Background: The leading causes of death in patients who died with a functioning allograft are cardiovascular diseases, which account for almost 40 percent of all deaths in this population.
Patients and methods: We studied prospectively basic data, hypomagnesaemia HbA1C, renal functions (serum creatinine, eGFR, and urea), and urinalysis. Then oral glucose tolerance test was performed as well, and HOMA index was calculated.
Results: The ratio of patients changed at Minute 120 of the test: there were 22 (37%) patients in the normal group, 26 (43%) patients in the IFG/IGT group, and 12 (20%) patients in the NODAT group. Regarding basic data, body mass index (p=0.854) did not influence the development of diabetes mellitus significantly. The results of laboratory tests showed significant difference in serum Mg level. HbA1C (p=0.009) and HOMA index (p=0.09) were significantly different. Renal function parameters, such as serum creatinine (p=0.001) and eGFR (p=0.0001), were also significantly different.
Conclusion: In our clinical study, given the frequency of NODAT and its relationship with cardiovascular risk, correcting hypomagnesemia soon after transplantation could translate into a significant decrease in vascular disease, which today is the primary cause of death in kidney transplant recipients.
kidney transplantation, hypomagnesemia, new onset diabetes after transplantation
New-onset diabetes after transplantation (NODAT) is a serious and frequent metabolic complication after renal transplantation [1,2]. This entity has been well defined since the publication of the International Consensus Guidelines in 2003. The factors contributing to the risk of NODAT and the strategies related to modifiable factors, with emphasis on practical issues are reviewed in this paper. Recognizing these factors may help clinicians to evaluate appropriate prevention strategies prospectively to minimize the risk of NODAT. Over the past 50 years, the concept of NODAT has evolved in terms of name and definition. Before 2003, de novo diabetes that developed after transplantation was described in various terms, most frequently as “new onset diabetes after transplantation” and suffered from a lack of consensus regarding its definition [3]. The most commonly used clinical definition was the requirement of insulin for a minimum period post-transplantation (often 30 days). This issue was addressed by the development of the 2003 Consensus Guidelines, developed by the American Diabetes Association (ADA) and the World Health Organization (WHO) [4]. A diagnosis of NODAT carries a threat to the renal allograft, as well as the same short- and long-term implications of type 2 diabetes seen in the general population. NODAT usually occurs early after transplantation and is usually diagnosed according to the general population guidelines. According to these guidelines, diabetes is present if the fasting blood glucose level is ≥ 7 mmol/L or if the blood glucose level measured 2-h following the oral administration of 75 g glucose, the oral glucose tolerance test (OGTT) is ≥11.1 mmol/L. Impaired fasting glucose (IFG) is defined as a fasting blood glucose level between 5.6 mmol/L and 6.9 mmol/L, whereas the normal value (N) for fasting blood glucose is <5.6 mmol/L or impaired glucose tolerance (IGT) (2-h values in the OGTT) is between 7.8 mmol/L and 11.0 mmol/L. OGTT was performed in each patient. Patients with blood glucose level ≥ 11.0 mmol/L were selected for the NODAT group. The general principle behind standardized NODAT incidence reporting is that the diagnostic criteria should reflect what is used in the general population. Hopefully, these developments will allow for more consistent reporting of NODAT in the future, leading in turn to more precise estimates of incidence rates.
The aim of our study was to compare the risk factors and incidence of NODAT and evaluate the body of evidence linking hypomagnesemia to clinical consequences in these specific populations, and we focuses on the relationship between hypomagnesemia and cardiovascular risk in kidney transplant recipients.
Our prospective study was performed in the Department of Surgery, University of Szeged, Hungary. Patients who had cadaver kidney transplantation within at least one year, were above the age of 18, had no diabetes mellitus in the past medical history, have not received steroid burst therapy, and had no cardiac disease in the past medical history were enrolled in the study. A total of 60 patients were involved in our study.
Basic data (age, gender, and BMI), time spent in hemodialysis, were analyzed. Laboratory tests were performed including serum Mg, HbA1C, renal functions (serum creatinine, estimated glomerular filtration rate (eGFR), and urea) and urinalysis (total protein and glucose secretion). Then OGTT was performed as well, during which glucose and insulin levels were measured 0 and 120 minutes after the administration of 75 g oral glucose, and then insulin resistance was calculated by using the homeostatic model assessment (HOMA) index (fasting glucose * fasting glucose / 22.5). Based on the value, the risk of insulin resistance (IR) can be determined, if the value is above 2, the patient is susceptible to have diabetes mellitus, and in case of a value above 4, the patient has IR.
Our study was approved by the Regional Human Biomedical Research Ethics Committee of the Albert Szent-Györgyi Clinical Center, University of Szeged (Reg. No.: 18/2017-SZTE). Each patient was provided comprehensive information regarding the study.
Magnesium physiology
Magnesium (Mg) is the fourth cation of the body and the second most prevalent intracellular cation [5]. Approximately half of total body Mg is located in bone [6], the remainder being contained in skeletal muscles and soft tissues [5]. Extracellular Mg represents only 1% of total body Mg [7] and is mostly found in serum with concentrations ranging between 0.65 to 1.05 mmol/L [8] and in red blood cells [4]. It is present in three different states: ionized Mg (55–70%), protein-bound Mg (20–30%), and Mg complexed with anions such as bicarbonate or phosphate (5–15%) [5].
Mg homeostasis is mainly dependent on intestinal absorption and renal excretion. In the intestine, absorption is modulated by luminal Mg concentration, at high concentrations, Mg is regulated by an active transcellular transport and passive paracellular diffusion; whereas in low concentrations, Mg is absorbed by an active transcellular pathway involving a Transient Receptor Potential Melastatin 6 (TRPM6) channel expressed on the small intestine cells [5].
Statistical methods
Continuous data were expressed as mean ± standard deviation, categorical data were expressed as number of cases and percentages. Univariate comparisons were performed by Welch’s ANOVA or Chi-square test for continuous or categorical variables, respectively. A p-value p<0.05 was regarded statistically significant. Statistical software IBM SPSS statistics version 24 (64bit) was used.
In our study, there were 30 patients in the normal group, 23 patients in the IFG group, and 7 patients in the NODAT at Minute 0 of OGTT. The ratio of patients changed at Minute 120 of the test: there were 22 (37%) patients in the normal group, 26 (43%) patients in the IFG/IGT group, and 12 (20%) patients in the NODAT group (Table 1). After the transplantation, every patient was administered steroid therapy. The risk factors of diabetes were examined to determine which factors influence the development of diabetes mellitus significantly. Regarding basic data, gender and age did not influence the development of diabetes mellitus significantly, although patients with NODAT spent more time in HD; the difference was not statistically significant (p=0.662). In our study, BMI (26.55 ± 3.84 vs. 28.58 ± 5.26; p=0.854) did not play a significant role in the development of NODAT (Table 2).
Table 1. Results of the OGTT
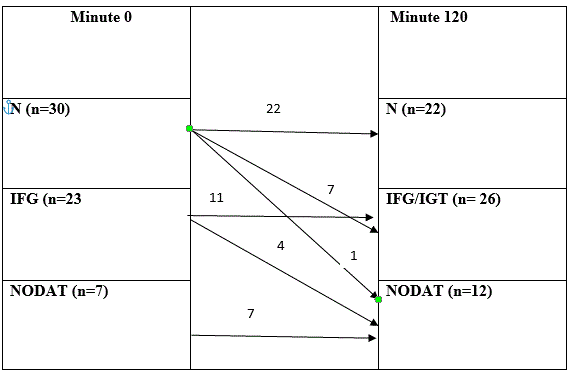
N-normal; IFG- Impaired fasting glucose; IGT- impaired glucose tolerance; NODAT- new onset diabetes after transplantation.
Table 2. Basic data of the different glucose metabolism groups
|
|
N
(n=22)
mean ± SD
|
IFG/IGT (n=26)
mean ± SD
|
NODAT (n=12)
mean ± SD
|
p value
|
|
age (years)
|
48.86 ± 5.83
|
48.38 ± 6.85
|
48.83 ± 7.16
|
0.616
|
|
gender (women/men)
|
7/15
|
10/16
|
5/7
|
0.824
|
|
BMI (kg/m2)
|
26.55 ± 3.84
|
26.43 ± 3.41
|
28.58 ± 5.26
|
0.854
|
|
hemodialysis (months)
|
22.91 ± 20.30
|
29.38 ± 36.16
|
20.58 ± 25.26
|
0.662
|
N-normal; IFG- Impaired fasting glucose; IGT- impaired glucose tolerance; NODAT- new onset diabetes after transplantation; BMI- body mass index; SD- standard deviation.
The results of laboratory tests showed no significant difference in serum magnesium (p=0.001). Renal function parameters, such as serum creatinine (p=0.001) and eGFR (p=0.0001) were significantly different, while urea (p=0.176) level did not differ significantly between groups with different carbohydrate metabolism. The urine total protein level was significantly different in the groups (p=0.0001). HbA1C (p=0.009) and HOMA index were considered to be the indicator of insulin resistance (p=0.09) were significantly different as well. Significant difference was found in the total urine protein level between the different glucose metabolic groups (Table 3). The analysis of the glucose content of the urine showed that there was no glucose in the urine except for 4 cases of patients having NODAT, and 3 (+) glucose was found in the urine in 2 cases. Patients having normal carbohydrate metabolism had no glucose in the urine (p=0.0001).
Table 3. Results of the blood and urine tests
|
|
N
(n=22)
mean ± SD
|
IFG/IGT (n=26)
mean ± SD
|
NODAT
(n=12)
mean ± SD
|
p value
|
magnesium
|
0.76 ± 0.09
|
0.72 ± 0.10
|
0.45 ± 0.08
|
0.001
|
se. creatinine (µmol/L)
|
131.45 ± 18.25
|
120.88 ± 32.69
|
247.33 ± 91.35
|
0.001
|
urea
(µmol/L)
|
10.31 ± 4.46
|
9.69 ± 6.71
|
14.02 ± 9.65
|
0.176
|
eGFR(mL/min/1.73m2)
|
50.90 ± 11.27
|
58.46 ± 17.94
|
27.25 ± 15.83
|
0.0001
|
urine total protein
|
14.10 ± 15.21
|
26.53 ± 22.48
|
207.18 ± 154.30
|
0.0001
|
HbA1C
|
5.59 ± 0.51
|
5.57 ± 0.43
|
6.62 ± 0.91
|
0.003
|
HOMA 1 index (IR)
|
1.96 ± 1.24
|
2.36 ± 3.20
|
6.68 ± 7.02
|
0.09
|
N-normal; IFG- Impaired fasting glucose; IGT- impaired glucose tolerance; NODAT- new onset diabetes after transplantation; eGFR- estimated glomerular filtration rate; IR- insulin resistance; HOMA- homeostatic model assessment.
After kidney transplantation, the incidence of NODAT was 20%. Chronic kidney disease (CKD) is frequently associated with hypermagnesaemia, which is usually mild or asymptomatic until end-stage renal disease (ESRD). In moderate CKD patients, increased fractional excretion of Mg compensates for a decline in renal function, providing a stable serum Mg within the normal range Clinical manifestations of hypomagnesemia are quite unspecific. Early signs include nausea, vomiting, anorexia and weakness [5]. Neuromuscular signs can also be present, including numbness, tingling, cramps, fasciculation seizures and neuropsychological disorders [5]. Severe hypomagnesemia has been associated with cardiac arrhythmia and coronary spasm [8-13]. In our clinical study, it was found that the recipients basic data were not significantly different concerning the onset of NODAT, and the difference in the BMI was also not significant, although the average BMI of patients having NODAT was higher compared to patients with normal carbohydrate metabolism [14-16]. The diabetogenic effect of glucocorticoids, mainly due to insulin resistance, is mediated by both impaired insulin-dependent glucose uptake in the peripheral tissues and enhanced gluconeogenesis in the kidney. We did not investigate the effect of steroids on NODAT because all patients received steroids after kidney transplantation.
Hypomagnesemia is frequently observed after kidney transplantation, in part to immunosuppressive regimens including calcineurin inhibitors (CNI) that induce Mg urinary waste. Hypomagnesemia was observed in 6.6% of patients treated with tacrolimus and in 1.5% of patients on cyclosporine [17]. The mechanisms leading to hypomagnesemia are not fully understood, but it has been shown that CNI induce a down-regulation of renal expression of the epidermal growth factor [18] and TRMP6 in the distal collecting tubule [19], leading to decreased Mg reabsorption. Sirolimus might induce hypomagnesemia through inhibition of Na-K-Cl co-transporter 2 expression in the thick ascending loop of Henle [20]. Renal Mg wasting has been shown to be similar between rats treated with sirolimus and those treated with cyclosporine or tacrolimus [21]. Many other factors influence Mg levels after kidney transplantation, such as post-transplantation volume expansion, metabolic acidosis, insulin resistance, decreased gastro-intestinal absorption due to diarrhea, low Mg intake and medication such as diuretics or proton pump inhibitors [22]. In a Japanese cohort of 728 subjects, lower serum Mg was significantly and independently associated with mean intima-media thickness (p = 0.004) and risk of ≥2 carotid plaques (p = 0.03) [23]. Hypomagnesemia was also reported to directly or indirectly affect vascular stiffness in the general population [24]. In another study, Mg supplementation improved endothelial dysfunction in patients with CHD [25].
Hypomagnesemia was reported to develop frequently within the first few weeks following transplantation [26], with a serum Mg level nadir in the second month post-transplantation [24]. Hypomagnesemia may persist for several years after kidney transplantation. In a cohort of 49 kidney transplant recipients, 22.4% of patients had hypomagnesemia 6 years after transplantation [20]. As observed in the general population, serum Mg levels were inversely correlated with glomerular filtration rate [22].
Hypomagnesemia is associated with a higher rate of NODAT but not with a reduced risk for graft failure. In regard to the function of the allograft, we found that there was significant difference in the serum creatinine (p=0.001) and eGFR levels (p=0.0001). NODAT leads to the deterioration of renal function and urinary proteinuria (p=0.0001).
If it is clear that lifestyle modification alone will be insufficient to control hyperglycemia, pharmacotherapy targeting glucose metabolism should be initiated. In the very early post-transplant phase, when corticosteroids are being rapidly tapered, additional pharmacotherapy may not be required if the hyperglycemia is mild. The choice between insulin and oral hypoglycemic agents depends on the severity, timing, and expected duration of hyperglycemia. Insulin therapy is safe, particularly when the graft function is not yet established or is unstable.
- First MR, Dhadda S, Croy R, Holman J, Fitzsimmons WE (2013) New-onset diabetes after transplantation (NODAT): an evaluation of definitions in clinical trials. Transplantation 96: 58-64. [Crossref]
- Lv C, Chen M, Xu M, Xu G, Zhang Y, et al. (2014) Influencing factors of new-onset diabetes after a renal transplant and their effects on complications and survival rate. PLoS One 9: e99406. [Crossref]
- Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, et al. (2003) International Expert Panel: New-onset diabetes after transplantation: International Consensus Guidelines. Proceedings of an international expert panel meeting. Transplantation 75: SS3-SS24.
- American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33: S62-S69.
- Jahnen-Dechent W, Ketteler M (2012) Magnesium basics. Clin Kidney J 5: i3-3i14. [Crossref]
- Weisinger JR, Bellorín-Font E (1998) Magnesium and phosphorus. Lancet 352: 391-396. [Crossref]
- Fawcett WJ, Haxby EJ, Male DA (1999) Magnesium: physiology and pharmacology. Br J Anaesth 83: 302-320. [Crossref]
- Tietz NW, Rinker AD, Morrison SR (1994) When is a serum iron really a serum iron? The status of serum iron measurements. Clin Chem 40: 546-551. [Crossref]
- England MR, Gordon G, Salem M, Chernow B (1992) Magnesium administration and dysrhythmias after cardiac surgery. A placebo-controlled, double-blind, randomized trial. JAMA 268: 2395-2402.
- Margreiter R (2002) European Tacrolimus vs. Ciclosporin Microemulsion Renal Transplantation Study Group Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: A randomised multicentre study. Lancet 359: 741-746.
- Ledeganck KJ, De Winter BY, Van den Driessche A, Jürgens A, Bosmans JL, et al. (2014) Magnesium loss in cyclosporine-treated patients is related to renal epidermal growth factor downregulation. Nephrol Dial Transplant 29: 1097-1102.
- Nijenhuis T, Hoenderop JG, Bindels RJ (2004) Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15: 549-557. [Crossref]
- Da Silva CA, de Bragança AC, Shimizu MHM, Sanches TR, Fortes MAZ, et al. (2009) Rosiglitazone prevents sirolimus-induced hypomagnesemia, hypokalemia, and downregulation of NKCC2 protein expression. Am J Physiol Renal Physiol 297: F916-F922.
- Borda B, Szederkényi E, Lengyel C, Morvay Z, Eller J, et al. (2011) Functional and histopathological changes in renal transplant patients with mew-onset diabetes and dyslipidaemia. Transplant Proc 43: 1254-1258.
- Sarno G, Muscogiuri G, De Rosa P (2012) New-onset diabetes after kidney transplantation: prevalence, risk factors, and management. Transplantation 93: 1189-1195.
- Andoh TF, Burdmann EA, Fransechini N, Houghton DC, Bennett WM (1996) Comparison of acute rapamycin nephrotoxicity with cyclosporine and FK506. Kidney Int 50: 1110-1117. [Crossref]
- Van Laecke S, Van Biesen W (2015) Hypomagnesaemia in kidney transplantation. Transplant Rev (Orlando) 29: 154-160. [Crossref]
- Hashimoto T, Hara A, Ohkubo T, Kikuya M, Shintani Y, et al. (2010) Serum Magnesium, Ambulatory Blood Pressure, and Carotid Artery Alteration: The Ohasama Study. Am J Hypertens 23:1292-1298.
- Kisters K, Gremmler B, Hausberg M (2006) Magnesium and arterial stiffness. Hypertension 47: e3. [Crossref]
- Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, et al. (2000) Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 102: 2353-2358.
- Van Laecke S, Van Biesen W, Verbeke F, De Bacquer D, Peeters P, et al. (2009) Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. Am J Transplant 9: 2140-2149.
- Huang JW, Famure O, Li Y, Kim SJ (2016) Hypomagnesemia and the Risk of New-Onset Diabetes Mellitus after Kidney Transplantation. J Am Soc Nephrol 27: 1793-1800.
- Hayes W, Boyle S, Carroll A, Bockenhauer D, Marks SD (2017) Hypomagnesemia and increased risk of new-onset diabetes mellitus after transplantation in pediatric renal transplant recipients. Pediatr Nephrol 32: 879-884.
- Osorio JM, Bravo J, Pérez A, Ferreyra C, Osuna A (2010) Magnesemia in Renal Transplant Recipients: Relation With Immunosuppression and Posttransplant Diabetes. Transplant Proc 42: 2910-2913.
- Sánchez-Fructuoso AI, Santín Cantero JM, Pérez Flores I, Valero San Cecilio R, Calvo Romero N, et al. (2010) Changes in magnesium and potassium homeostasis after conversion from a calcineurin inhibitor regimen to an mTOR inhibitor-based regimen. Transplant Proc 42: 3047-3049.
- Van Laecke S, Nagler EV, Taes Y, Van Biesen W, Peeters P, et al. (2014) The effect of magnesium supplements on early post-transplantation glucose metabolism: A randomized controlled trial. Transpl Int 27: 895-902.

