanid S, Hutchings S (2019) Comparative bioavailability study of a new orodispersible formulation of ibuprofen versus two existing oral tablet formulations in healthy male and female volunteers. Clin Ther 41: 1486-1498. [Crossref]
- Weiser T, Schepers C, Mück T, Lange R (2019) Pharmacokinetic properties of ibuprofen (IBU) from the fixed‐dose combination IBU/caffeine (400/100 mg; FDC) in comparison with 400 mg IBU as acid or lysinate under fasted and fed conditions—data from 2 single‐center, single‐dose, randomized crossover studies in healthy volunteers. Clin Pharmacol Drug Dev 8: 742-753. [Crossref]
- Yakasai IA (2003) Effect of sodium/potassium salt (potash) on the bioavailability of ibuprofen in healthy human volunteers. Eur J Drug Metab Pharmacokinet 28: 93-99. [Crossref]
- Mehlisch DR, Sykes J (2013) Ibuprofen blood plasma levels and onset of analgesia. Int J Clin Pract Suppl 178: 3-8 [Crossref]

